Affiliation:
Rheumazentrum Ruhrgebiet, Ruhr-University Bochum, 44649 Herne, Germany
†These authors contributed equally to this work.
Email: david.kiefer@elisabethgruppe.de
ORCID: https://orcid.org/0000-0003-1602-7649
Affiliation:
Rheumazentrum Ruhrgebiet, Ruhr-University Bochum, 44649 Herne, Germany
†These authors contributed equally to this work.
Affiliation:
Rheumazentrum Ruhrgebiet, Ruhr-University Bochum, 44649 Herne, Germany
†These authors contributed equally to this work.
ORCID: https://orcid.org/0000-0002-9156-5095
Explor Musculoskeletal Dis. 2023;1:11–19 DOI: https://doi.org/10.37349/emd.2023.00003
Received: August 05, 2022 Accepted: January 03, 2023 Published: February 24, 2023
Academic Editor: Peter Mandl, Medical University of Vienna (MUW), Austria
Gout often presents as acute arthritis but may also present with chronic joint inflammation. For the diagnosis of an acute gout attack with its typical symptoms, the differentiation towards a bacterial joint infection is critical and mandatory. The detection of intracellular uric acid crystals in the synovial fluid of affected joints is important for the initial diagnosis of gout. In the case of a chronic course with polyarticular joint involvement, the differentiation from other inflammatory rheumatic diseases such as rheumatoid arthritis (RA) can be challenging. The case presented here is of interest because the patient initially had characteristic clinical symptoms of tophaceous gout including a typical medical history—even though rheumatoid factor and anti-citrullinated protein antibodies (anti-CCP) were positive. The course of the disease and the critical evaluation of all findings also, and most interestingly, including histological results finally suggested a main diagnosis of RA.
Arthritis due to gout, spondyloarthritis, and rheumatoid arthritis (RA) is, with a prevalence of roughly 1% for RA and a range from < 1% to 6.8%, depending on different study populations for gout, the most common causes of arthritis worldwide [1–6]. An acute gouty attack with its typical symptoms, in form of severe pain with redness and swelling of the typically affected joints, represents a diagnostic challenge only in so far as a bacterial infection has to be excluded, and the gain of synovial or tophaceous material is sometimes not trivial. This matters for the diagnosis, since the ultimate proof of gout does rely on the intraarticular detection of urate crystals. The diagnosis is more difficult in chronic disease stages with a polyarticular course, and in some cases even in the tophaceous stage [7]. In this case, it is often not so easy to distinguish chronic gout from RA [8–11]—especially since both diseases may, reportedly, occur together [12, 13]. This case report is meant to serve as a basis to discuss differential diagnostic problems of gouty arthritis.
Classical gout (arthritis urica) is characterized by an acute attack of mon- or oligoarthritis with redness, swelling, and severe pain which may occur after excessive alcohol and/or meat intake or during/after fasting. The serum uric acid level is often but not always elevated in acute attack. Normally the differential diagnosis is more difficult. There is quite some tropism in gout: the first metatarsophalangeal (MTP) joint is frequently affected, but other joints such as knee or ankle joints may also be affected. In the early phases of the disease, gout occurs mainly as mono- or oligoarthritis, mainly in the lower extremities, and later increasingly as polyarthritis, also in the upper extremities. Extra-articular manifestations include bursitides of the elbow (bursitis olecrani) and knee (bursitis praepatellaris) are quite common—as are insertional tendinopathies of the Achilles and patellar tendons. The chronic, tophaceous and/or erosive course of gouty arthritis usually occurs only after long persistent hyperuricemia [7]. This results in the tissue deposition of sodium urate, which may occur intra- and periarticularly but also in other soft tissues where it potentially becomes visible as gout tophi. In the chronic course of the disease, the frequency of gout attacks increases and the recurrent arthritis does not infrequently lead to joint destruction resulting in functional deficits. In acute and chronic gout, inflammatory serum markers such as C-reactive protein (CRP) are usually markedly elevated. Chronic renal insufficiency may be the cause of hyperuricemia but also comorbidity like cardiovascular disease and metabolic syndrome which commonly occur together.
In addition to the acute management with colchicine, non-steroidal anti-inflammatory agents (in case of normal renal function) and glucocorticoids administered both, intraarticularly and systemically, anti-interleukin-1 (anti-IL-1) directed biologic agents may be indicated and used in more severe cases. This part of the treatment strategy is acute and anti-inflammatory aiming to reduce pain and abolish inflammation. In parallel, preventive therapeutic strategies are started to permanently lower serum uric acid levels to reduce gout attacks and decrease the number and size of tophi. For this purpose, urate-lowering treatment with xanthine oxidase inhibitors such as allopurinol and febuxostat is most commonly used.
The management of gout can be challenging for multiple reasons [14]. To ascertain the diagnosis of gout, the current gold standard is the detection of uric acid crystals in the synovial fluid, in periarticular tissues, or directly in tophi. Unfortunately, arthrocentesis is often not possible for technical and other reasons such as patients’ pain. Imaging techniques such as arthrosonography have been shown to potentially support a diagnosis of gout since the detection of the so-called double contour phenomenon at the articular cartilage as well as topheous deposits and/or bony erosions, preferentially located peri- or intraarticularly—so-called punched-out lesions are suggestive of being caused by gout [8]. Another, relatively new imaging technique, dual-energy computed tomography (DECT), is a diagnostic tool that can detect uric acid crystals based on their specific X-ray absorption behavior [14–16].
RA is a chronic inflammatory rheumatic disease that typically manifests as symmetric polyarthritis. Wrists, metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints, and MTP joints are frequently affected [17]. However, almost all synovial joints can be involved. RA usually manifests with joint pain and swelling, often associated with morning stiffness exceeding 30 min [18]. Extra-articular manifestations such as pulmonary involvement are rather frequent but other features such as rheumatoid nodules and vasculitis are less common. RA usually runs a chronic course potentially leading to joint destruction, tendon rupture, deformities, and functional loss leading to reduced physical activity and participation. Risk factors for an aggressive erosive course of RA are in particular seropositivity with autoantibodies to immunoglobulin G (rheumatoid factor) and/or to anti-citrullinated protein antibodies (anti-CCP) as well as smoking and elevated acute phase reactants [17, 19, 20].
Immunomodulatory drugs are the backbone of treatment which are supposed to reduce inflammation and prevent structural damage to joints and tendons in RA. In case this is proven, they are named disease-modifying antirheumatic drugs (DMARDs). They are conventional DMARDs (cDMARDs), such as methotrexate with a not completely clarified mode of action, or biologic DMARDs (bDMARDs), such as the tumor necrosis factor (TNF) inhibitors and targeted synthetic DMARDs (tsDMARDs) such as the Janus-kinase (JAK) inhibitors.
Some clinical differences between chronic tophaceous gout and RA are listed in Table 1. The initial therapeutic strategies comprise non-steroid anti-inflammatory drugs (NSAIDs) and glucocorticoids usually lead to rapid improvement of symptoms. However, since the other targeted therapeutic strategies differ substantially a solid confirmation of the diagnosis is mandatory and much wanted by the responsible rheumatologist and/or general practitioner [14, 21].
Summary of similarities and differences between acute and chronic gout and RA
| Symptoms and diagnostics | Acute gout attack | Chronic tophaceous gout | RA |
|---|---|---|---|
| Clinical pattern | Frequently monoarthritis, large joints such as knee, ankle, and MTP joint | Frequently polyarticular, soft tissue tophi | Symmetrical, mostly small joints (MCP, PIP, MTP, wrists), ulnar deviation, swan neck deformity, buttonhole deformity, rheumatoid foot deformity |
| Symptoms | Redness, swelling, pain, possibly fever | Redness, swelling, pain, possibly fever | Swelling, pain, stiffness |
| Extraarticular manifestation | Subcutaneous edema due to inflammation | Bursa, tendon and soft tissue involvement, extraarticular tophi | Bursa, tendons, vessels, serous membranes, pulmonary involvement, vasculitis |
| Joint ultrasound | Double contour sign, synovitis, positive power doppler signal | Double contour sign, synovitis, positive power doppler signal, effusion tophi, punched-out lesions | Synovitis, positive power doppler erosions, structural damage |
| X-ray | Initially often no bony changes, soft tissue swelling | Tophi, soft tissue tophi, punched-out lesions | Soft tissue swelling, structural changes like erosions, subluxations, deformation, ankyloses |
| Dual energy computed tomography (CT) | Urat crystals are detectable, but in early stages (usually < 6 weeks) the DECT may show false negative results | Urat crystals are detectable if the mass of crystals is high enough | Osteitis in the form of bone marrow edema, synovitis, synovial fluid, structural changes |
| Synovial fluid analysis | Inflammatory synovial fluid (usually increased leukocyte count and a high percentage of granulocytes and rhagocytes) and detection of negative birefringence crystals in polarized light microscopy | Inflammatory synovial fluid with mostly increased cell count and detection of urate crystals in polarized light microscopy | Inflammatory synovial fluid with mostly increased cell count |
| Histology rheumatoid nodules/gouty tophus | Evidence of urate crystals | Evidence of urate crystals | Central fibrinoid necrosis surrounded by histiocytes and epithelioid cells |
History: A 47-year-old man presented to a specialized hospital with pain and swelling of elbows, wrists, and feet which had first appeared about 3 years ago. The complaints were first described as more like acute attacks and later as both, attack-like and persistent. The patient also reported morning stiffness of about 30 min and alcohol abuse, preferably vodka, on a daily basis, frequent use of soft drinks, and a meat-enriched diet. Clinically, podagra of the right big toe and swelling of both elbows with palpable solid structures in the olecranon bursa and the forearm were suggestive of gout tophi (Figure 1). Informed consent for publication was obtained from the participant.
The uric acid level was normal (6.3 mg/dL), and CRP was slightly elevated (1.1 mg/dL, normally < 0.5 mg/dL) with a rather normal erythrocyte sedimentation rate (ESR) of 22 mm/1 h. Rheumatoid factor (418 IU/mL, normally < 14 IU/mL) and anti-CCP antibodies were detected in high titers (236 IU/mL, normally < 40 IU/mL).
A conventional radiograph revealed tophus-suspect soft tissue compaction lateral to the 5th MTP in both feet accentuated on the right, as well as tophus-suspect soft tissue compaction medial to the first MTP on the right (Figure 2). By magnetic resonance imaging (MRI), an oval structure, approximately 3 cm in size, was seen ventral to the right proximal tibia in the subcutaneous adipose tissue, which morphologically appeared as an intermediate to low signal on a T1 weighted image, possibly an intratenosynovial tophus (Figure 3). In addition, peritenosynovial inflammation and a soft tissue spot suggestive of another tophus at the right flexor hallucis longus tendon were seen. However, no structure suggestive of urate in that region was detected by DECT. A massive tophus-suspect soft tissue compression or swelling dorsal to the left elbow, and, correspondingly, on the right proximal ulna could, unfortunately, not be punctured because the patient didn’t consent.
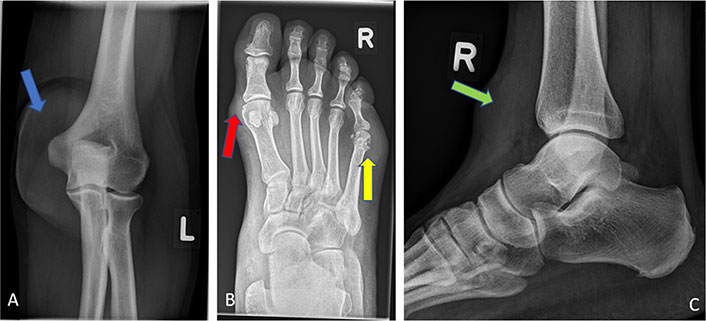
X-ray. A) X-ray (anterior-posterior) of the left elbow without erosive changes but massive tophus suspected soft tissue thickening (blue arrow); B) radiograph of the right foot anterior-posterior with tophus suspected soft tissue thickening lateral to the MTP5 joint and erosions/usures of the MTP5 head (yellow arrow). Tophus suspicious soft tissue thickening medial of MTP1 and MTP1 head with medial small erosions (red arrow); C) radiograph of the lateral upper ankle with tophus and soft tissue thickening ventral to the right-proximal tibia in subcutaneous fat (green arrow)
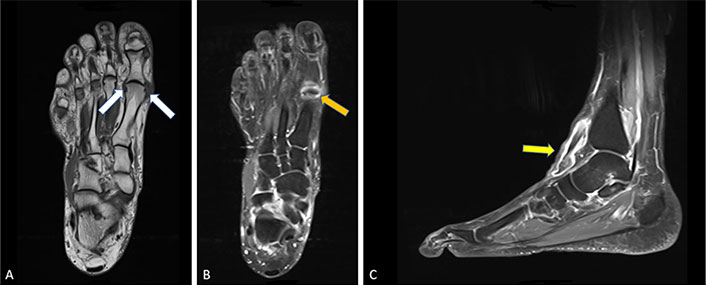
MRI of the right foot. A) T1 weighting transversal: small marginal erosion head MTP1 medial, soft tissue plus compatible with tophus medial of head MTP1 (white arrows); B) T1 with contrast transversal: synovialitis MTP1 (orange arrow); C) T1 with contrast sagital: severe tenovaginitis of tibialis anterior tendon intratenosynovial signal-poor structures as in intratenosynovial tophus (yellow arrow)
In addition, X-rays of the forefeet showed small and larger erosions on the left and right MTP1 joint, as well as on the left basophalanx base DII and right MTP5 (Figure 2).
The summarization of all findings of the clinical pictures was interpreted as typical for advanced erosive tophaceous gout.
A therapy with celecoxib [200 mg once a day (OD)], prednisolone (decreasing from 30 mg), colchicine, and pantoprazol 40 mg/day was started. Allopurinol [300 mg twice a day (BID)] had already been initiated by the primary care physician several weeks ago. There was a rapid clinical improvement of pain and reduction of inflammation. However, due to the positive rheumatoid factors, the radiographically detected erosions, and the negative DECT, there was residual diagnostic uncertainty and, since an elective bursectomy of the left elbow had to be performed within a few days, a biopsy was taken during the operation (Figure 4). The pathologic evaluation of the biopsy sample revealed chronic florid abortive granulomatous bursitis, most compatible with rheumatoid granuloma, with, again, no evidence of uric acid crystals (Figure 5). Thus, the diagnosis was changed to seropositive RA, and accordingly, therapy with methotrexate was started. In parallel, inpatient treatment of the alcoholic disease was initiated. The patient presented with just a few complaints but no more arthralgia in an outpatient control visited 3 months later.
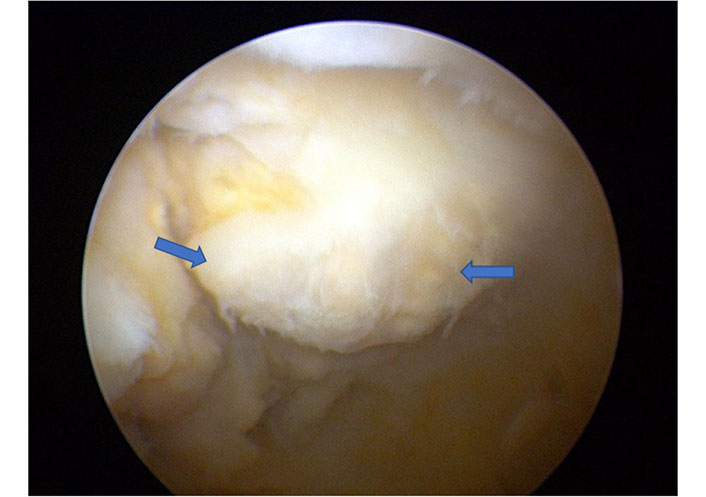
Arthroscopic findings of the left elbow with nodular soft tissue changes (blue arrows)
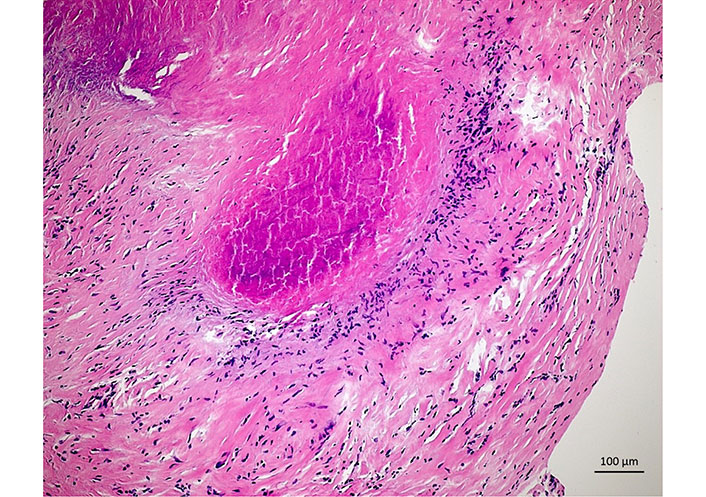
Histology. Histology of left elbow: portions of a fibrous bursal wall with rich capillarization and follicularly organized fibrin clots, marginal small fibrinoid necrosis zone due to wall-like granulation tissue, overall consistent with rheumatoid granuloma
The reason for publishing this case is to draw attention to the from time to time difficult differential diagnosis between chronic gout and RA. Our clinical findings of this patient initially seemed to favor a diagnosis of gout but the histology taken from the left olecranon bursa told a different story making RA the more likely diagnosis. However, a mixed picture and the presence of two diagnoses in consideration of the lifestyle of this patient and the initially typical podagra are still possible and cannot be excluded. This case reminds us that, even with rather typical manifestations of a clinical picture, the possible differential diagnosis has to be critically discussed, and even though histology is usually not needed to diagnose RA, in special cases as here, it might be useful. Since the clinical picture of RA and gout, especially in chronic cases, can be rather similar, imaging techniques such as MRI and DECT can be helpful for the differential diagnosis but arthrocentesis to be able to examine synovial fluid and sometimes even a biopsy to allow for histologic analyses may still be important for a correct diagnosis. The patient presented here may possibly even suffer from both diseases which may become more apparent in the course of the disease. At present, the patient is in remission regarding his RA and his uric acid level has remained < 5 mg/dL.
For many years the common opinion was that gout and RA rarely coexist but only a few studies have been performed. Several hypotheses related to the pathogenesis were put forward some decades ago, none of which turned out to be true. These included the binding of rheumatoid factor to urate crystals could block their surface activity [22, 23], or hyperuricemia directly inhibiting the secondary polyarthritis [24], for example, because of antioxidant effects [25]. In conclusion, there is no evidence of whether and how the pathogenetic effects of these two diseases could possibly influence each other. However, data is suggesting that RA and gout do coexist and some even found an increased prevalence compared to the general population.
Indeed, in one population-based retrospective study which confirmed the rare coexistence of these two diseases a lower prevalence of gout was found in RA patients compared to the general population [26]. In contrast, in a population-based cross-sectional study from Israel using a medical database with > 11,000 RA patients and > 50,000 controls the proportion of patients with concomitant gout (1.61%) was significantly higher than in controls (0.92%), and, by multivariate analysis, RA was even found to be associated with gout [odds ratio (OR) = 1.72, 95% confidence interval (CI) = 1.45–2.05], however, database analyses like this are known to methodologically suffer from the general lack of ascertainment which seems to be especially critical with the study question asked here [12]. In a longitudinal study with almost 2,000 RA patients, a high frequency of hyperuricemia (17%) and gout (6%) was reported [27]. A study from New Zealand on patients with tophaceous gout (the majority being Polynesian or Maori) revealed a prevalence of anti-CCP antibodies of 4%—this is relatively rare but may occur in patients with tophaceous gout [28]. Both, monosodium urate crystals and rheumatoid nodules, were detected in the biopsy material of the knee joint of a 42-year-old patient presenting with multiple subcutaneous nodules and intermittent episodes of acute arthritis [29]. Using DECT, it was recently shown that many RA patients with concomitant hyperuricemia had periarticular monosodium urate crystal deposits in hand and foot joints [30]. However, whether these findings contribute to the clinical symptoms of the patients is not clear. In addition, this may be similar to other diseases such as osteoarthritis.
Several case reports and series of RA and gout appearing concomitantly have been published, in some cases, the RA started earlier, and in others, it was gout [13, 22–24, 31–34]. This supports the hypothesis that the simple explanation is that they may just occur together because of their relatively high prevalence in the population (both about 1%).
These data indicate that, contrary to popular beliefs, RA and gout do coexist in patients [31], and this may even be more frequent than previously thought. Thus, this knowledge and awareness are important for finding solutions in difficult clinical cases and for good treatment decisions.
In summary, the differential diagnosis between RA and chronic gout, both rather frequent rheumatic diseases, can be challenging, and the diseases may even occur in parallel—with no rules about which one comes first. The here presented case describes the clinical problems and challenges associated with the differential diagnosis of RA vs. gout. The case also shows that the first diagnosis doesn’t always stay the same in the course of the disease and that in the management of rheumatic diseases, all available evidence may be needed to solve clinical problems and draw meaningful conclusions.
anti-CCP: anti-citrullinated protein antibodies
DECT: dual-energy computed tomography
DMARDs: disease-modifying antirheumatic drugs
MRI: magnetic resonance imaging
MTP: metatarsophalangeal
RA: rheumatoid arthritis
DK: Conceptualization, Writing—original draft, Writing—review & editing. JE: Conceptualization, Writing—original draft, Writing—review & editing. JB: Conceptualization, Writing—original draft, Writing—review & editing.
The authors declare that they have no conflicts of interest.
Not applicable.
Informed consent to participate in the study was obtained from all participants.
Informed consent to publication was obtained from relevant participants.
Not applicable.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
