Affiliation:
1Rheumatology Department, Hospital Universitario Infanta Leonor, 28031 Madrid, Spain
2Department of Medicine, Universidad Complutense de Madrid, 28040 Madrid, Spain
3Crystal-induced Arthritis Study Group, Spanish Society of Rheumatology (GEACSER), 28001 Madrid, Spain
Email: ecalvoa@hotmail.com
ORCID: https://orcid.org/0000-0002-3095-0969
Affiliation:
1Rheumatology Department, Hospital Universitario Infanta Leonor, 28031 Madrid, Spain
3Crystal-induced Arthritis Study Group, Spanish Society of Rheumatology (GEACSER), 28001 Madrid, Spain
ORCID: https://orcid.org/0009-0002-8278-8251
Affiliation:
3Crystal-induced Arthritis Study Group, Spanish Society of Rheumatology (GEACSER), 28001 Madrid, Spain
4Osakidetza, OSI EEC, Cruces University Hospital, Rheumatology Division, 48903 Barakaldo, Spain
5Department of Medicine, Medicine and Nursing School, University of the Basque Country, 48940 Leioa, Spain
ORCID: https://orcid.org/0000-0002-5268-1894
Affiliation:
3Crystal-induced Arthritis Study Group, Spanish Society of Rheumatology (GEACSER), 28001 Madrid, Spain
6Rheumatology Department, Hospital Universitario La Paz, 28046 Madrid, Spain
ORCID: https://orcid.org/0000-0002-2200-0859
Explor Musculoskeletal Dis. 2025;3:100793 DOI: https://doi.org/10.37349/emd.2025.100793
Received: December 29, 2024 Accepted: April 27, 2025 Published: May 22, 2025
Academic Editor: Valderilio Feijó Azevedo, Federal University of Paraná, Brazil
The article belongs to the special issue Pharmacological and Non-Pharmacological Management of Gout
Aim: To evaluate the efficacy and tolerability of febuxostat (FBX) and benzbromarone (BNZ) combination therapy in patients with difficult-to-treat (D2T) gout.
Methods: This observational study was performed at two centers and included patients fulfilling the 2015 European Alliance of Associations for Rheumatology/American College of Rheumatology (EULAR/ACR) gout classification criteria, with clinical tophi and suboptimal response to standard urate-lowering therapy. A two-step treatment regimen was implemented: a 6-month dose escalation of the FBX dose followed by add-on BNZ. Demographic, clinical, and laboratory data—including cardiovascular risk factors (CVRFs), history of nephrolithiasis, liver enzymes, and estimated glomerular filtration rate (eGFR)—were recorded. Changes in serum urate (SUA) and eGFR were analyzed using paired t-tests.
Results: The study population comprised 15 patients (87% male, median age 59 years) with longstanding gout [median 15 years, range 3–31; interquartile range (IQR) 8–25]. Baseline SUA was 10.3 ± 1.7 mg/dL; mean eGFR was 63.7 ± 23.6 mL/min. CVRFs were common (hypertension, 93%; dyslipidemia, 73%: major adverse cardiovascular events, 13%; diabetes, 7%). At 12 months, SUA had decreased significantly to 2.9 ± 1.1 mg/dL (Δ = 7.4 mg/dL; p < 0.01), with FBX alone contributing to a Δ of 5.4 mg/dL and BNZ an additional Δ of 2.1 mg/dL (both p < 0.01). Tophi resolved in 60% of patients. No serious adverse events or significant changes in liver or renal function were observed. One unrelated death was recorded.
Conclusions: FBX + BNZ was effective and well-tolerated in patients with severe D2T gout, achieving a substantial reduction in SUA and clinically significant dissolution of tophi.
Urate crystal arthritis, or gout, is the most common cause of non-traumatic joint inflammation, and its prevalence and incidence have been increasing in recent decades [1]. Gout results from elevated serum urate (SUA) levels that exceed the saturation threshold and persist over time, leading to the nucleation, growth, and accumulation of monosodium urate (MSU) crystals in tissues. Gout is a debilitating disease that significantly impacts patients’ quality of life and ultimately leads to functional impairment [2, 3].
Pharmacological treatment of gout using the treat-to-target (T2T) approach with urate-lowering treatments (ULTs) aims to dissolve MSU crystal deposits by reducing SUA levels to < 6 mg/dL (< 5 mg/dL in severe gout) [4]. The current standard of care (SoC) consists primarily of monotherapy with xanthine oxidase inhibitors (XOIs), such as allopurinol (ALO, usually the first-line ULT) or febuxostat (FBX). Uricosurics, including benzbromarone (BNZ), lesinurad (LSN), and probenecid (PBN), may also be used when available. Pegloticase, a recombinant uricase with a strong hypouricemic effect, is typically reserved for severe XOI-refractory tophaceous gout or in cases with contraindications [5, 6]. However, its use is limited by availability, cost, and infusion-related reactions [7].
Although the combination of XOIs and uricosurics is included in international gout management guidelines [5, 6], the few studies that have assessed its efficacy and safety mostly involved ALO [8–19]. Combination therapy with two potent ULTs, such as FBX and BNZ [BNZ being more widely available than LSN (withdrawn from the European market) and better tolerated than PBN at medium-high doses] [20], may be a therapeutic alternative for patients with severe gout, which is characterized by large subcutaneous tophi, recurrent attacks, and comorbidities. Severe gout is also referred to as difficult-to-treat (D2T) gout or D2T gouty arthritis, based on criteria used in clinical trials that led to European Medicines Agency approval of canakinumab for gout (≥ 3 attacks per year and contraindications, refractoriness, or intolerance to SoC) [21–23].
This was an observational study including patients with severe D2T gout treated at the Hospital Universitario Infanta Leonor (HUIL), Madrid; and Hospital Universitario Cruces (HUC), Barakaldo. All patients met the 2015 European Alliance of Associations for Rheumatology/American College of Rheumatology (EULAR/ACR) classification criteria for gout [24], had subcutaneous tophi, and poor therapy to SoC with XOI monotherapy. Poor therapy was defined as insufficient resolution of tophi and/or ≥ 3 gout attacks in the previous 12 months despite prophylactic treatment with non-steroidal anti-inflammatory drugs (NSAIDs), colchicine, or glucocorticoids [21–23]. Patients with severe renal impairment, active liver disease, or previous allergy or intolerance to FBX or BNZ were excluded. Combination therapy in this study was based on FBX rather than ALO, as FBX was selected for its greater urate-lowering potency and favorable pharmacokinetics, especially in patients with mild-to-moderate renal impairment, which is common in severe gout.
The treatment protocol was agreed upon prospectively by both centers based on prior clinical experience. It consisted of a two-step regimen: an initial 6-month phase comprising escalation of the dose of FBX, followed by the addition of BNZ with progressive uptitration. For patients already receiving FBX, the dose was increased to the highest tolerated level. For new FBX users, treatment was initiated at 40 mg/day with progressive escalation to the maximum tolerated dose. In the second phase, BNZ was added to FBX in all patients at 50 mg/day, with escalation to 100 mg/day if tolerated (aiming for higher doses to enhance urate lowering and tophus resolution). All patients received colchicine (0.5 mg/day) prophylaxis for at least 12 months to reduce the risk of attacks, in accordance with international recommendations [5, 6]. Prednisone or low-dose NSAIDs were used as alternatives in patients with intolerance. Despite prophylaxis, any gout flares occurring during follow-up were managed according to those recommendations, using NSAIDs, colchicine, glucocorticoids, or interleukin-1 (IL-1) blockers as clinically appropriate, based on patient factors and preferences.
Demographic, clinical, and laboratory data were collected. These included cardiovascular risk factors (CVRFs), major cardiovascular events (MACEs: myocardial infarction, stroke, hospitalization for unstable angina, coronary revascularization), history of nephrolithiasis, estimated glomerular filtration rate (eGFR), and liver function test results. Clinical evaluations included joint examination and tophi measurement (caliper, ultrasound, and, in selected cases, spectral computed tomography). Tophi resolution was evaluated using the same methods during follow-up visits. Blood tests were performed at baseline, month 1, and then every 3 months. Adherence to therapy was monitored using institutional electronic pharmacy dispensing records.
Descriptive analyses were performed using frequencies and percentages in the case of qualitative variables. For quantitative variables, measures of central tendency (mean or median) and dispersion [standard deviation (SD) or interquartile range (IQR)] were reported based on the data distribution. A paired samples t-test or a Wilcoxon test was performed to assess changes in quantitative variables. Statistical analyses were performed using SPSS version 21.0, and significance was set at p < 0.05.
The study was approved by the Ethics Committee of OSI EEC at Cruces University Hospital (CEIC-E03/45) and adhered to the principles of the Declaration of Helsinki. Written informed consent was obtained from all participants.
Data on 15 patients [HUC 9, HUIL 6 (87% male)] were collected from a database of over 2,000 patients. The median age was 59 years (range, 43–93), and the mean number of attacks per year was 5.7 ± 3.7. Disease was longstanding (median duration 15 years, range 3–31, IQR 8–25), baseline SUA was high (mean 10.3 ± 1.7 mg/dL), and the prevalence of CVRFs was high (hypertension, 93%; dyslipidemia, 73%; MACE, 13%; diabetes, 7%). Impairment of kidney function varied (mean eGFR 63.7 ± 23.6 mL/min; one patient had a history of nephrolithiasis). Baseline demographic and clinical characteristics are presented below (Table 1).
Baseline demographic and clinical characteristics of patients with severe difficult-to-treat gout (n = 15)
| Category | Data |
|---|---|
| Male sex, n (%) | 13 (87%) |
| Age, median (range) years | 59 (43–93) |
| Gout duration, mean (SD) years | 16.2 (8.8) |
| Attacks per year, mean (SD) | 5.7 (3.7) |
| Tophi, n (%) | 15 (100%) |
| Alcohol, n (%) | 8 (53%) |
| Hypertension, n (%) | 14 (93%) |
| Dyslipidemia, n (%) | 11 (73%) |
| Diabetes mellitus, n (%) | 1 (7%) |
| MACE, n (%) | 2 (13%) |
| Diuretics, n (%) | 8 (53%) |
| Urolithiasis, n (%) | 1 (7%) |
| Baseline SUA, mean (SD) mg/dL | 10.3 (1.7) |
| Baseline eGFR, mean (SD) mL/min | 63.7 (23.6) |
SD: standard deviation; MACE: major cardiovascular event (myocardial infarction, stroke, hospitalization for unstable angina, coronary revascularization); SUA: serum urate; eGFR: estimated glomerular filtration rate
All patients had received SoC but were unable to control inflammation despite receiving ULT and prophylactic treatment (colchicine, NSAIDs, or prednisone). Several patients required tetracosactide or anakinra. Before initiating FBX + BNZ, 80% had received XOIs [27% ALO (median 200 mg/day; range 100–300), and 53% FBX (median 100 mg/day; range 40–120)]. At three months, FBX doses were 80, 120, and 160 mg/day in 11, 3, and 1 patients, respectively. At six months, the doses were 80, 120, and 160 mg/day in 2, 12, and 1 patient, respectively. BNZ was then added, increasing from 50 to 100 mg/day, with 73% receiving 100 mg/day by the end of follow-up.
After a median follow-up of 18 months (range, 9–44), all patients completed the first 6-month phase of treatment (FBX monotherapy). Median BNZ exposure to BNZ was 12 months (range, 3–38), with 93.3% (14/15) of patients receiving BNZ for at least 6 months.
At 12 months, SUA had decreased significantly [Δ = 7.4 mg/dL; 95% confidence interval (CI), 6–8.7; p < 0.01] from baseline (10.3 ± 1.7 mg/dL; see Figure 1). Adding BNZ to FBX resulted in an additional reduction in SUA (Δ = 2.1 mg/dL; 95% CI, 1.2–2.9; p < 0.01), following the initial decrease observed with FBX monotherapy (Δ = 5.4 mg/dL; 95% CI, 4.4–6.4; p < 0.01), ultimately lowering SUA to 2.9 ± 1.1 mg/dL (2.7 ± 1.2 mg/dL at the last observation). At 12 months, 80% had achieved SUA < 5 mg/dL, 73.3% < 4 mg/dL, and 13.3% < 2 mg/dL, increasing to 93.3%, 93.3%, and 33.3%, respectively, at the end of follow-up.
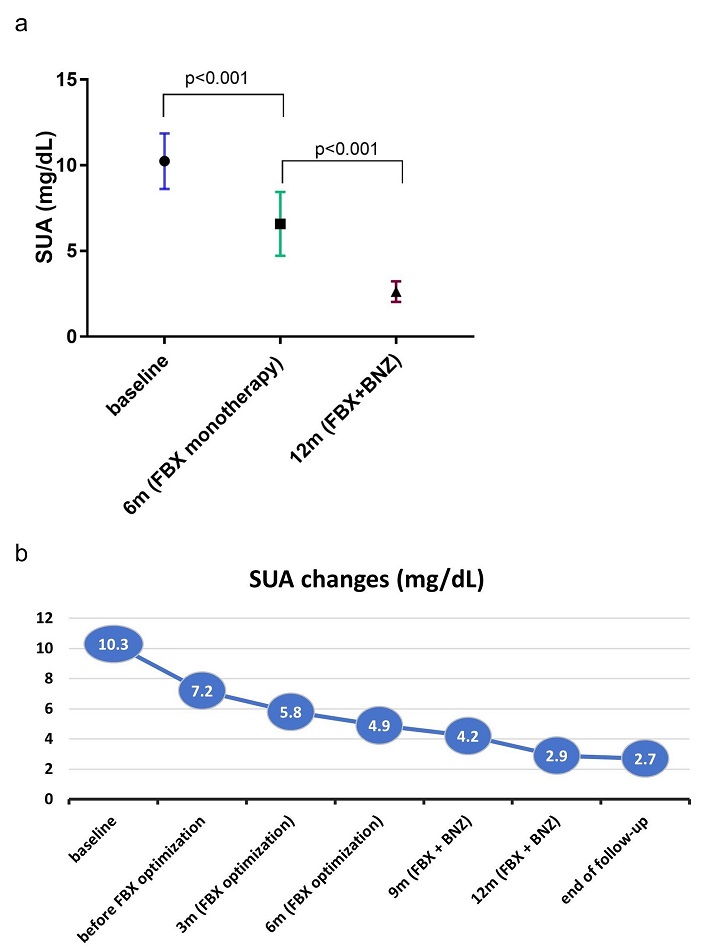
Changes in serum urate during sequential combination therapy. At 12 months, SUA had decreased significantly (Δ = 7.4 mg/dL; 95% CI, 6–8.7; p < 0.01), with FBX alone contributing to a Δ of 5.4 mg/dL and BNZ an additional Δ of 2.1 mg/dL (both p < 0.01). (a) Reduction in SUA after initial uptitration of FBX over 6 months. Data are expressed as mean ± SD. (b) Additional decrease in SUA after addition of BNZ. SUA: serum urate; FBX: febuxostat; BNZ: benzbromarone; CI: confidence interval; SD: standard deviation
A significant reduction in MSU deposition was observed, with complete resolution of tophi in 9 patients (60%) at 12 months (Figure 2). No significant differences were found in resolution of tophi between BNZ doses, although a trend towards lower SUA values was observed in those receiving 100 mg/day.
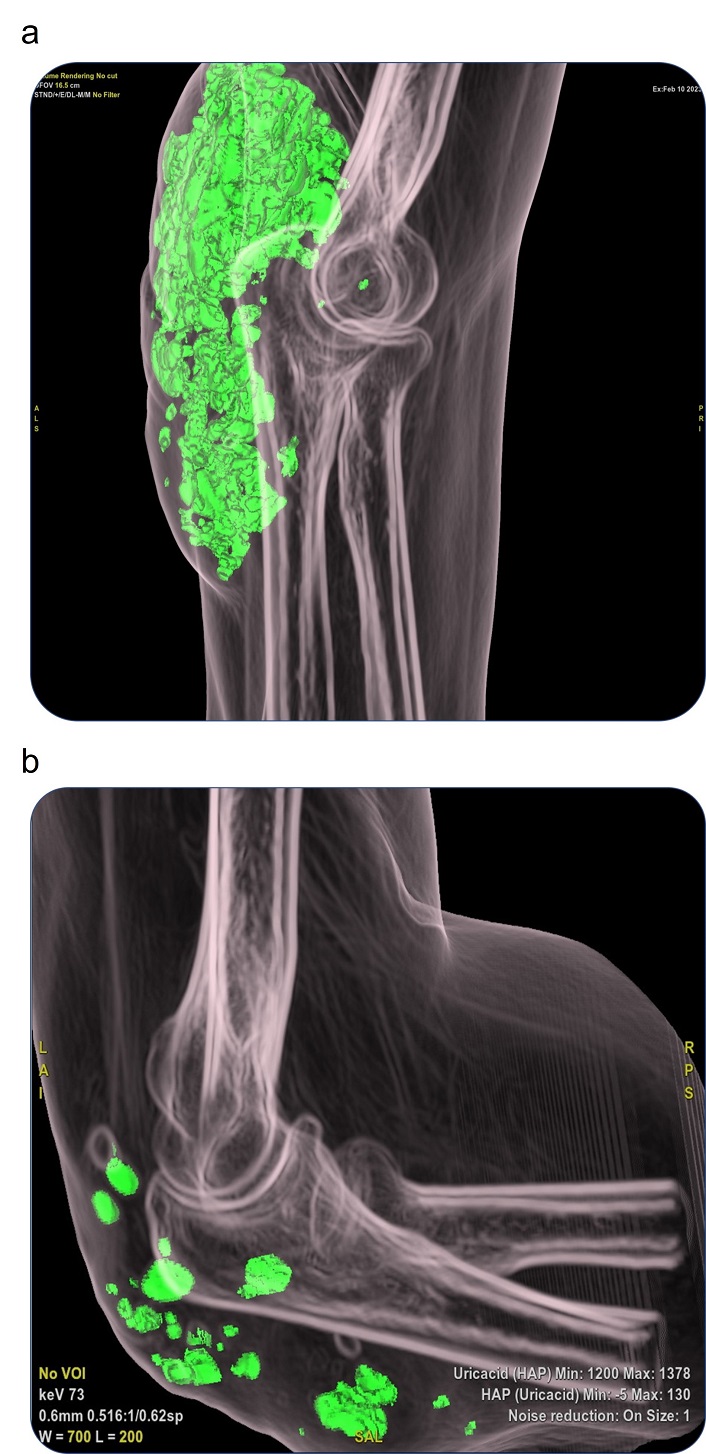
Radiological evidence showing resolution of urate deposit in a representative patient with severe tophaceous gout. The SUA level at month 12 was 2.9 mg/dL. (a) Spectral CT image at baseline showing MSU deposits (green areas) at the elbow. (b) Same site after 12 months of FBX + BNZ showing marked reduction in urate burden. SUA: serum urate; CT: computed tomography; MSU: monosodium urate; FBX: febuxostat; BNZ: benzbromarone
Treatment was well tolerated, with a low incidence of adverse events (< 10%), all of which were non-severe. Three patients withdrew from the study, although not because of treatment-related adverse events. BNZ was discontinued in only one case (6.7%) owing to intolerance. The addition of BNZ to FBX in the second phase of treatment resulted in no adverse events or significant changes in liver enzyme or renal function values, either at 12 months (mean eGFR12m 55.2 ± 22.1 mL/min vs. eGFR6m 60.1 ± 21.3 mL/min; p = 0.98) or at the end of follow-up (mean eGFRfinal 61.8 ± 21.6 vs. eGFR6m 60.1 ± 21.3 mL/min; p = 0.26). Similarly, we found no significant differences when comparing renal function at baseline and end of follow-up (mean eGFRfinal 61.8 ± 21.6 mL/min vs. eGFRbaseline 63.7 ± 23.6 mL/min; p = 0.47). During follow-up, 6 patients (40%) experienced gout flares, which were managed with NSAIDs (n = 1), colchicine (n = 1), glucocorticoids (n = 3), or IL-1 blockade (n = 1). None of the flare episodes required hospitalization. One unrelated death was recorded (sudden death in an elderly patient with valvular heart disease).
In this study, standard-dose FBX/BNZ combination therapy was well tolerated by patients with severe D2T gout, leading to a significant reduction in SUA and rapidly dissolving tophi. ULT aims to prevent attacks and eliminate tophi by lowering SUA. While SoC includes XOIs (ALO or FBX) and uricosurics (BNZ, PBN, and LSN), some patients remain refractory or intolerant to conventional treatment [21, 25].
No unified and validated term has been proposed for D2T gout. However, the condition is certainly prevalent and poses a therapeutic challenge. Globally, the T2T strategy has proven successful in gout; however, some patients with severe tophaceous D2T gout require stringent SUA targets (< 5 mg/dL, ideally < 4 mg/dL) for complete dissolution of MSU crystals [26–28]. This also has significant implications for prognosis, given the link between tophi and mortality [29, 30].
Most patients in our study met intended SUA targets with the ‘FebBenz’ approach at 12 months (80% < 5 mg/dL, 73.3% < 4 mg/dL) and end of follow-up (93.3% < 4 mg/dL, 33.3% < 3 mg/dL). Achieving such a reduction often necessitates combination therapy, as XOIs decrease uric acid formation while uricosurics enhance excretion [5]. Beyond efficacy, uricosurics may confer endothelial benefits in hyperuricemia [31].
However, unlike other inflammatory arthropathies such as rheumatoid arthritis, where the combination of several drugs is common, studies on ULT combinations in gout are scarce, focusing primarily on ALO [8–19]. Although both drugs have demonstrated significant efficacy as monotherapy in patients affected by gout, with BNZ being superior to PBN, studies on the FBX + uricosuric combination, typically at low doses, suggest superior efficacy [12, 17, 18]. Xue et al. [13] reported enhanced urate-lowering with FBX + BNZ, positioning this combination therapy as a potential alternative to standard ULT regimens [20, 32]. Our study revealed an even greater reduction in SUA (10.3 to 2.7 vs. 9.4 to 5.6 mg/dL) with higher doses (FBX 80–160 vs. 20–40 mg/day, BNZ 50–100 vs. 25 mg/day) and longer follow-up [18 (9–44) vs. 3 months]. Our cohort had older patients [59 (43–93) vs. 40 (32–46) years], all with tophi (100% vs. 17%), and a longer disease duration (mean 16 vs. 4 years), higher baseline SUA (mean 10.3 vs. 9.4 mg/dL), greater prevalence of CVRFs (hypertension, 93% vs. 23%; dyslipidemia, 73% vs. 43%; diabetes, 7% vs. 5%; major adverse cardiovascular events, 13% vs. 4%), and worse renal function (eGFR 63.7 ± 23.6 vs. 100.2 ± 14.1 mL/min).
Compared with Xue et al. [13], we recorded a higher loss to follow-up (20% vs. 12%) [13]. However, our safety outcomes were favorable, with no serious adverse events or significant changes in renal or hepatic function, even with prolonged exposure to BNZ [12 (3–38) vs. 3 months] and the use of higher doses of FBX + BNZ (see above). Notably, initial XOI therapy mitigates the renal urate burden (SUA × GFR) more effectively than initial treatment with a uricosuric agent. Additionally, stepwise addition of uricosurics enables monitoring of SUA and assessment of the risk of nephrolithiasis (via urine pH and urine uric acid levels). In our cohort, no nephrolithiasis events were reported.
Lastly, with LSN withdrawn, the ‘FebBenz’ approach may be a cost-effective alternative to uricases (pegloticase/rasburicase), which are limited by cost, availability, and immunogenicity [33].
The strengths of our study include its multicenter design, homogeneous baseline characteristics (severe tophaceous gout, high SUA, and failure of SoC), real-world D2T patient data (including middle-aged and elderly patients, with a high prevalence of CVRFs, chronic kidney disease, and other comorbidities), ambitious SUA targets, and the longest reported follow-up for ULT combination therapy.
Our study is limited by its observational design, small sample size, and losses to follow-up (influenced by the COVID-19 pandemic, BNZ supply shortages, and patient multimorbidity) [27], which may prevent generalizability. Nevertheless, the statistically significant reduction in SUA (p < 0.01) corresponds to clinically meaningful outcomes (resolution of tophi in 60%). While the uncontrolled design prevents causal inferences, the mechanistic plausibility of the synergistic action of FBX + BNZ supports our findings. Future randomized trials with adjusted analyses for confounders are needed to validate these results and evaluate long-term efficacy and safety.
In conclusion, we found the combination of FBX + BNZ to be effective and well-tolerated in patients with D2T gout, resulting in intensive reduction in SUA and rapid dissolution of tophi. The combination of FBX + BNZ may serve as a therapeutic alternative for patients with severe gout.
ALO: allopurinol
BNZ: benzbromarone
CI: confidence interval
CVRFs: cardiovascular risk factors
D2T: difficult-to-treat
eGFR: estimated glomerular filtration rate
FBX: febuxostat
HUC: Hospital Universitario Cruces
HUIL: Hospital Universitario Infanta Leonor
IL-1: interleukin-1
IQR: interquartile range
LSN: lesinurad
MACEs: major cardiovascular events (myocardial infarction, stroke, hospitalization for unstable angina, coronary revascularization)
MSU: monosodium urate
NSAIDs: non-steroidal anti-inflammatory drugs
PBN: probenecid
SoC: standard of care
SUA: serum urate
T2T: treat-to-target
ULTs: urate-lowering treatments
XOIs: xanthine oxidase inhibitors
Preliminary findings from this study were presented at the following conferences, although this manuscript completes the study with a comprehensive analysis that has not been previously reported: 1) Madrid Regional Rheumatology Society (SORCOM) Congress 2023 https://www.sorcom.es/images/Anales/Revista-Anales-Congreso-SORCOM-2023.pdf; 2) European Alliance of Associations for Rheumatology (EULAR) Congress 2024 https://doi.org/10.1136/annrheumdis-2024-eular.5897; 3) European Crystal Network (ECN) Workshop 2024 https://doi.org/10.3390/gucdd2030021; 4) Spanish Society of Rheumatology (SER) Congress 2024 https://static.elsevier.es/reuma/reumacongreso2024b.pdf. The authors retain copyright for this material and grant the journal permission for reuse.
Special thanks to Mr. J.A. Ángel Sesmero (Clinical Nurse Specialist, Rheumatology Department, Hospital Universitario Infanta Leonor) and the Radiology Department of Hospital Universitario Cruces (Dr. B. Souto-Canteli). We also thank Mr. Thomas O’Boyle, on behalf of the Spanish Foundation of Rheumatology, for his valuable assistance in providing medical writing and editorial support during the preparation of the manuscript.
ECA: Conceptualization, Investigation, Writing—original draft, Writing—review & editing, Supervision, Funding acquisition. CMGG: Investigation, Writing—original draft, Writing—review & editing. FPR: Conceptualization, Investigation, Writing—review & editing, Validation, Supervision. MNN: Data curation, Methodology, Writing—review & editing. All authors read and approved the submitted version.
Enrique Calvo-Aranda: Speaker for the Spanish Foundation for Rheumatology, Asacpharma, SOBI, AbbVie, and Alfasigma. Claudia María Gómez-González: the author declares that there are no conflicts of interest. Fernando Pérez-Ruiz: Advisor for Amgen, Arthrosi Therapeutics, Cristalys, LG, and Protalix; Speaker for the Spanish Foundation for Rheumatology and Menarini; Research grants from the Cruces Rheumatology Association; Editorial work for the Spanish Foundation for Rheumatology, Open Exploration Publishing, and Wolters-Kluwer; the Editor-in-Chief and Guest Editor of Exploration of Musculoskeletal Diseases had no involvement in the decision-making or the review process of this manuscript. Marta Novella-Navarro: Speaker for AbbVie, Lilly, Alfasigma, and UCB.
The study was approved by the Ethics Committee of OSI EEC at Cruces University Hospital (CEIC-E03/45) and complied with the principles of the Declaration of Helsinki.
The patients gave their written informed consent to participate.
Not applicable.
De-identified data supporting this study are available from the corresponding author upon reasonable request.
This work was supported by the Spanish Foundation of Rheumatology [FERBT2025]. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3137
Download: 55
Times Cited: 0
Benjamin Plotz ... Michael H. Pillinger
Hamish Farquhar ... Lisa K. Stamp
Naomi Schlesinger, Dan Kaufmann
Mark D. Russell, James B. Galloway
Robert T. Keenan ... Michael H. Pillinger
Robin Christensen ... Lisa K. Stamp
Edward Roddy ... Christian D. Mallen
Philip L. Riches ... Amrey Krause
Emilie Schurenberg ... Kenneth G. Saag
Orsolya I. Gaal ... Tania O. Crișan
