Affiliation:
1Department of Oral Pathology and Microbiology, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences (SIMATS), Saveetha University, Chennai 600077, Tamil Nadu, India
Email: 152107003.sdc@saveetha.com
ORCID: https://orcid.org/0009-0003-2087-6472
Affiliation:
1Department of Oral Pathology and Microbiology, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences (SIMATS), Saveetha University, Chennai 600077, Tamil Nadu, India
ORCID: https://orcid.org/0000-0003-1921-8336
Affiliation:
1Department of Oral Pathology and Microbiology, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences (SIMATS), Saveetha University, Chennai 600077, Tamil Nadu, India
ORCID: https://orcid.org/0000-0002-5426-3506
Affiliation:
2Department of Woman, Child and General and Specialist Surgery, University of Campania “Luigi Vanvitelli”, 80138 Naples, Italy
3Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences (SIMATS), Saveetha University, Chennai 600077, Tamil Nadu, India
Affiliation:
4Department of Biomedicine and Prevention, University of Rome “Tor Vergata”, 00100 Rome, Italy
Affiliation:
5Multidisciplinary Department of Medical-Surgical and Odontostomatological Specialties, University of Campania “Luigi Vanvitelli”, 80138 Naples, Italy
Affiliation:
6Department of Biomedical and Surgical and Biomedical Sciences, Catania University, 95123 Catania, Italy
Affiliation:
5Multidisciplinary Department of Medical-Surgical and Odontostomatological Specialties, University of Campania “Luigi Vanvitelli”, 80138 Naples, Italy
Email: giuseppe.minervini@unicampania.it
Explor Immunol. 2025;5:1003228 DOI: https://doi.org/10.37349/ei.2025.1003228
Received: July 07, 2024 Accepted: July 16, 2025 Published: November 17, 2025
Academic Editor: Cunte Chen, South China University of Technology, China
The article belongs to the special issue The Role of Immune Checkpoint Molecules in Cancer and Hematological Malignancies
Background: Oral squamous cell carcinoma (OSCC), a significant health burden in developing nations, is linked to risk factors such as tobacco use, alcohol consumption, betel nut chewing, HPV infection, and genetic susceptibility. A hallmark of OSCC is impaired T cell function, driven in part by the PD-L1/PD-1 immune checkpoint pathway, which enables tumor immune evasion and progression. Despite growing interest in immunotherapy, a focused synthesis of PD-L1 expression and its clinical implications in OSCC remains limited. This review aims to consolidate existing evidence on PD-L1 in OSCC, evaluating its expression patterns, correlation with disease progression, and therapeutic relevance.
Methods: A systematic search was conducted across multiple databases to identify studies examining PD-L1 expression in OSCC and its relationship with clinicopathological parameters and immune response.
Results: The findings revealed a higher PD-L1 positivity in female patients, non-smokers, and non-drinkers. Positive PD-L1 expression rate correlated with poor differentiation, lymph node metastasis, and advanced TNM stage. Although it didn’t significantly impact overall survival, higher PD-L1 expression was observed in HPV-positive patients and correlated with increased CD8+ TIL levels.
Discussion: Understanding the role of PD-L1 in OSCC elucidates immune evasion mechanisms and offers insights into potential treatments, such as checkpoint inhibitors, for personalized therapies and innovative cancer treatments. This comprehensive synthesis provides valuable insights into the complex interplay between PD-L1 expression and OSCC progression, laying the groundwork for additional studies in this area.
Oral squamous cell carcinoma (OSCC) is one of the most common oral cancers, and it predominantly occurs in developing nations [1]. The origin of OSCC is the squamous epithelial cells lining the oral cavity, which include the tongue, lips, buccal mucosa, floor of the mouth, hard palate, and oropharynx. It is known due to its aggressive nature, tendency for local invasion, and high metastasis rate to regional lymph nodes, which significantly contribute to its morbidity and mortality [2]. With a higher frequency seen in underdeveloped nations, OSCC is the sixth most frequent cancer worldwide. Key risk factors include the use of smokeless and smoking tobacco, alcohol consumption beyond the permissible limits, betel nut chewing, and human papilloma virus (HPV) infection, particularly HPV-16 [3]. Contributing factors also include genetic predispositions, poor oral hygiene, and chronic irritation or trauma to the oral mucosa. The frequency of OSCC is much higher in males than in females, which may be due to the fact of more hazardous behavioral practices and risk factors among men. A series of genetic mutations and epigenetic changes contribute to the pathophysiology of OSCC, converting normal oral epithelial cells into cancerous cells. The main molecular pathways involved in OSCC comprise the p53 tumor suppressor pathway, the retinoblastoma (Rb) pathway, and a few signaling cascades like EGFR, MAPK, and PI3K/AKT [3, 4]. The changes result in losing the normal checks that limit the growth of cells, developing cell resistance to programmed cell death (apoptosis), supplying tumor tissue with new blood vessels, and enhancing the ability to invade other tissues [3].
Programmed death-ligand 1 (PD-L1), also known as B7-H1, together with its receptor (PD-1), are key element of the immune checkpoint pathway, which plays a significant role in regulating immune responses [5]. Under physiological conditions, this pathway is essential for maintaining immune homeostasis and preventing autoimmunity by inhibiting T-cell activity [6]. However, the tumor cells exploit such a machinery by PD-L1 overexpression, which leads to the suppression of the cytotoxic T-cell responses, thereby allowing an unfettered tumor progression. Although extensive research has been done to determine whether the expression levels of PD-1 and PD-L1 proteins in tumor tissues correspond with the biological behavior and clinical features of head and neck squamous cell carcinoma (HNSCC), their precise role and clinical relevance in OSCC remain ambiguous [7]. In OSCC, tumor cell-expressed PD-L1 interacts with PD-1 receptors on T cells to deliver inhibitory signals that lead to the reduction in T-cell proliferation, cytokine secretion, and cytotoxic activity. This immune checkpoint interaction facilitates tumor immune evasion, making PD-1/PD-L1 blockade a promising therapeutic strategy [8]. Nivolumab and pembrolizumab, two well-known PD-1 inhibitors, have been approved by the FDA to treat a number of malignancies, including HNSCC, which includes OSCC [9].
In addition to PD-1 inhibitors, several PD-L1 inhibitors (PD-L1-I), such as durvalumab and atezolizumab, are undergoing clinical trials to assess their efficacy in treating HNSCC and other cancers. These treatments have been extremely effective in shrinking tumors and in achieving the remission phase, thus representing a significant leap in cancer immunotherapy. The clinical application of PD-L1-I in OSCC represents a major advancement in its management. Clinical trials such as CheckMate141 and KEYNOTE-012 have shown that these inhibitors can improve overall survival (OS) and reduce adverse effects compared to standard therapies [10].
In summary, the integration of PD-L1-I into the treatment paradigm for OSCC signifies a major advancement in cancer immunotherapy, providing new avenues for effective and targeted treatment [9, 11]. Several systematic reviews have published findings on PD-L1 expression in OSCC, however, a thorough integrative analysis that covers its expression, prognostic implications, and correlation with disease progression is still lacking. The present review is committed to mend this significant gap by consolidating the current evidence base, identifying variances among different research studies, and providing potential suggestions for future research direction.
A comprehensive and systematic search was conducted across four major electronic databases: PubMed, Scopus, Web of Science, and Embase. The search strategy utilized a combination of MeSH terms and free-text keywords, including: “PD-L1,” “programmed death-ligand 1,” “oral squamous cell carcinoma,” “OSCC,” “immune checkpoint,” “immune evasion,” and “immunotherapy.” Boolean operators (AND, OR) were applied to refine and expand the search scope. References of eligible articles were also screened manually to identify additional relevant studies.
Systematic reviews focusing on the role of the PD-L1 pathway in OSCC were included. Only peer-reviewed journal publications were considered, with no restrictions on publication date or language.
Studies not directly related to the PD-L1 pathway or OSCC, studies detailing HNSCC without mention of the total number of OSCC cases included, and non-peer-reviewed articles, conference abstracts, and case reports were excluded.
Two reviewers independently extracted data using a predefined template, including study characteristics (author, year, country), population details, number of OSCC cases, PD-L1 detection methods, clinicopathological correlations, and reported outcomes. Key findings were synthesized qualitatively, identifying consistent patterns, methodological limitations, and divergent results.
Results were interpreted in a context of the current understanding of the PD-L1 pathway in OSCC. Implications for clinical practice and further directions of study were highlighted based on the synthesized evidence.
Since this review involved an analysis of data that has already been published, ethical clearance was not necessary.
The review process comprised screening titles and abstracts of systematic reviews, full-text assessment, data extraction, and quality assessment. Discrepancies were resolved through consensus among review team members.
Reporting adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
A total of 22 systematic reviews were obtained during the initial search. After complete screening, a total of 6 articles were reviewed (Figure 1).
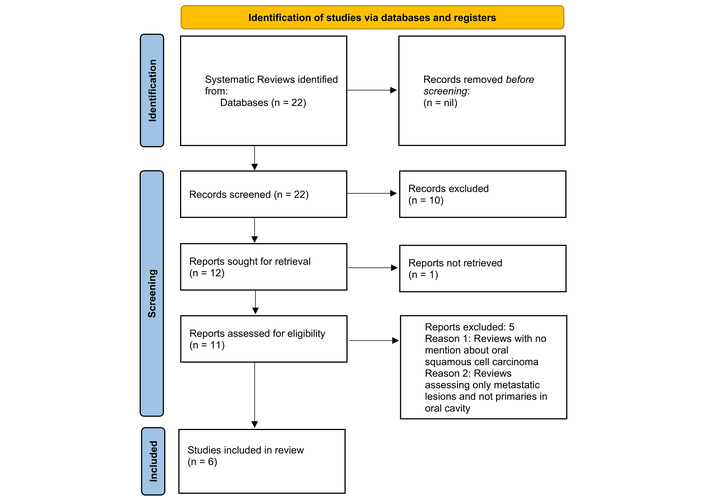
PRISMA 2020 flow diagram showcasing systematic reviews included from searches of databases and registers only. PRISMA flow chart displaying the review process, detailing the number of records identified, screened, excluded, and included in the final analysis. Adapted from “PRISMA” (http://www.prisma-statement.org/). Accessed June 25, 2024. © 2024–2025 the PRISMA Executive. Distributed under a Creative Commons Attribution (CC BY 4.0) license.
The data were systematically collected from a series of reviews and subsequently organized into a detailed table (Table 1). This table was designed to provide a clear and comprehensive overview of the collected information.
The final articles included in the review, along with their respective data.
| Author and year | Country | Databases searched | No. of OSCC studies included | Parameters | Main findings | Methodology |
|---|---|---|---|---|---|---|
| He et al., 2020 [12] | China | PubMed, Web of Science, EMBASE, Cochrane Library, and CNKI | 23 | PD-L1 and clinicopathological features | High PD-L1 linked to adverse clinicopathological traits; no impact on overall survival (OS). | PD-L1 expression was detected using immunohistochemistry (IHC) on formalin-fixed, paraffin-embedded (FFPE) tissue, evaluated in tumor cells. |
| Yang et al., 2018 [13] | China | PubMed, Embase, Web of Science, Cochrane Library, and Scopus | 9 | PD-L1 and prognosis | Positive PD-L1 may predict better progression-free survival (PFS) in advanced HNSCC; interaction with CD8+ tumor infiltrating lymphocytes (TILs) noted. | PD-L1 was detected via IHC on FFPE tissue sections, with expression assessed in both tumor and immune cells. |
| Tang et al., 2019 [14] | China | PubMed, Embase, and Cochrane | 9 | PD-L1, clinicopathological features; and survival | PD-L1 overexpression is associated with females; disease-free survival (DFS) significance only in the oropharyngeal squamous cell carcinoma (OPSCC) subgroup. | PD-L1 was assessed via IHC on FFPE tissue sections, with expression evaluated in tumor cells. |
| Lenouvel et al., 2020 [15] | Spain | PubMed, Embase, Web of Science, and Scopus | 26 | PD-L1, clinicopathological features, and survival | PD-L1 correlated with poor disease-specific survival (DSS)/DFS; it was higher in females, non-smokers, and advanced tumors. | PD-L1 expression was measured using IHC with specific antibody clones on FFPE samples, evaluated in both tumor and immune cells. |
| Patel et al., 2020 [16] | South Carolina | PubMed (NLM NIH), Scopus (Elsevier), Embase (Elsevier), Web of Science (Clarivate), and Cochrane Library (Wiley) | 6 | PD-L1, OS, and HPV | PD-L1 expression linked to better response and OS; HPV status had no survival impact. | PD-L1 detection was performed using IHC on FFPE tissue sections, with evaluation in tumor cells. |
| Troiano et al., 2019 [17] | Italy | PubMed, Scopus, Web of Science | 11 | PD-L1 and OS | High PD-L1 is not linked to poor prognosis; findings varied across studies. | IHC was used on FFPE specimens to detect PD-L1 expression, evaluated in tumor cells. |
Data extracted from the systematic reviews conducted on PD-L1 receptors and OSCC. OSCC: oral squamous cell carcinoma; PD-L1: programmed death-ligand 1; HNSCC: head and neck squamous cell carcinoma; HPV: human papilloma virus.
Multiple studies have consistently shown that female patients with OSCC exhibit higher PD-L1 expression compared to their male counterparts (Table 2). He et al. [12] found a notably higher rate of positive PD-L1 expression in females than in males. Tang et al. [14] highlighted a greater probability of enhanced PD-L1 expression in female OSCC patients through subgroup meta-analysis. Lenouvel et al.’s [15] meta-analysis of 14 investigations revealed that females were more likely to have higher PD-L1 overexpression. Similarly, Troiano et al. [17] observed that female patients were twice as likely to exhibit high PD-L1 expression as males. This consistent pattern suggests a potential gender-related immunoregulatory mechanism.
The studies that analyzed the association between gender and PD-L1 expression.
| Study | Gender showing higher % positivity | Key findings |
|---|---|---|
| He et al. [12] | Females | Higher PD-L1 expression in females (52.4%) compared to males (44.9%). |
| Tang et al. [14] | Females | OR = 0.56 (95% CI: 0.41–0.77). Greater probability of enhanced PD-L1 expression in female OSCC patients. |
| Lenouvel et al. [15] | Females | OR = 0.69 (95% CI: 0.53–0.91, p = 0.008). Females are more likely to have higher PD-L1 overexpression. |
| Troiano et al. [17] | Females | OR = 0.5 (95% CI: 0.36–0.69, p < 0.0001). Female patients are twice as likely to exhibit high PD-L1 expression as males. |
Displaying the association between gender and PD-L1 expression. PD-L1: programmed death-ligand 1; OR: odds ratio; CI: confidence interval; OSCC: oral squamous cell carcinoma.
From Table 3, the study by Lenouvel et al. [15] evaluated PD-L1 overexpression in smokers versus non-smokers and drinkers versus non-drinkers. Involving six studies with 676 patients, it found that non-smokers had elevated PD-L1 overexpression compared to smokers. However, the stability of this finding was questioned by sensitivity analysis. Additionally, five studies with 591 patients showed that non-drinkers exhibited higher PD-L1 overexpression compared to drinkers. Despite this statistically significant outcome, the robustness was challenged due to substantial heterogeneity among the studies.
Results of the association between habits and PD-L1 expression.
| Comparison | Number of studies | Total patients | Odds ratio | 95% confidence interval (CI) | Key findings | Stability of findings |
|---|---|---|---|---|---|---|
| Smokers vs. Non-smokers | 6 | 676 | 0.45 | 0.27–0.75 | Non-smokers had elevated PD-L1 overexpression. | Stability questioned by sensitivity analysis. |
| Drinkers vs. Non-drinkers | 5 | 591 | 0.4 | 0.16–0.97 | Non-drinkers exhibited elevated PD-L1 overexpression compared to drinkers. | Robustness challenged by sensitivity analysis due to 69% heterogeneity. |
Displaying the association between habits and PD-L1 expression. PD-L1: programmed death-ligand 1.
Table 4 shows three studies that examined PD-L1 expression across various clinical characteristics in OSCC and HNSCC patients (Table 4). He et al. [12] found that poor/moderate differentiation was linked to higher PD-L1 expression, with strong correlations observed with tumor node metastasis (TNM) stage, distant metastasis, and lymph node metastasis. Tang et al. [14] reported no significant correlation of PD-L1 expression with age, grade, lymph node metastasis, tumor stage, or recurrence, but noted increased expression in lower-grade malignancies in OSCC. Lenouvel et al. [15] found no significant correlation with age, grade, T status, tumor risk, or M status but observed a significant correlation with advanced cancer stage when excluding small studies. High heterogeneity and sensitivity analysis were noted in these studies.
Results of the association between tumor staging, grading, and PD-L1 expression.
| Study | Clinical characteristics evaluated | Key findings | Stability and additional insights |
|---|---|---|---|
| He et al. [12] | Histological differentiation, TNM stage, lymph node metastasis, distant metastasis, and race | Poor/moderate differentiation is linked to higher PD-L1 expression. Strong correlation with TNM stage, distant metastasis, and lymph node metastasis. | Subgroup analysis showed differences in PD-L1 expression based on race. |
| Tang et al. [14] | Age, grade, lymph node metastasis, tumor stage, recurrence | No significant correlation with age, grade, lymph node metastasis, tumor stage, or recurrence. In OSCC, lower-grade malignancies showed increased PD-L1 expression. | N/A |
| Lenouvel et al. [15] | Age groups, tumor grade, T status, tumor risk, M status, lymph node involvement, cancer stage | No significant correlation with age, grade, T status, tumor risk, or M status. Significant correlation with advanced cancer stage when small studies were excluded. | High heterogeneity noted; leave-one-out sensitivity analysis confirmed findings. |
Displaying the association between tumor staging, grading, and PD-L1 expression. PD-L1: programmed death-ligand 1; TNM: tumor node metastasis; OSCC: oral squamous cell carcinoma.
Five studies evaluated the correlation between PD-L1 expression and survival outcomes in OSCC and HNSCC patients (Table 5). He et al. [12] found no significant correlation between PD-L1 expression and OS in OSCC patients, with no differences across racial subgroups. Yang et al. [13] observed no significant difference in OS, disease-free survival (DFS), or disease-specific survival (DSS) in HNSCC patients, though an improvement in progression-free survival (PFS) was noted. Tang et al. [14] reported no significant relationship between PD-L1 expression and OS or DFS in HNSCC, except for better DFS in oropharyngeal squamous cell carcinoma (OPSCC) patients. Lenouvel et al. [15] found no significant impact on OS, DFS, or PFS, but noted a significant result for DSS despite high heterogeneity. Troiano et al. [17] found no correlation between PD-L1 expression and lymph node metastases, OS, DFS, or DSS, with significant variation among the studies. Overall, these studies highlight a general lack of significant correlations between PD-L1 expression and survival outcomes, with some variability and specific contexts showing differing results.
Results of the association between PD-L1, survival, and prognosis.
| Study | Number of trials | Key findings | Stability and additional insights |
|---|---|---|---|
| He et al. [12] | 17 | No significant correlation between PD-L1 expression and OS. Subgroup analysis showed no significant relationship for Asian or Caucasian individuals with OSCC. | Significant variability; random-effects model used. |
| Yang et al. [13] | 19 | No significant difference in OS between PD-L1-positive and PD-L1-negative HNSCC patients. No significant association with OS, DFS. Improvement in PFS observed. No significant difference in DSS. | Considerable heterogeneity; random-effects model used. |
| Tang et al. [14] | 9 | No significant relationship between PD-L1 expression and OS or DFS in HNSCC. Better DFS noted in OPSCC patients. | Subgroup analysis showed better DFS in OPSCC patients. |
| Lenouvel et al. [15] | 13 | No significant impact on OS, DFS, PFS, or LRFS. Significant result observed for DSS despite heterogeneity. | Notable heterogeneity; significant result for DSS despite variability. |
| Troiano et al. [17] | 11 | No correlation between PD-L1 expression and lymph node metastases, OS, DFS, or DSS. | Significant variation was found among the studies. |
Displaying the association between survival and prognosis and PD-L1 expression. PD-L1: programmed death-ligand 1; OS: overall survival; OSCC: oral squamous cell carcinoma; HNSCC: head and neck squamous cell carcinoma; DFS: disease-free survival; PFS: progression-free survival; DSS: disease-specific survival; OPSCC: oropharyngeal squamous cell carcinoma; LRFS: locoregional recurrence free survival.
Table 6 shows the studies that evaluated the association between PD-L1 and HPV status. He and colleagues [12] investigated the association between PD-L1 expression and the presence of HPV infection, finding that HPV-positive patients had a significantly higher rate of PD-L1 positivity, especially among Asian populations. Yang et al. [13] found that higher PD-L1 expression was significantly associated with HPV-positive HNSCC, but PD-L1 positivity did not significantly improve OS in these cases. Patel et al. [16] compared survival rates between HPV-positive and HPV-negative patients, observing no significant difference in OS between the two groups. Additionally, PD-L1 positive patients had a slightly improved median PFS.
Results of studies that analyzed the association between HPV and PD-L1.
| Study | Study focus | Key findings | Inference |
|---|---|---|---|
| He et al. [12] | Association between PD-L1 expression and HPV | PD-L1 positivity was 44.8% in HPV-negative and 56.7% in HPV-positive patients, RR = 1.30 (95% CI: 1.04–1.62, p = 0.019), and higher PD-L1 positivity in Asian HPV-positive patients (RR = 1.22, p = 0.027). | HPV infection is significantly associated with higher PD-L1 expression, especially in Asians. |
| Yang et al. [13] | Predictive significance of PD-L1 in HPV-positive HNSCC | Higher PD-L1 expression in HPV-positive cases (OR = 1.99, p < 0.001), but no significant effect on OS (HR = 1.04, p = 0.88). | PD-L1 expression is elevated in HPV+ HNSCC but not linked to improved survival. |
| Patel et al. [16] | Survival rates of HPV-positive vs HPV-negative | Similar OS at 6, 12, and 18 months between HPV-positive and HPV-negative groups, PD-L1+ (≥ 1%) patients had slightly longer median PFS (3.34 months). | No significant OS difference by HPV status; PD-L1+ may be associated with a modest improvement in PFS. |
Displaying the association between HPV and PD-L1 expression. HPV: human papilloma virus; PD-L1: programmed death-ligand 1; RR: relative risk; CI: confidence interval; HNSCC: head and neck squamous cell carcinoma; OR: odds ratio; OS: overall survival; HR: hazard ratio; PFS: progression-free survival.
Table 7 shows the results of the studies that evaluated the expression of PD-L1 and its association with TILs. Yang et al. [13] investigated PD-L1’s predictive significance in HNSCC patients with varying levels of CD8+ TILs, finding that PD-L1 expression had no impact on PFS or OS in patients with high CD8+ TIL levels. However, high PD-L1 expression was linked to worse OS in HNSCC patients with low CD8+ TILs. Lenouvel et al. [15] examined the relationship between PD-L1 overexpression and the presence of CD8, CD4, and PD-1 in the tumor microenvironment, discovering that PD-L1 overexpression was positively correlated with higher levels of these immune cells. These findings suggest that PD-L1 expression negatively impacts OS in HNSCC patients with low CD8+ TIL levels and indicate a complex interaction between PD-L1 and immune cells in the tumor microenvironment.
Association between PD-L1 and TILs.
| Study | Study focus | Key findings | Inference |
|---|---|---|---|
| Yang et al. [13] | Predictive significance of PD-L1 in HNSCC patients with variable CD8+ TIL levels | PD-L1 expression had no impact on PFS or OS in patients with high CD8+ TILs; in contrast, high PD-L1 was linked to worse OS in patients with low CD8+ TILs (HR: 1.90); no significant difference in PFS across CD8+ TIL levels. | PD-L1 expression negatively impacts OS in HNSCC patients with low CD8+ TIL levels, but not in those with high CD8+ TIL levels. |
| Lenouvel et al. [15] | Relationship between PD-L1 overexpression and presence of CD8, CD4, and PD-1 in the tumor microenvironment | PD-L1 overexpression was positively correlated with CD8+ TILs (OR: 3.63), CD4+ TILs (OR: 3.25), and PD-1 expression (OR: 33.36). | PD-L1 overexpression is linked to increased immune cell infiltration, indicating complex immunoregulatory interactions. |
Displaying the association between TILs and PD-L1. PD-L1: programmed death-ligand 1; TILs: tumor infiltrating lymphocytes; HNSCC: head and neck squamous cell carcinoma; PFS: progression-free survival; OS: overall survival; HR: hazard ratio; OR: odds ratio.
These studies collectively suggest that while PD-L1 expression correlates with higher levels of immune cell infiltration, its impact on survival outcomes may depend on the density of specific TIL populations, particularly CD8+ TILs.
The greater PD-L1 expression in females suffering from OSCC might be the consequence of different biological and genetic factors. Hormones, and more specifically the influence of estrogen, are likely to play a major role. Estrogen acts as an immune modulator; therefore, it may increase the PD-L1 expression significantly. Furthermore, genetic differences, including several variations in the immune-related genes and X-chromosome linked factors, might contribute to the increased PD-L1 expression in females. Overall, these hormonal influences, along with genetic factors, can affect the internal tumor microenvironment, leading to a higher prevalence of PD-L1 overexpression in female patients.
Although higher PD-L1 expression is predominant in female OSCC patients according to multiple studies, the significance of this finding for clinical purposes is inconclusive. Elevated PD-L1 levels may reflect sex-specific immunoregulatory mechanisms, potentially influenced by estrogen signaling, which has been shown to upregulate PD-L1 expression in various cancers. While the clinical significance remains to be fully established, these findings may have implications for tailoring immunotherapeutic strategies, as gender-specific immune responses could influence treatment outcomes in OSCC. Yet, the current studies fail to find a consistent link between this manner of expression and the way OS, progression of the disease or response to immunotherapy vary in females as compared to males. Further studies are needed to validate the influence of sex-based PD-L1 expression on clinical outcomes or allocation of particular treatments [18–21].
Numerous underlying mechanisms attribute to the differential PD-L1 expression between smokers and non-smokers as well as between drinkers and non-drinkers. Smoking leads to alterations in the tumor microenvironment owing to its contribution to chronic inflammation and oxidative stress. This microenvironment might trigger immune escape of tumor cells, which results in the suppression of PD-L1 expression to evade detection by the immune system. Additionally, the carcinogens present in tobacco can cause genetic mutations and epigenetic changes, affecting the regulatory pathways of PD-L1 expression. In contrast, non-smokers may have a different inflammatory and immune landscape, leading to higher PD-L1 expression as part of their immune response to tumor presence [21].
Alcohol consumption downregulates immune responses by altering cytokine production. This immunosuppression might result in lower PD-L1 expression in drinkers due to a less active immune surveillance mechanism. Individuals who abstain from alcohol may show higher PD-L1 expression due to a more preserved and responsive immune system. This enhanced immune activity may trigger upregulation of PD-L1 as a feedback mechanism to regulate immune responses [22].
Both smoking and alcohol consumption cause chronic systemic changes affecting overall immune function and the tumor microenvironment. These lifestyle factors could influence not only the direct regulation of PD-L1 but also other immune checkpoints and pathways interacting with PD-L1 expression. The heterogeneity in study findings suggests that individual variations in genetics, environment, and the complex interplay of multiple factors lead to differences in PD-L1 expression patterns among smokers, non-smokers, drinkers, and non-drinkers [23].
The association involving PD-L1 expression and staging of the tumor, along with grading, can be explained by the interactions between tumor biology and immune evasion mechanisms. Poorly and moderately differentiated tumors are more likely to exhibit higher PD-L1 expression compared to well-differentiated tumors. This might be due to the more aggressive nature of poorly differentiated tumors, which often develop mechanisms to escape immune surveillance, including upregulating PD-L1 to inhibit T-cell activity. High PD-L1 expression in these tumors helps them avoid destruction by the immune system, facilitating tumor growth and metastasis [24].
Positive PD-L1 expression has been significantly linked to advanced clinical features such as lymph node involvement, distant metastasis, and higher TNM stages. Advanced-stage tumors often upregulate PD-L1 to enhance immune evasion. This allows them to suppress antitumor immunity, particularly at metastatic sites where the immune cell activities are more pronounced [25].
The different levels of PD-L1 expression in relation to tumor stages and grades indicate its potential as a prognostic marker to detect tumor aggressiveness. In addition to this, it points to the importance of PD-L1 as a candidate marker for immunotherapy, especially for patients who are in the later stages of the disease [26].
The lack of significant association between PD-L1 expression and survival outcomes, including OS, DFS, and DSS, across multiple studies suggests a variation between PD-L1 and tumor biology [26].
This evidence implies that merely considering PD-L1 expression as a predictor in HNSCC might be insufficient. Other parameters, including tumor heterogeneity, immune microenvironment, and treatment modalities, probably have a greater impact on patient outcomes. Additionally, the high heterogeneity observed among studies underscores the necessity for standardized approaches and extensive, rigorously controlled investigations to validate the prognostic utility of PD-L1 expression in HNSCC [27, 28].
Overall, PD-L1 is still a viable target to counteract the immune system’s resistance in HNSCC, although its use as a prediction marker concerning the survival of patients seems to be quite insignificant according to the available data. Additional studies are needed to specify the association between the expression of PD-L1 in patients and their prognosis, thus opening up new treatment options for OSCC and other types of tumors [29].
The finding of an association between HPV infection and PD-L1 expression in OSCC may signify that there is a link between viral etiology and immune checkpoint activation. The evidence base indicates that PD-L1 positivity among HPV-positive OSCC patients is higher, giving rise to the hypothesis that HPV is instrumental in the immune microenvironment. HPV infection-driven alterations in the tumor microenvironment may lead to the upregulation of PD-L1 as a mechanism by which cells escape immune surveillance [30]. HPV infection-driven changes within the tumor microenvironment may favor upregulation of PD-L1 as part of an immune surveillance escape mechanism. This increase in PD-L1 expression constitutes the creation of an immunosuppressive environment, enabling tumor cells to escape from immune detection and eradication. Despite the association of HPV infection with PD-L1 expression, however, the role of PD-L1 as a prognostic marker in HPV-positive OSCC is uncertain, with a need for further mechanistic and clinical investigation regarding this relationship. The predictive value of PD-L1 expression in HPV-positive OSCC is still undetermined. Therefore, while there are comparable OS rates with their HPV-negative counterparts, PD-L1 expression can influence PFS. Nevertheless, data on low PD-L1 expression and survival outcomes according to HPV status are still scant. The findings indicate the complicated interplay of HPV infection, expression of PD-L1, and the patient’s prognosis in OSCC. This warrants further investigations in order to fully understand the clinical implications and therapeutic potential of these factors [31].
PD-L1, present on both the malignant as well as the immune cells, engages with its counterpart receptor PD-1 on TILs, leading to T-cell exhaustion or anergy. The binding of PD-L1 with PD-1 on TILs hampers T-cell activation and T-cell function regimen allowing cancer cells to remain undetected by the immune system and evade destruction [32].
Tumors with high density of immune cell infiltration display increased PD-L1 expression levels, and this may be seen as an ‘aggressive’ immune response against the tumor. The tumor microenvironment’s CD8+ cytotoxic T lymphocytes (CTLs) are evidence that the immune system is making an effort to detect and kill cancer cells. In this case, the production of PD-L1 might serve as a counter-regulatory mechanism to control the magnitude of the immune response and avoid causing undue tissue damage [32, 33].
On the contrary, in instances where CD8+ TIL count is low, PD-L1 expression may still assist the tumors in evading the immune system by suppressing the cytotoxic activity of the T cells. This leads to an immunosuppressive microenvironment that, in turn, promotes tumor progression and metastasis. Furthermore, PD-1-positive TILs reflect a condition of T-cell exhaustion due to continuous exposure to antigens, leading to functional impairment and loss of anti-tumor activity [34].
As a whole, the association of PD-L1 expression with TIL density reflects the dynamic balance between anti-tumor immunity and immune evasion within the tumor microenvironment (Figure 2) [35].
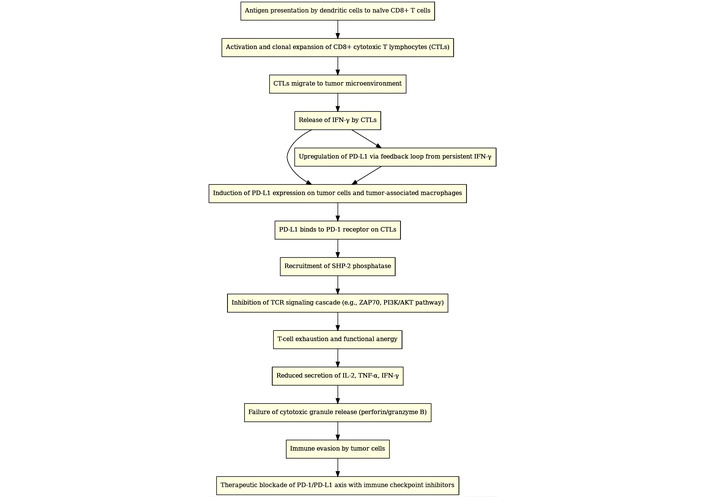
Interrelationship of PD-L1 expression and TILs in immunoregulation and immunotherapy. It presents a streamlined cellular pathway flowchart that elucidates the intricate mechanism underlying PD-L1 expression and the interaction with TILs within the tumor microenvironment. This diagram is structured to illustrate the sequential and interrelated biological processes that contribute to the immune evasion tactics employed by tumors. PD-L1: programmed death-ligand 1; PD-1: programmed death-1; TILs: tumor infiltrating lymphocytes.
Treatment strategies involving PD-L1-I have shown promise in improving patient outcomes (Figure 3). OSCC is characterized by its aggressive nature and resistance to conventional therapies, making immunotherapy an appealing alternative. Clinical trials have been conducted to test the effectiveness of PD-L1-I, such as pembrolizumab and nivolumab, in treating OSCC [36].
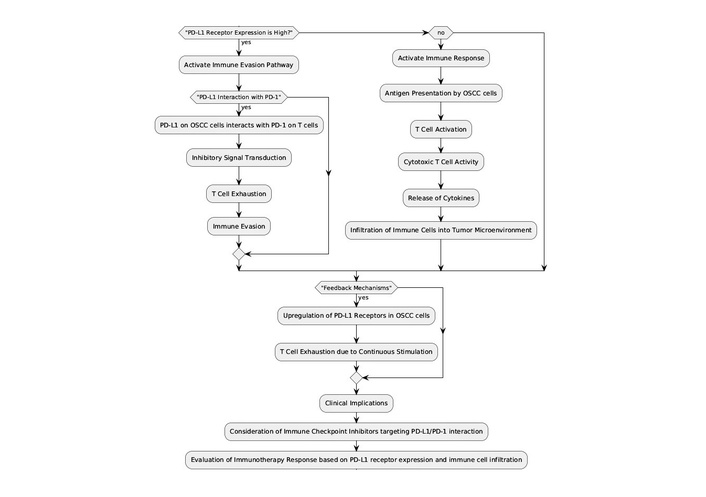
Immune checkpoint pathway and clinical implications in OSCC. This flowchart illustrates the mechanism of PD-L1 receptor expression in OSCC cells and its interaction with PD-1 on T cells, leading to immune evasion. It details the sequence from antigen presentation by OSCC cells, T cell activation, and the subsequent inhibitory signal transduction that results in T cell exhaustion. Clinical implications include the consideration of immune checkpoint inhibitors targeting the PD-L1/PD-1 interaction to evaluate immunotherapy response. PD-L1: programmed death-ligand 1; PD-1: programmed death-1; OSCC: oral squamous cell carcinoma.
One approach involves utilizing PD-L1 inhibitors as monotherapy in patients with recurrent or metastatic OSCC who have failed conventional treatments. Clinical trials have proven to yield sustained responses and enhance survival rates in some patients, particularly those with high PD-L1 expression levels. These results provide evidence of the potential of PD-L1-I as a useful tool in the treatment of OSCC patients with the least number of therapeutic options [37].
Besides that, the combined therapies that have PDL-1 inhibitors in common with other drugs, for example, chemo or targeted therapies, are also being considered. These combination approaches aim to enhance the immune response while simultaneously targeting other pathways involved in OSCC progression. Few studies suggest that combination therapies may offer improved response rates and survival outcomes compared to monotherapy alone [18, 19].
Moreover, present research efforts are focused on locating prognostic biomarkers for more precise identification of patients who will benefit from anti-PD-L1 therapy. Mainly, a high PD-L1 expression has been linked to good treatment responses; however, not every patient with high PD-L1 levels is therapy responsive. Hence, there is a pool of biomarkers other than PD-L1, such as tumor mutational burden (TMB) or immune cell infiltration, which are now thoroughly researched for elaborate patient selection and better treatment outcomes [38].
Emerging evidence continues to reinforce the central role of PD-L1 in modulating anti-tumor immune responses and shaping therapeutic outcomes. Recent studies demonstrate that enhancing PD-L1-targeted strategies—whether through adjunctive approaches like phototheranostics or by accounting for tumor-resident microbiota—can significantly improve immune activation and treatment efficacy. These findings underscore the therapeutic potential of refining PD-L1 modulation in the tumor microenvironment and highlight the importance of integrating such innovations into future clinical frameworks [39–41].
In conclusion, PD-L1 expression in OSCC is modulated by a range of biological, hormonal, and environmental factors—including sex, HPV status, lifestyle behaviors, and tumor differentiation—highlighting its complex role in tumor immune evasion. Tumors with poor differentiation and advanced stage often exhibit higher PD-L1 expression, suggesting its potential as a marker of tumor aggressiveness. However, while being linked to a few clinicopathological features, PD-L1 has not been a sole predictor of OS, DFS, and response to immunotherapy. Variability of results across studies echoes the challenges in relying solely on PD-L1 expression to be a consistent prognostic or predictive biomarker in OSCC. Procedurally, PD-L1 inhibitors have started marking the road as one of the most remarkable means of therapy with recurrent or metastatic OSCC patients as a special beneficiary group, unresponsive to conventional treatments. The outcome of monotherapy with PD-L1 inhibitors has been long-lasting in some cases, especially in those with elevated PD-L1 expression. Additionally, combination therapies that integrate PD-L1 blockade with chemotherapy or targeted agents are showing potential to improve outcomes by targeting multiple oncogenic and immunosuppressive pathways.
Ongoing research is increasingly focused on identifying more precise biomarkers—such as TMB or immune cell infiltration—to better predict which patients will benefit from PD-L1-targeted therapies. These efforts aim to refine patient selection and improve therapeutic outcomes.
In summary, while PD-L1 remains a key player in OSCC immune evasion and a valuable therapeutic target, its prognostic significance remains limited. Future studies should prioritize standardized methodologies, robust biomarker development, and deeper exploration of PD-L1 biology to optimize its clinical utility in OSCC management.
DFS: disease-free survival
DSS: disease-specific survival
HNSCC: head and neck squamous cell carcinoma
HPV: human papilloma virus
OPSCC: oropharyngeal squamous cell carcinoma
OS: overall survival
OSCC: oral squamous cell carcinoma
PD-1: programmed death-1
PD-L1: programmed death-ligand 1
PD-L1-I: programmed death-ligand 1 inhibitors
PFS: progression-free survival
TILs: tumor infiltrating lymphocytes
TMB: tumor mutational burden
TNM: tumor node metastasis
NK: Conceptualization, Data curation, Writing—original draft, Writing—review & editing. KR: Conceptualization, Writing—original draft, Writing—review & editing, Supervision. PR: Conceptualization, Validation, Writing—review & editing, Supervision. MMM: Writing—original draft, Writing—review & editing. RF: Writing—original draft. DR: Visualization. MC and GM: Conceptualization, Validation, Writing—review & editing, Supervision. All authors read and approved the final manuscript.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
The primary data for this systematic review were sourced online from databases listed in the methods. Reference articles are accessible on PubMed, Scopus, Web of Science, and Embase. Additional supporting data are available from the corresponding author upon request.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1484
Download: 25
Times Cited: 0
Luis Cabezón-Gutiérrez ... Vilma Pacheco-Barcia
Raffaele Pellegrino ... Antonietta Gerarda Gravina
Lingli Zhao ... Gaoli Niu
Rawaa AlChalabi ... Ahmed AbdulJabbar Suleiman
Qing Bao ... Hailin Tang
