Affiliation:
1Centre of Molecular Medicine and Diagnostics, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences (SIMATS), Saveetha University, Chennai 600077, Tamil Nadu, India
ORCID: https://orcid.org/0000-0003-4574-0136
Affiliation:
2School of Biotechnology, Jawaharlal Nehru University, New Delhi 110067, India
Email: raviverma@jnu.ac.in
ORCID: https://orcid.org/0000-0003-1859-5973
Affiliation:
3Department of Biochemistry, All India Institute of Medical Sciences, New Delhi 110029, India
ORCID: https://orcid.org/0000-0001-7505-6023
Affiliation:
1Centre of Molecular Medicine and Diagnostics, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences (SIMATS), Saveetha University, Chennai 600077, Tamil Nadu, India
Email: vishnupriya@saveetha.com
ORCID: https://orcid.org/0000-0002-5071-9860
Explor Immunol. 2025;5:1003227 DOI: https://doi.org/10.37349/ei.2025.1003227
Received: March 18, 2025 Accepted: September 19, 2025 Published: November 17, 2025
Academic Editor: Diego A. Bonilla, Dynamical Business & Science Society-DBSS International SAS, Colombia
Tumor-infiltrating lymphocytes (TILs) play a critical role in the ability of the immune system to combat cancer, offering a foundation for personalized immunotherapies. However, the effectiveness of TILs is often reduced by problems like becoming less active, the tumor making the immune system weaker, and not lasting long in the tumor environment. Recent advancements in single-cell technologies, including single-cell RNA sequencing (scRNA-seq), single-cell T-cell receptor sequencing (scTCR-seq), and mass cytometry (CyTOF), have revolutionized our understanding of TIL heterogeneity and dynamics. These tools offer new perspectives on the diverse phenotypes, functional states, and spatial organization of TILs, enabling the identification of key exhaustion markers, regulatory pathways, and neoantigen-specific clones. Concurrently, genetic reprogramming strategies have emerged to address TIL limitations by reversing exhaustion, enhancing metabolic resilience, and improving persistence in vivo. This review explores the synergistic integration of single-cell technologies and genetic engineering in refining TIL-based therapies. We talk about how spatial transcriptomics can help us understand how TILs work in different areas of the body and how changing their epigenetics can help them become more effective at fighting cancer. Additionally, we highlight emerging approaches to overcome immunosuppressive barriers in the tumor microenvironment (TME), including targeting regulatory immune cells, neutralizing suppressive cytokines, and enhancing antigen presentation. Together, these strategies promise to unlock the full therapeutic potential of TILs, paving the way for more effective and durable cancer immunotherapy.
In the field of oncology, cancer immunotherapy represents a revolutionary therapeutic strategy that enables the use of the immune system to fight against malignancies. Tumor-infiltrating lymphocytes (TILs) in the tumor microenvironment (TME) have recently been shown to play an important role in anti-tumor immunity. In contrast to circulating immune cells, TILs have been explicitly primed to recognize tumor antigens, allowing them to directly attack and eliminate cancerous cells. Their infiltration into tumors is frequently associated with better patient outcomes, providing a promising therapeutic target for therapy [1, 2]. However, the success of TIL-based treatments has been inconsistent and faced many problems, like the mixed types of TILs, the immune-suppressing nature of the TME, and the exhaustion of TILs from being exposed to the same antigens for too long [3, 4].
Contributing to this hurdle is the TME, which induces a suppressive milieu that inhibits the activity of TILs. Different tumors have been shown to use many ways to weaken the immune system, such as attracting regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), producing checkpoint molecules like PD-L1, and releasing suppressive cytokines, to avoid being attacked by the immune system [5]. Moreover, TIL exhaustion, which refers to a gradual loss of effector function, significantly interferes with therapeutic effectiveness. When T cells get tired, they increase the number of certain receptors (like PD-1, TIM-3, and LAG-3) and change their gene activity and structure [6, 7].
Recent progress in single-cell technologies provides another tool for dissecting TIL heterogeneity at a very high resolution. Techniques like single-cell RNA sequencing (scRNA-seq), single-cell T-cell receptor sequencing (scTCR-seq), and mass cytometry (CyTOF) have helped us understand the different types of TILs, their functions, and the TME in great detail. For example, scRNA-seq has found new kinds of T cells, such as pre-exhausted progenitor-like T cells, which can still grow and may be important for treatment [8, 9]. Similarly, scTCR-seq has been used to study the different types of TILs and how they respond to tumors, helping to understand how many tumor-fighting T cells are present and how this relates to treatment outcomes [10].
CyTOF allows scientists to analyze more than 40 protein markers in individual cells at the same time, which helps them study the different types of TILs and how they interact with the tumor environment. This led to the discovery of TIL groups that have a limited number of clones and a stronger ability to kill cancer cells (like CD8+ T cells that produce more harmful substances, such as granzyme B and perforin) [11]. These findings have inspired novel strategies to endogenously and exogenously genetically and epigenetically reprogram TILs, including overcoming significant limitations of cell function such as T cell exhaustion and insufficiency of TIL anti-tumor activity. Genetic engineering methods, like using CRISPR-Cas9 to target blockers or increase the levels of helper molecules, have made TILs work better [12]. On the other hand, changing the way genes are expressed, especially by reversing the changes that lead to exhaustion, has been shown to be an effective way to boost TILs [7].
Herein, we review the convergence of single-cell technologies and genetic engineering to accelerate TIL-based cancer immunotherapy. Recent revolutionary advances in single-cell analysis platforms have greatly enhanced the ability to characterize immune cells and explore the molecular interactions that govern their functions. This wide range of advanced technologies helps researchers understand the differences within TIL groups and create personalized TIL treatments for patients that can improve results in medical care.
However, TILs have various intrinsic limitations that undermine their therapeutic potential for clinical use. TME is a limitation. These cells are a mixed group that includes T cells that attack tumors, Tregs, and other immune cells, each with different functions. The identification and isolation of tumor-specific TILs from this diverse pool is technically challenging but vital for successful therapy [13, 14]. T cell exhaustion is another key issue produced by persistent antigen exposure in the TME. Exhausted TILs have reduced ability to kill cancer cells and have high amounts of inhibitory receptors like PD-1, LAG-3, and TIM-3. Changes in gene activity worsen this unhealthy condition by weakening their ability to fight and making it difficult to fix [15].
In addition, immunosuppressive TME is another level of complexity. Low IL-6 levels in TILs occur because the tumor continuously makes a lot of TGF-β and IL-10, along with having immune-suppressing cell types present [5, 16]. MDSCs and Tregs within tumors are also known to inhibit TIL activity and proliferation. Moreover, TILs have limited in vivo persistence and expansion upon reinfusion. While TILs are grown outside the body to get them ready for treatment, this process can change their characteristics and make them less effective [17]. The third significant barrier to the use of new materials is manufacturing difficulties. TIL-based therapies are created using complicated processes in special facilities, which makes them expensive and hard to produce in large amounts for more patients [18, 19]. The challenges and potential solutions for TIL-based therapies are summarized in Table 1.
Key challenges and strategies in TIL-based therapies.
| Challenge | Description | Potential solution |
|---|---|---|
| TIL heterogeneity | Diverse population with varying anti-tumor and immunosuppressive roles | Single-cell technologies of subset identification and enrichment |
| T cell exhaustion | Reduced effector function due to chronic antigen exposure | Epigenetic reprogramming to reverse exhaustion markers |
| Tumor microenvironment | Suppressive factors like TGF-β and hypoxia inhibit TIL activity | Genetic engineering for resistance to suppressive signals |
| Low TIL expansion rates | Insufficient TIL numbers after ex vivo culture | Optimized expansion protocol using cytokines and co-stimulants |
| Loss of persistence in vivo | Rapid TIL depletion post infusion | Engineering for enhanced persistence and memory-like phenotypes |
TIL: tumor-infiltrating lymphocyte.
These are just some of the challenges and innovative strategies for addressing them. High-throughput single-cell analysis methods, such as scRNA-seq and scTCR-seq, now make it possible to accurately identify and select specific types of tumor-fighting TILs. Methods that identify specific markers, like PD-1 and CD39, have proven effective in isolating certain groups of cells from the tumor environment that can be used for treatment [20, 21]. Genetic engineering tools like CRISPR-Cas9 primarily counteract T cell exhaustion by knocking out inhibitory receptors. Other treatments, like histone deacetylase inhibitors (HDACis), have also been studied for their potential to revive tired T cells and help them maintain their ability to fight tumors [7, 22]. Combination therapies are being developed to diminish the suppressive TME. For example, early research and clinical tests showed that combining TIL therapy with other immune checkpoint inhibitors works better together by blocking the signals that suppress the immune response in the TME. Additionally, researchers have found substances such as IL-2, IL-15, and IL-21 to enhance the growth, survival, and effectiveness of TILs. Additionally, changing the genes of T cells, like in chimeric antigen receptor (CAR)-TILs, has become a new way to improve how TILs work and help them target the TME better. Despite these advancements, the manufacture of TILs continues to evolve. Mechanized and multi-closed-system processing will reduce costs and production periods while maintaining high-quality cellular products. Additionally, cryopreservation regulations are optimized to ensure that TILs maintain their viability when frozen and exposed to clinical processes. Also, biomarkers like TMB, TCR clonality, and where TILs are located in the tissue help choose the right patients, making sure they are the ones who will benefit the most.
TIL-based immunotherapies have not worked well for many types of cancer, but their success is hidden by big problems like immune suppression in the tumor area, TIL exhaustion, and difficulties in making them. To tackle these issues and improve how TILs work, how tumors resist treatment, and how TILs are made is very important. Overall, these methods not only make the most of TILs’ ability to treat cancer but also highlight their important role in cancer therapy, as shown by new research supporting their use in clinics and the FDA approvals of TIL treatments.
TIL-based cell immunotherapy is a potential approach that leverages the immune system’s inherent ability to attack cancer. TILs are a group of T cells taken from tumor tissue, grown and activated outside the body, and then put back into the patient. TILs can recognize and target tumor-specific antigens, thus showing enormous potential. This therapy is based on the rationale for fighting cancer cells [17]. TIL-based therapies have been particularly successful in treating cancers that have many examples of mutations, including melanoma, non-small cell lung cancer, epithelioid ovarian cancer, and triple-negative breast cancer [18].
The workflow of TIL-based immunotherapy involves several key steps. This results in the complete removal of the tumor tissue, which is then broken down into a mixture of single cells that include TILs. These TILs are subsequently expanded with IL-2 to increase their numbers [19]. The TILs are then increased to the right amount for treatment and put back in the patient after a special preparation that helps them last longer and work better. Finding methods to separate and examine TILs with single-cell technologies, along with ways to modify their genes, has led to a better process for selecting and improving specific TIL groups that attack tumors and boost their effectiveness. Single-cell technologies have transformed the study of TILs, offering a unique view of their heterogeneity and functional states. scRNA-seq lets scientists see which genes are active in each individual cell, providing a clear picture of the different TIL groups and their roles in the tumor environment [23, 24]. Similarly, scTCR-seq helps identify different TCR types and understand how many of each type are present and what they target in TIL groups. scRNA-seq enables profiling of gene expression at the single-cell level and provides a high-resolution map of TIL subsets and their function in the TME [25]. In the same vein, scTCR-seq allows for the characterization of TCR clonotypes and the determination of clonality and specificity of TIL populations [26]. CyTOF works alongside these methods by analyzing many proteins at once, which helps to find out how TILs are functioning and what characteristics they have in complicated data [27]. Genetic and epigenetic modifications have emerged as powerful tools to further augment the therapeutic potential of TILs. Researchers are using methods like CRISPR-Cas9 to stop pathways that reduce TIL activity, such as PD-1, to make them work better and resist treatments that block immune checkpoints [12]. To do this, researchers are looking into ways to change the epigenetics of T cells, like altering histones and editing DNA methylation, to help T cells regain their strength against tumors [28].
However, there are still major challenges to achieving wider success, such as T cell exhaustion, low survival rates in the body, and a tumor environment that suppresses the immune response [4]. Nonetheless, further development of TIL-based therapies through combination strategies (for example, immune checkpoint inhibitors, cytokine support, and CAR-engineered TILs) is ongoing. These advancements could broaden the use of TIL therapy and enhance the results in patients with solid tumors [29]. Here, we review recent advances in the integration of single-cell technologies and genetic engineering to optimize TIL-based immunotherapy for cancer. We review how single-cell analyses have illuminated the complexity of TIL populations, informed the design of novel genetic and epigenetic reprogramming approaches, and set the stage for next-generation, patient-specific TIL therapies. Figure 1 summarizes the pipeline and rationale for harvesting, expanding, and re-infusing TILs in the adoptive cell therapy.
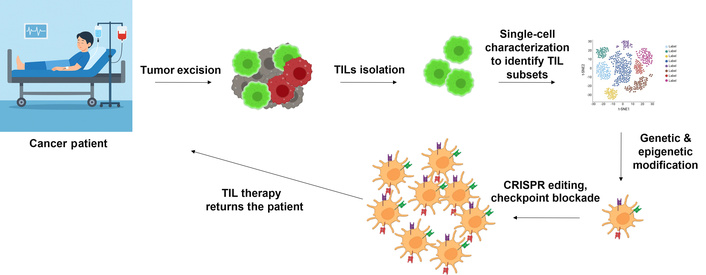
Illustration of tumor-infiltrating lymphocytes (TILs) isolation from the surgically removed tumor tissue, and TIL therapy returns to the patient after genetic and epigenetic modification of TILs.
Understanding the different functions and characteristics of TILs will help us use them better in cancer immunotherapy. Recent advances in single-cell technologies have paved the way for detailed analyses of TIL subsets, their activation states, and TME. Such approaches have helped elucidate the complexity of TIL populations and have yielded actionable insights to improve their therapeutic performance.
One of the most potent methods for profiling the transcriptome of individual TILs is scRNA-seq, which enables the characterization of different TIL subpopulations and their functional states. One example comes from scRNA-seq studies in which researchers have characterized exhausted TIL phenotypes that are associated with high expression of inhibitory receptors, such as PD-1 and LAG-3, which could serve as potential targets for therapeutic intervention [30, 31]. Similarly, scTCR-seq has been important for understanding the different types and amounts of TCRs, which helps to find the growth of TIL clones that respond to tumors when treated with receptors, helping to identify the growth of TIL clones that react to tumors in response to immunotherapies [26]. scTCR-seq has been pivotal for elucidating TCR diversity and clonality, identifying the expansion of tumor-reactive TIL clones as a response to immunotherapies.
CyTOF, or mass cytometry, works alongside transcriptomic techniques by allowing the analysis of many proteins in individual cells at the same time. Using this technology to study TILs has helped identify various types of T cells by looking at their surface markers and proteins inside the cells, which helps tell apart the different groups of effector, memory, and Tregs [27]. For example, CyTOF analysis of TILs from melanoma showed a group of CD8+ T cells that had a greater ability to kill cancer cells, which was linked to better patient outcomes [18].
Spatial transcriptomics complements this toolkit by maintaining the spatial context of TILs within the tumor tissue. This has helped us better understand how TILs interact with other immune cells or support cells in the tumor environment, allowing us to find the specific areas that reduce TIL activity [22, 30]. Using spatial transcriptomics together with scRNA-seq helped us understand TIL biology in a complete way, connecting molecular details to the tissue’s structure.
As individual technologies, single-cell approaches have improved our knowledge of TIL heterogeneity and its clinical relevance. By bringing together different types of data, researchers can find better ways to change genes and gene activity, addressing issues like TIL fatigue, energy problems, and poor tumor antigen recognition [31]. These approaches may be helpful in developing next-generation TIL-based, patient-specific therapies. Figure 2 presents an overview of TIL integration with single-cell technologies. Thanks to single-cell technologies, we can now study the differences, functions, and interactions of TILs in the TME like never before. The use of these technologies enables detailed characterization of TIL subsets and opens novel avenues for more efficient immunotherapy strategies.
Integration of single-cell sequencing technology to improve upon TIL-based immunotherapies. TIL: tumor-infiltrating lymphocyte.
scRNA-seq gives a clear picture of the TIL transcriptome, allowing us to examine various conditions of TILs, including active, memory, and exhausted types. So far, this method has revealed important signs of exhaustion, like PD-1, TIM-3, and LAG-3, which are linked to a decrease in their ability to fight tumors. Cytotoxic CD8+ T cell gene activity, indicated by the levels of granzyme B, perforin, and interferon-gamma (IFN-γ), highlights their importance in fighting tumors. Research has shown that when Tregs produce FOXP3 and CTLA-4, it reduces the body’s ability to fight tumors [8, 32].
When combined with scRNA-seq, the TCR repertoire in patients can help us understand how certain immune cells grow and recognize new cancer markers. Several studies have shown that TILs are frequently polyclonal, indicating that clonal expansion occurs, which reiterates their active role in tumor eradication. Linking certain TCRs to recognizing neoantigens has shown the possible benefits of using these clones in treatment [33].
CyTOF combines flow cytometry and mass spectrometry to allow the analysis of many protein markers in individual cells at the same time. This technology has revealed the phenotypic heterogeneity and functional states of TILs among diverse tumor types. CyTOF has identified different states of activation and exhaustion in TILs, leading to the discovery of unique TIL groups and new surface markers for further study [34].
Single-cell technologies are great at showing the differences between individual cells, while spatial transcriptomics helps us understand where in the tumor tissue specific gene expressions happen. These studies have also revealed spots where many TILs are interacting with tumor cells and talking to other cells in the TME [35, 36]. In Table 2, various single-cell technologies used for TIL research and their therapy applications are organized and shown in an updated Figure 3 [37–39]. Although scRNA-seq, scTCR-seq, and CyTOF each contribute uniquely to TIL research, their combined use provides a more comprehensive understanding of cellular phenotypes. A summary of their individual advantages and current limitations is presented in Table 2.
Single-cell technologies in TIL research.
| Technology | Strengths | Limitations | Application in TIL therapy |
|---|---|---|---|
| scRNA-seq | High-resolution transcriptome profiling identifies functional states | No direct protein expression; expensive; complex data analysis | Stratifying patients, identifying exhaustion targets |
| scTCR-seq | Tracks clinical diversity and neoantigen specificity | Requires integration with scRNA-seq for full functional insights | Selection of tumor-reactive clones for adoptive therapy |
| CyTOF | Multiplex protein profiling identifies phenotypic subsets | Lower throughput than scRNA-seq; limited to known protein markers | Optimizing TIL enrichment protocols |
| Spatial transcriptomics | Maps the spatial localization of gene expression in situ | Limited resolution; currently lower gene coverage than scRNA-seq | Targeting TIL-enriched tumor niches |
scRNA-seq: single-cell RNA sequencing; scTCR-seq: single-cell T-cell receptor sequencing; CyTOF: mass cytometry; TIL: tumor-infiltrating lymphocyte.
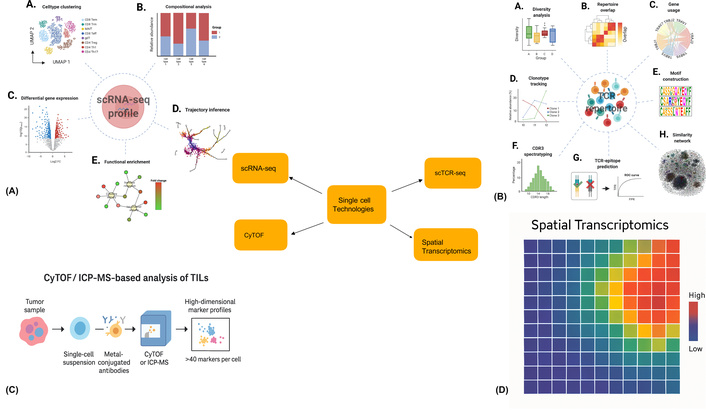
Overview of single-cell technologies applied in TIL analysis. (A) scRNA-seq enables high-resolution clustering of immune cell subtypes, such as CD4+ T cells, CD8+ T cells, NK cells, dendritic cells, monocytes, and B cells, using UMAP dimensionality reduction. Reprinted from [83]. © 2022 The Authors. CC BY 4.0. (B) Compositional analysis of single-cell datasets reveals shifts in immune cell proportions across different tumor conditions (e.g., control, treated, T1, T2) using stacked bar plots. Reprinted from [83]. © 2022 The Authors. CC BY 4.0. (C) Differential gene expression (DGE) analysis highlights up- or down-regulated transcripts between clusters, aiding identification of exhaustion or activation signatures in TILs. (D) Trajectory inference traces the developmental or activation paths of T cells across pseudotime, useful for mapping transitions from naive to exhausted or memory phenotypes. scRNA-seq: single-cell RNA sequencing; scTCR-seq: single-cell T-cell receptor sequencing; CyTOF: mass cytometry; TIL: tumor-infiltrating lymphocyte.
At the same time, single-cell technologies have helped us understand the different types of TILs, which may include useful groups and active states. These findings not only advance our knowledge of the tumor-immune ecosystem but also point to specific targets for intervention. Based on this knowledge, new genetic and epigenetic approaches to improve TIL functionality, prevent exhaustion, and optimize their anti-tumor activity are being investigated. Combining single-cell analyses with genetic reprogramming approaches using ectopic TIL is a critical step toward next-generation, precision-engineered TIL therapies.
TILs have long been proven to be effective in cancer immunotherapy, and genetic/epigenetic reprogramming can greatly enhance their functional efficacy. Such strategies overcome major challenges, such as T cell exhaustion, TME, and poor post-infusion persistence. Genome-editing tools, such as CRISPR-Cas9, have converged with epigenetic modulators to spur the evolution of next-generation TIL therapies.
Genetic changes and adjustments to gene activity have opened up exciting new ways to make TILs work better by changing how they function, how long they last, and how they interact with their environment. One such tool that has proven indispensable in addressing this challenge is CRISPR-Cas9 genome editing, which enables accurate genetic engineering to enhance TIL functionality. For example, removing genes that make inhibitory receptors like PD-1, LAG-3, or TIM-3 can help T-cells recover from exhaustion and improve their functions [40]. Cytokine receptor subunits, like IL-2 and IL-7R, are also very important because they help TILs grow and survive in the body [15]. CRISPR-modified TILs that have had specific genes removed, including those linked to TGF-β or adenosine signaling, can better resist signals from the tumor environment that weaken their function, allowing them to work more effectively for a longer time [41]. Improvements also include methods for growing edited TILs outside the body, where more growth from cytokines results in a greater potential for treatment [42].
On the other hand, synthetic biology approaches (such as the design of CAR-TILs) are reshaping the field of adoptive cell therapy. TILs naturally find and target tumors, and CARs can identify tumor markers; so, using CARs in TILs could merge their natural ability to locate tumors with a strong ability to recognize tumor markers in regular TILs [43]. CAR-TILs do not depend on major histocompatibility complex (MHC) compatibility, making them applicable to a broader range of patient populations. A significant challenge, however, is to maintain TILs’ intrinsic tumor specificity in the context of adding CAR constructs [44].
Another promising way to restore TIL functionality is through epigenetic reprogramming, where dynamic changes in gene expression occur without altering the underlying DNA sequence. A key focus has been on reversing T cell exhaustion, a signature of dysfunctional TILs. Methods like using HDACis to make chromatin more accessible have helped restore the functions of TILs by reactivating genes that fight tumors [45]. Similarly, DNA methyltransferase inhibitors (DNMTis) help remove chemical tags from DNA that are involved in T cell exhaustion, which helps to refresh TILs in the tumor environment [46].
The importance of changing how genes are expressed to improve TIL function has become a hot topic, with research indicating that how open or closed chromatin is closely related to T cell exhaustion and the ongoing presence of tumors. SHP-1 signaling, which has recently been found to play an important role in how T cells respond to melanoma, could be changed at the epigenetic level to overcome resistance and improve the effectiveness of checkpoint treatments [47]. In this situation, using a mix of changing gene activity, blocking immune checkpoints, and using AI to predict outcomes has shown promise in customizing treatment for melanoma.
TILs represent another promising avenue for improving memory-like phenotypes. Memory-like T cells are known to last longer, adapt better, and fight tumors more effectively than fully developed effector T cells [48]. These procedures involve altering the expression of genes related to memory-like traits in TILs under specially controlled laboratory conditions [49].
Together with the epigenetic approach, these genetic strategies (Table 3) have opened a wide avenue for overcoming major hurdles in TIL-based therapies. These approaches can rejuvenate spent TILs, restore their persistence and function, and improve clinical outcomes of adoptive cell therapy for cancer. Figure 4 illustrates the genetic approaches discussed in the references [50–52].
Key genetic and epigenetic targets in TIL reprogramming.
| Category | Target | Effect |
|---|---|---|
| Genetic engineering | PD-1, LAG-3, TIM-3 | Knockout reverses exhaustion, enhances effector functions |
| Genetic engineering | IL-2R, IL-7R | Overexpression improves proliferation and persistence |
| Genetic engineering | CAR integration | Enhances antigen specificity and bypasses MHC restrictions |
| Epigenetic reprogramming | HDACs, DNMTs | Modulation restores effector gene accessibility, reverses exhaustion |
| Epigenetic reprogramming | Memory-associated genes | Enhances persistence and memory-like phenotypes |
TIL: tumor-infiltrating lymphocyte; MHC: major histocompatibility complex.
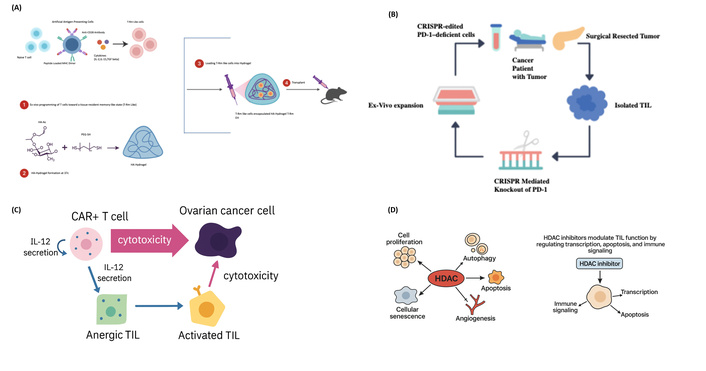
Mechanistic approaches enhancing tumor-infiltrating lymphocyte (TIL) function in the tumor microenvironment. (A) Hydrogel-based delivery system to promote tissue-resident memory T cell generation. T cells are first exposed ex vivo to tissue-imprinting cytokines to induce a T-Rm like phenotype, then encapsulated in HA-based hydrogel and injected intratumorally. This system promotes retention and efficacy of memory T cells within solid tumors. Inspired by Li et al. [50]. Adapted from [50]. © 2024 The Authors. CC BY 4.0. (B) Ex vivo CRISPR editing of TILs to knock out PD-1 expression. TILs are isolated from resected tumors and expanded ex vivo. CRISPR-Cas9 editing is used to generate PD-1-deficient TILs, which are re-infused to overcome immune exhaustion and enhance tumor killing. Adapted from [51]. © 2022 The Authors. CC BY 4.0. (C) IL-12-mediated reactivation of anergic TILs by CAR+ T cells. CAR-engineered T cells secrete IL-12 upon encountering tumor antigens, promoting both direct cytotoxicity and bystander activation of anergic TILs into cytotoxic effector cells. This strategy potentiates both adoptive and endogenous T cell responses. (D) HDAC inhibitors modulate TIL function by regulating transcription, apoptosis, and immune signaling. Schematic representation showing the dual role of HDACs in regulating cell proliferation, autophagy, apoptosis, angiogenesis, and senescence, and how HDAC inhibitors restore TIL functionality by enhancing transcriptional accessibility, promoting apoptosis, and supporting immune signaling. Figure concept inspired by Shanmugam et al. [52]; diagram fully redrawn with original elements.
Although methods to change genes and epigenetics have shown great promise in improving the performance, longevity, and targeting of TILs, the TME still poses a challenge. The TME is a tough environment for TIL-based therapies because it has many immunosuppressing cells, harmful substances, and physical barriers. This environment not only makes it hard for reprogrammed TILs to enter and stay in the tumor but also weakens their ability to fight cancer. So, understanding and finding ways to get past these challenges is essential for making the most of improved TILs, which requires both modifying the cells and changing the TME.
TIL therapy is a promising frontier in cancer immunotherapy, but it faces significant challenges due to TME-dependent barriers. TME is a complex and dynamic entity composed of stromal cells, immune cells, blood vessels, and an extracellular matrix (ECM) that poses significant hurdles and impairs TIL function. It is indispensable to understand the immune evasion mechanisms that exist within this hostile microenvironment to improve TIL-based therapeutic strategies. TME is immunosuppressive and mediates various mechanisms to dampen the action of TILs. Regulatory immune cells are among these cell types. Tregs are a well-known type of immune cell that can reduce the growth and activity of TILs, using substances like TGF-β and IL-10 [53]. Moreover, Tregs also compete with growth factors, such as IL-2, which further inhibits the expansion of TIL [54]. MDSCs also stop TILs from working by releasing substances like arginase-1, nitric oxide, and reactive oxygen species [55].
Soluble factors in the TME also play a role in TIL dysfunction. TGF-β and IL-10 reduce the ability of TILs to kill cancer cells and produce important signaling molecules [56]. Indoleamine 2,3-dioxygenase (IDO) changes tryptophan into kynurenine, which stops TILs from growing and working properly [57]. Hypoxia in the TME intensifies these immunosuppressive effects. Changes caused by low oxygen levels, influenced by hypoxia-inducible factors (HIFs), also make it harder for TILs to move into the tumor area [58]. Also, higher levels of AMPKα1 are linked to increased glucose uptake because tumor cells are competing for the small amount of glucose available in the TME [59].
Tumor cells develop sophisticated mechanisms to escape immune surveillance, thereby decreasing the effectiveness of TILs. MHC class I molecules play a crucial role in this process [60]. Changes in the components that help present tumor antigens can also interfere with how tumors show their antigens, allowing them to avoid detection by the immune system [61]. Immune checkpoints, like PD-L1 found on tumor cells, affect TILs by increasing PD-L1 levels, which causes TILs to become less effective when they interact with PD-1 on their surface [62]. The presence of other negative receptors, like TIM-3 and LAG-3, works together to increase TIL exhaustion and dysfunction. Also, the thick layer of ECM made by fibroblasts creates physical obstacles that limit TIL entry and movement [63]. Monoclonal antibodies that reduce Tregs and MDSCs have been shown to be effective in early research and clinical trials [64]. Switching exhausted TILs and recovering effector functions using immune checkpoint inhibitors can also be performed through TIL therapy [65]. The potential of TGF-β and IDO inhibitors to improve TIL activity has been demonstrated by neutralizing soluble immunosuppressive factors. TILs can also be improved using gene-editing methods like CRISPR-Cas9, which help them resist signals that suppress their activity [66]. Helping TILs survive in low-oxygen areas by adding pathways that trigger hypoxia and enzymes that break down ECM components has been shown to improve TIL entry into tumors and their ability to stay there [58]. Researchers are looking into different ways to improve antigen presentation, such as using epigenetic drugs to boost MHC class I release and developing neoantigen-based vaccination strategies [67].
The immunosuppressive TME can pose a barrier to tumor rejection, and tumor immune evasion mechanisms further complicate this rejection challenge. Thus, there is a need for optimized manufacturing protocols to produce highly functional TILs capable of overcoming these hurdles. TIL manufacturing aims to create a strong group of cells that can fight tumors by using new methods to separate and grow these cells effectively, while also considering the challenges posed by the TME. The combination of looking at where TILs come from and adjusting TIL bone marrow methods is key to making TIL therapy better in clinical settings. Table 4 summarizes the conditions in the TME and the possible solutions to compensate for this.
Tumor microenvironment challenges and the proposed solution for each condition.
| Challenge | Mechanism | Proposed solution |
|---|---|---|
| Regulatory immune cells | Tregs and MDSCs secrete inhibitory cytokines | Depletion via monoclonal antibodies |
| Soluble immunosuppressive factors | TGF-β, IL-10, and IDO suppress TIL activity | Use inhibitors to neutralize suppressive signals |
| Hypoxia and metabolic constraints | Hypoxia and glucose deprivation impair TILs | Engineered TILs with hypoxia-resistance pathways |
| Antigen presentation loss | Own regulation of MHC class I molecules | Epigenetic drugs to restore MHC expression |
| Physical barriers | Dense ECM limits infiltration | Combine with ECM-degrading enzymes |
MDSCs: myeloid-derived suppressor cells; IDO: indoleamine 2,3-dioxygenase; TIL: tumor-infiltrating lymphocyte; MHC: major histocompatibility complex; ECM: extracellular matrix.
The problems caused by the TME and how tumors avoid the immune system highlight the need for better ways to produce TILs that are strong enough to break through these obstacles. By using new ways to separate the cells, better methods to grow them, and making genetic changes, TIL manufacturing aims to create strong cell groups that can still fight tumors even in the tough environment of the TME. The smooth connection between recognizing the difficulties of the TME and improving TIL manufacturing methods is essential for making TIL therapy more effective in treating patients. Isolated and expanded TIL therapy is a successful treatment for certain solid cancers, and it is essential to know how to create TILs that are active and healthy for these therapies to work well. To this end, researchers have undertaken novel manufacturing paradigms that emphasize TIL isolation optimization, ex vivo expansion, and genetic or epigenetic reprogramming of TIL. The ultimate goal of these developments is to increase cell viability and potent anti-tumor activity and to enable scalable, broad clinical translation.
This starts with the isolation of viable TILs from tumor tissues. We achieved this by combining mechanical disruption with enzyme digestion. These are typically supplemented with enzymes such as collagenase and DNase to digest the stromal matrix and release immune cells. To get the most TILs from a tumor sample, we use enzyme digestion along with mechanical methods like cutting and blending [68]. Focusing on tumor-reactive TILs is an important step in separating a specific group of immune cells that can fight tumors from a larger mix of immune cells. Historically, such enrichment has been achieved via cytokine-based expansion in IL-2-rich medium. Recently, new methods that involve stimulating immune cells with neoantigens have been created to boost the number of tumor-fighting TILs, leading to better treatment outcomes [69]. In vitro expansion provides a method to obtain adequate amounts of TIL for therapeutic use. A pre-rapid expansion protocol (pre-REP) usually initiates this process, allowing small-scale cultures to proliferate heterogeneous TIL populations. Cytokines, such as IL-15 and IL-21, which promote T-cell memory formation, have been proposed as alternatives to IL-2 to enhance long-term persistence [70].
The process of transferring and growing TILs, which uses a lot of IL-2, anti-CD3 antibodies, and feeder cells, can sometimes lead to over 90% growth of TILs, often resulting in a 1,000-fold increase in TIL growth within two to three weeks. This reliable route is the basis of clinical-grade TIL production [69, 70]. The quality of TILs also matters as much as their quantity. Mixing certain cytokines, like IL-15 and IL-21, helps grow more basic, stem-like TILs that last longer and work better against tumors. IL-2, in synergy with IL-7, on the other hand, promotes the survival and functions of CD8+ T cells [49]. However, cultural conditions also play an important role. Being in low-oxygen conditions outside the body has been shown to help TIL survive and mimic the environment of tumors, which may prepare the cells better for working effectively inside the body. In addition, using serum-free media results in less variability, which allows a smoother transition to clinical manufacturing [59].
During TIL manufacturing, we can use genetic and epigenetic modifications to mitigate unique barriers such as immune suppression and exhaustion. CRISPR-Cas9 is a strong gene-editing tool that can accurately remove genes that prevent immune responses, like PD-1, or add new synthetic receptors, such as CARs, which help target tumor markers better. Epigenetic reprogramming is a promising therapeutic strategy. Adding HDACis to the growth solution helps reactivate important genes, and making the DNA more accessible can enhance the development of long-lasting TILs that work well against tumors [71].
Automation and bioreactor systems are transforming the scalability of TIL therapies. For example, automated closed systems perform the entire manufacturing process, from isolation to harvest, reducing the risk of contamination and ensuring the same product. Bioreactors made for perfusion help grow a lot of TILs at once and allow for continuous tracking of how the cells are growing. Bioreactors enhance reproducibility and scalability by providing customizable parameters, two of the most daunting bottlenecks in TIL therapy [68]. Much work has been done to translate TIL immunotherapy from bench to bedside. Table 5 summarizes the innovations in TIL techniques, which have enabled significant improvements in the technology for clinical uses.
Advances in TIL manufacturing.
| Stage | Innovation | Impact |
|---|---|---|
| TIL isolation | Enzymatic digestion and enrichment | Higher yield of tumor-specific TILs |
| Ex vivo expansion | Cytokine combinations (IL-15, IL-21) | Development of stem-like TILs with enhanced persistence |
| Genetic modification | CRISPR-Cas9 editing | Knockout of inhibitory receptors to boost efficacy |
| Epigenetic reprogramming | HDAC inhibitors | Expansion of memory-like TILs with improved functionality |
| Automation | Closed automated systems | Reduced labor costs and contamination risks |
| Bioreactor technology | Perfusion-based culture systems | Scalable production with real-time monitoring |
TIL: tumor-infiltrating lymphocyte.
TIL therapy has evolved from experimental studies to commercially approved immunotherapies with outstanding potential. Since October 2023, Lifileucel, a TIL-based immunotherapy developed by Iovance Biotherapeutics, has been approved by the U.S. FDA for patients with advanced melanoma who have not responded to other treatments. This approval represents an important landmark achievement towards making TIL therapy a standard therapy for patients with cancer, underscoring that its safety and efficacy have been thoroughly tested in rigorous clinical trials [72]. In preclinical studies, TME is represented by immune suppression and TIL exhaustion. Preclinical studies have found that using TILs that specifically target these unique tumor markers helps to clear tumors more effectively in mouse models. CAR-engineered TILs have been very effective against solid tumors because they can avoid MHC limitations and better recognize tumor antigens [23].
After Lifileucel was approved, researchers are now looking into using TIL therapy for more types of cancer, such as non-small cell lung cancer, cervical cancer, and triple-negative breast cancer. There are current efforts to improve TIL therapy by using checkpoint inhibitors, neoantigen vaccines, and oncolytic viruses. Table 6 depicts the pivotal developments in TIL-based immunotherapy for various cancers.
Key milestone in TIL immunotherapy development.
| Cancer type | Trial name/ID | Therapy used | Status | Key findings |
|---|---|---|---|---|
| Metastatic melanoma | NCT02360579 (Lifileucel) | TIL + IL-2 | Completed | Objective response rate (ORR) ~31.4%, complete response (CR) ~5.9%, partial response (PR) ~25.5%; median duration of response (DOR) ~36.5 months, median overall survival (OS) ~13.9 months, 5 years OS ~19.7% |
| HPV related cancer | NCT03108495 | TIL | Terminated | Demonstrated CRs in 2/9 patients (in one cohort) |
| Non-small cell lung cancer | NCT04614103 | Neo-TILs | Recruiting | The ORR was 21.4% (n = 28) with one CR and five PRs |
| Triple-negative breast cancer | NCT04111510 | Neoantigen-TILs | Completed | Higher density of sTILs (a marker for TILs) was associated with a higher pathological CR (pCR) rate and better OS |
| Gastrointestinal (GI) cancer | NCT01174121 | Autologous TILs | Recruiting | Showed objective responses in 23.5% of patients with treatment-refractory metastatic GI cancers |
| Cervical cancer | NCT01585428 | TIL + IL-2 | Completed | Objective tumor responses occurred in 5/18 (28%) patients |
| Ovarian cancer | NCT03287674 | TIL + nivolumab | Completed | Safety demonstrated; increased TIL persistence |
| Refractory ovarian cancer, colorectal cancer, or pancreatic ductal adenocarcinoma | NCT03610490 | TIL + IL-2 | Terminated | Disease control rate (DCR) of 62.5% (10 out of 16 patients) |
TIL: tumor-infiltrating lymphocyte.
TIL therapy is at the forefront of immuno-oncology, ready to tackle current challenges, boost efficacy, and expand its application across various malignancies. Such innovations include genetic engineering, combination therapies, modulation of the TME, manufacturing, and non-oncological applications. This development has led to multiplex gene editing using genetic engineering approaches, such as CRISPR-Cas9, to modify multiple genes simultaneously to improve the function of TILs. Taking away markers that make TILs less effective, such as PD-1 and LAG-3, and adding resistance to substances that weaken the immune response, like TGF-β, greatly enhances how long TILs survive and how effectively they attack tumors [12], for instance. Synthetic biology techniques improve TIL performance by using logic gates to specifically target tumor-related antigens and creating synthetic circuits that control the release of cytokines to reduce overall toxicity [73]. There is also the synergistic potential to combine TIL therapy with other next-generation therapies. Personalized neoantigen vaccines can prepare the immune system and improve the targeting and effectiveness of TIL in early research studies [74, 75]. Oncolytic viruses contribute to this by allowing the release of tumor antigens while promoting the penetration of TIL [76]. Using immune checkpoint inhibitors like anti-PD-1 and anti-CTLA-4, along with TIL therapy, works better together, helping to reduce fatigue in the immune cells and making the treatment last longer [77]. TIL therapy is significantly hampered by the TME. Changing the thick, low-oxygen areas of tumors helps TILs get in and survive better, with new methods aimed at improving the surrounding tissue and blood flow in tumors [78]. By using ways that help cells adjust to low oxygen, such as turning on HIF, the effectiveness of TILs is increased in the low-oxygen areas commonly found in tumors [79]. TIL therapy is becoming more scalable and efficient owing to innovations in the manufacturing and delivery methods. Automated, closed systems ensure that production is streamlined and products are more consistent, as well as bioreactor technologies designed to optimize TIL expansion in real time [80]. Also, using CRISPR editing to stop the body from rejecting donor T cells in allogeneic TIL therapies gives hope for ready-to-use solutions that are cheaper and faster to produce [81]. The potential for TIL therapy is not limited to oncology. In the case of infectious diseases, researchers are looking into TIL-like therapies to attack cells infected by viruses in long-lasting infections, like HIV and hepatitis B. Similarly, altered TILs that reduce harmful immune responses might provide new treatment choices for autoimmune diseases like rheumatoid arthritis and lupus. Specific predictive biomarkers can predict custom TIL therapy based on all these factors. TMB, neoantigen load, and immune infiltration profiles have been explored to improve patient selection [82]. Similarly, follow-up studies are intended to understand the long history of TIL responses and late-onset toxicities and guide protocol refinement. To make TIL therapy accessible to all patients, strategies for lowering its price consist of simplifying the manufacturing processes and developing allogeneic products. Government and non-profit initiatives can subsidize treatment in low-income regions. Local partnerships, as well as the establishment of clinical trials and regulatory pathways, will accelerate the global uptake of this disruptive therapy.
TIL therapy significantly transforms the fight against cancer, providing hope to patients with treatment-resistant tumors. Despite these challenges, advancements in genetic engineering, TME modification, and manufacturing scalability are paving the way for wider application and improved patient outcomes. As ongoing studies have shown how well it works for different types of cancer, TIL therapy is set to become a key part of personalized cancer treatment alongside the latest immunotherapy methods. TIL therapy has gone from experimental studies to commercially approved immunotherapy with enormous potential. Since October 2023, Lifileucel, a TIL-based immunotherapy developed by Iovance Biotherapeutics, has been approved by the U.S. FDA for patients with advanced melanoma who have not responded to other treatments. This move represents an important landmark achievement towards making TIL therapy a standard therapy for patients with cancer, underscoring that its safety and efficacy have been thoroughly tested in rigorous clinical trials.
CAR: chimeric antigen receptor
CyTOF: mass cytometry
ECM: extracellular matrix
HDACis: histone deacetylase inhibitors
HIFs: hypoxia-inducible factors
IDO: indoleamine 2,3-dioxygenase
MDSCs: myeloid-derived suppressor cells
MHC: major histocompatibility complex
scRNA-seq: single-cell RNA sequencing
scTCR-seq: single-cell T-cell receptor sequencing
TILs: tumor-infiltrating lymphocytes
TME: tumor microenvironment
Tregs: regulatory T cells
We thank Saveetha University and our respective guides and co-guides for their continued support and feedback during the preparation of this manuscript.
CK: Conceptualization, Methodology, Investigation, Formal analysis, Data curation, Visualization, Writing—original draft. RV: Methodology, Writing—review & editing. AS: Writing—review & editing. VVP: Conceptualization, Supervision, Project administration, Writing—review & editing. All authors read and approved the final manuscript.
The authors declare that they have no competing interests.
Not applicable.
Not applicable.
Not applicable.
All the data discussed is publicly available from cited repositories, such as GEO and TCGA.
This research received no specific grant from any funding agency, commercial entity, or not-for-profit organization. The authors declare that they did not receive any financial support for the preparation of this article.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1639
Download: 30
Times Cited: 0
