Open Access
Meta-Analysis
Work-related musculoskeletal disorder prevalence among African nurses: systematic review and meta-analysis
Philippe Gorce, Julien Jacquier-Bret
Published: February 12, 2026 Explor Musculoskeletal Dis. 2026;4:1007116
This article belongs to the special issue Prevalence and Risk Factors of Work-related Musculoskeletal Disorders

Open Access
Editorial
Biosimilars: state of the art in the treatment of rheumatic diseases
Valderilio Feijó Azevedo
Published: February 03, 2026 Explor Musculoskeletal Dis. 2026;4:1007115
This article belongs to the special issue Biosimilars: State of the Art in the Treatment of Rheumatic Diseases

Open Access
Editorial
Magnetic Resonance Neurography: redefining the diagnostic frontier in musculoskeletal disease
Theodoros Soldatos
Published: February 02, 2026 Explor Musculoskeletal Dis. 2026;4:1007114
This article belongs to the special issue Magnetic Resonance Neurography: Advances, Techniques, and Clinical Applications

Open Access
Case Report
Xanthoma simulating gouty tophus (case report of atypical cholesterol crystal deposition)
Maxim Sergeevich Eliseev ... Maria Nikolaevna Chikina
Published: December 24, 2025 Explor Musculoskeletal Dis. 2025;3:1007113
This article belongs to the special issue Evaluation and Outcomes in the Management of Gout

Open Access
Mini Review
Lycopene supplementation in rheumatic diseases: a comprehensive review
Jozélio Freire de Carvalho, Ana Tereza Amoedo Martinez
Published: December 09, 2025 Explor Musculoskeletal Dis. 2025;3:1007112
This article belongs to the special issue Complementary and Integrative Medicine in Rheumatology: Evidence, Therapies, and Clinical Impact
Open Access
Case Report
Ultrasound-guided dextrose hydrodissection for multiple peripheral entrapment neuropathies in scleroderma: a case presentation
Helen Gharaei ... Ziba Bagherian
Published: December 08, 2025 Explor Musculoskeletal Dis. 2025;3:1007111
Open Access
Mini Review
Current concepts on the intervention for adhesive capsulitis
Abeer Alomari, Philip Peng
Published: December 05, 2025 Explor Musculoskeletal Dis. 2025;3:1007110
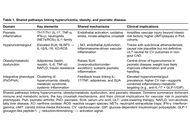
Open Access
Editorial
Hyperuricemia-gout, psoriatic disease, and what to expect from advanced anti-obesity therapies
Rubén Queiro-Silva
Published: November 25, 2025 Explor Musculoskeletal Dis. 2025;3:1007109
This article belongs to the special issue Evaluation and Outcomes in the Management of Gout
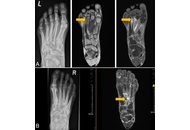
Open Access
Review
The significance of insufficiency fractures in rheumatic musculoskeletal diseases
Jürgen Braun, Björn Bühring
Published: November 12, 2025 Explor Musculoskeletal Dis. 2025;3:1007108
This article belongs to the special issue Multifaceted Imaging in Rheumatic and Musculoskeletal Diseases
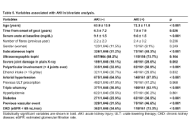
Open Access
Original Article
Acute kidney injury in gout: prevalence and risk factors through two decades
Nuria Perez-Herrero ... Fernando Perez-Ruiz
Published: November 10, 2025 Explor Musculoskeletal Dis. 2025;3:1007107
This article belongs to the special issue Evaluation and Outcomes in the Management of Gout
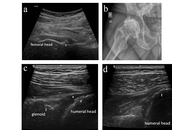
Open Access
Perspective
Musculoskeletal ultrasound in the management of immune checkpoint inhibitor arthritis
Gregory J. Challener ... Minna J. Kohler
Published: October 22, 2025 Explor Musculoskeletal Dis. 2025;3:1007106
This article belongs to the special issue Multifaceted Imaging in Rheumatic and Musculoskeletal Diseases

Open Access
Original Article
Prevalence of vitamin D deficiency among Northern Muslim women with chronic musculoskeletal pain residing in Port Harcourt, Rivers State, Nigeria: a cross-sectional study
Gogo James Owo ... Karibo Amakiri Okari
Published: October 14, 2025 Explor Musculoskeletal Dis. 2025;3:1007105
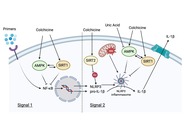
Open Access
Review
New perspectives on the NLRP3 inflammasome—colchicine and the suppression of inflammatory pathways in metabolic syndrome associated diseases
Benjamin Plotz ... Michael H. Pillinger
Published: September 29, 2025 Explor Musculoskeletal Dis. 2025;3:1007104
This article belongs to the special issue Pharmacological and Non-Pharmacological Management of Gout
Open Access
Review
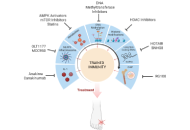
Targeting innate immune memory: a new paradigm for gout treatment
Orsolya I. Gaal ... Tania O. Crișan
Published: August 28, 2025 Explor Musculoskeletal Dis. 2025;3:1007103
This article belongs to the special issue Pharmacological and Non-Pharmacological Management of Gout
Open Access
Case Report
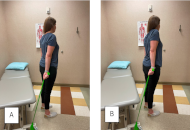
Mechanically loaded neurodynamics for post-surgical rehabilitation of arterial thoracic outlet syndrome in an endurance athlete: a case report
Thomas Wyatt
Published: August 26, 2025 Explor Musculoskeletal Dis. 2025;3:1007102
Open Access
Mini Review
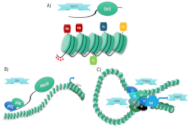
Long noncoding RNA: control of chromatin structure in muscle differentiation
Rodolfo Daniel Ávila-Avilés
Published: August 19, 2025 Explor Musculoskeletal Dis. 2025;3:1007101

Open Access
Review
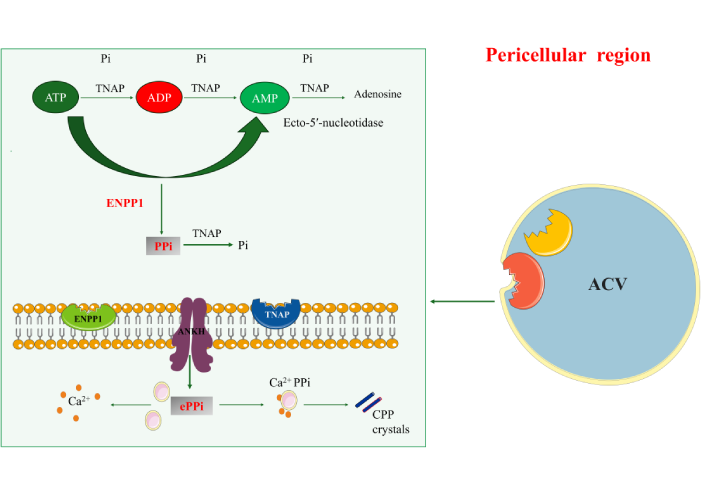
Treatment of calcium pyrophosphate crystal deposition disease: a mini-review
Ebru Atalar, Hatice Bodur
Published: August 18, 2025 Explor Musculoskeletal Dis. 2025;3:1007100
This article belongs to the special issue Calcium Pyrophosphate Deposition Disease
Open Access
Review
Calcium pyrophosphate deposition (CPPD) disease: a review of pathophysiology, clinic and diagnosis
Gamze Dilek ... Kemal Nas
Published: August 11, 2025 Explor Musculoskeletal Dis. 2025;3:100799
This article belongs to the special issue Calcium Pyrophosphate Deposition Disease

Open Access
Review
Biologics and biosimilars in musculoskeletal diseases: addressing regulatory inconsistencies and clinical uncertainty
Lauren N. McGrath ... Steven R. Feldman
Published: July 21, 2025 Explor Musculoskeletal Dis. 2025;3:100798
This article belongs to the special issue Biosimilars: State of the Art in the Treatment of Rheumatic Diseases

Open Access
Review
Risk factors of neoplastic disease in patients with systemic rheumatic disorders
Eugeniusz J. Kucharz
Published: July 16, 2025 Explor Musculoskeletal Dis. 2025;3:100797
 Previous
Previous