Affiliation:
Japan Tissue Engineering Co. Ltd., Gamagori City 443-0022, Aichi, Japan
Email: shinobu_yanada@jpte.co.jp
ORCID: https://orcid.org/0000-0002-6857-0128
Explor Musculoskeletal Dis. 2025;3:100792 DOI: https://doi.org/10.37349/emd.2025.100792
Received: December 28, 2024 Accepted: May 14, 2025 Published: May 21, 2025
Academic Editor: Fernando Pérez-Ruiz, Cruces University Hospital, Spain
The article belongs to the special issue Cell Therapy and Tissue Engineering for Musculoskeletal Conditions: From Pre-clinical Studies to Clinical Trials
Bone marrow mesenchymal stem cells (BMSCs) are multipotent progenitor cells with the capacity to differentiate into various mesenchymal lineages, including osteogenic, chondrogenic, and adipogenic tissues, rendering them promising candidates for regenerative medicine. This review delves into current foundational and preclinical research concerning BMSCs, with a particular emphasis on the use of genetically modified rat-derived BMSCs expressing green fluorescent protein (GFP) to facilitate in vivo cell tracking during tissue repair. It also examines various administration strategies, including intra-articular injections and magnetically guided cell targeting, to evaluate their therapeutic efficacy. Emerging evidence highlights the pivotal role of BMSCs in regenerating musculoskeletal tissues, including muscle, meniscus, and cartilage. Notably, the application of external magnetic fields (EMF) to direct magnetically labeled BMSCs to injury sites has demonstrated encouraging outcomes in cartilage repair. Furthermore, advances in BMSC culture techniques, single-cell genetic analysis, and tissue engineering methodologies may further augment their therapeutic potential. Preclinical and early-phase clinical studies underscore the promise of BMSCs as a minimally invasive therapeutic modality in orthopedic and regenerative medicine. Further research is essential to refine their applications and optimize delivery strategies, such as the use of internal magnetic fields generated by magnetized material implanted in damaged knee cartilage, to ensure long-term efficacy and safety.
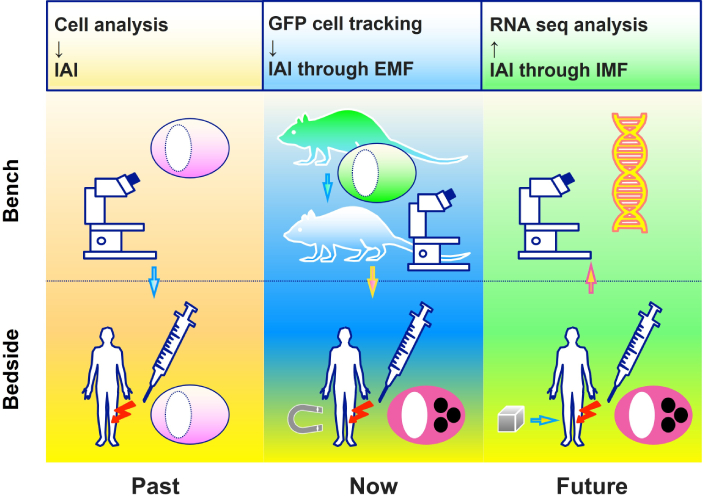
Bone marrow mesenchymal stem cells (BMSCs) are pivotal in the fields of pluripotency, immunomodulation, and tissue regeneration. We aimed to elucidate the fundamental biological properties of BMSCs, particularly their multipotent differentiation potential and delineate their therapeutic relevance in orthopedic medicine. Key preclinical methodologies are explored, including the use of green fluorescent protein (GFP) transgenic rats, magnetic field-facilitated cell aggregation, and investigations into telomere biology in the context of BMSC differentiation dynamics.
Residing within the bone marrow niche, BMSCs are indispensable to the advancements in regenerative medicine and tissue engineering. In 1968, Friedenstein et al. [1] successfully isolated these nonhematopoietic, fibroblast-like adherent cells from bone marrow, demonstrating their capacity to differentiate into osteogenic and adipogenic lineages. Caplan [2], in 1991, introduced the term “mesenchymal stem cells (MSCs)” to highlight their multipotency and regenerative capabilities. Subsequently, Johnstone et al. [3] provided compelling evidence that aggregated bone marrow-derived stromal cells could undergo chondrogenic differentiation. Caplan [4] later modified the hypothesis and stated that BMSCs primarily act as signaling entities involved in tissue repair, thereby enabling their use in cell-based therapeutic approaches for cartilage and bone regeneration.
Subsequent investigations have significantly advanced the understanding of BMSC biology, particularly regarding their immunomodulatory functions and interactions within inflammatory microenvironments. The paracrine effects of BMSCs, which secrete a diverse array of bioactive molecules such as transforming growth factor-beta (TGF-β), hepatocyte growth factor (HGF), and prostaglandin E2 (PGE2), play a pivotal role in promoting tissue regeneration, immune regulation, and maintaining immune homeostasis [5–7]. Moreover, their ability to suppress immune rejection by modulating T-cell proliferation underscores their potential for allogeneic transplantation [8].
Recent technological advancements have introduced innovative strategies to enhance the therapeutic efficacy of BMSCs. The use of GFP-expressing rats has enabled precise in vivo tracking of BMSC migration and differentiation, facilitating a rigorous assessment of their regenerative capabilities [9]. Additionally, magnetic field-mediated cell aggregation techniques have been employed to improve the targeted delivery and retention of BMSCs at injury sites, thereby maximizing their therapeutic impact [10, 11]. These advancements are particularly valuable in addressing persistent challenges, such as poor engraftment and limited post-transplantation cell viability.
Concurrently, research into BMSC telomere dynamics has yielded critical insights into their proliferative capacity and long-term functionality. Telomere attrition, a consequence of serial passaging in vitro, poses a significant barrier to their clinical utility, necessitating the optimization of culture conditions to preserve stem cell characteristics [12, 13]. Promising strategies aimed at modulating telomerase activity have emerged, offering potential solutions to enhance the viability and functional capacity of BMSCs [14].
Clinically, BMSC-based interventions have demonstrated considerable promise in orthopedic applications, including cartilage repair, osseous defect reconstruction, and tendon regeneration [15, 16]. In preclinical models of osteoarthritis, intra-articular BMSC administration has demonstrated efficacy in attenuating cartilage degeneration [17]. Furthermore, MSC-derived extracellular vesicles have exhibited protective effects on the extracellular matrix (ECM) [18]. Nevertheless, several barriers continue to hinder the clinical translation of BMSC therapies. Key challenges include donor variability, heterogeneity within cell populations, and the lack of standardized culture protocols, which warrant further investigation [19]. Additionally, challenges related to large-scale manufacturing and regulatory approval must be addressed to ensure the reproducibility, safety, and efficacy of BMSC-based therapeutics [20].
The characteristics of BMSCs are defined by their multipotency, self-renewal capacity, immunomodulatory abilities, secretion of bioactive factors, and promotion of angiogenesis. Their differentiation into various tissue types, such as bone, cartilage, and adipose tissue, is pivotal for in situ tissue regeneration and organ repair following cell transplantation. These attributes have been the subject of extensive research [21–24].
Fluorescent proteins have transformed the visualization and tracking of cells and tissues. Among them, GFP, first identified by Shimomura et al. [25], is widely favored for its strong fluorescence, high stability, and minimal toxicity. GFP has advanced biological research, enabling the observation and analysis of various biological processes. Its integration into the genomes of numerous animal models, including rats, has expanded research capabilities. GFP rats serve as indispensable tools across diverse fields, such as cytogenetics, neuroscience, immunology, and developmental biology.
GFP rats provide distinct advantages in regenerative medicine, including the real-time tracking and evaluation of transplanted cell dynamics to assess therapy efficacy, comprehensive analysis of tissue regeneration and repair, optimization of therapeutic protocols by studying cell behavior at specific sites like knee joints, and the development of noninvasive imaging techniques for quantitatively assessing regenerative processes and therapeutic outcomes. Table 1 outlines their applications in orthopedic research.
Applications of GFP rats in orthopedic research
| Category | Study focus | Reference |
|---|---|---|
| In vivo cell tracking | Monitoring of BMSC migration and differentiation | Han et al. [26] 2012 |
| Cartilage repair | Demonstrated enhanced ECM synthesis via GFP-labeled BMSCs | Yin et al. [27] 2016 |
| Bone healing | Assessed BMSC migration to bone injury sites | Li et al. [28] 2018 |
| Tendon healing | Evaluated tendon integration with GFP rat derived BMSCs | Safi et al. [29] 2018 |
| Functional recovery following spinal cord injury | Assessed BMSC migration to spinal cord injury sites | Yi et al. [30] 2019 |
| Gene expression analysis | Functional genomic profiling of GFP rat derived BMSCs | He et al. [31] 2006 |
| Scaffold integration | Compatibility and differentiation potential of GFP rat derived BMSCs within scaffolds | Rooney et al. [32] 2008 |
| Angiogenesis | Role of GFP-BMSCs in enhancing neovascularization for tissue engineering | Zhang et al. [33] 2006 |
GFP: green fluorescent protein; BMSCs: bone marrow mesenchymal stem cells; ECM: extracellular matrix
Studies were initiated using BMSCs isolated from GFP rats, inspired by Hakamata et al. [34], who introduced GFP rats as tools for organ transplantation research in 2001. At that time, no studies had tracked transplanted cells to verify their efficacy in orthopedic contexts. GFP rat-derived BMSCs were used in foundational studies targeting orthopedic tissues such as cartilage, bone, meniscus, and muscle.
GFP rats enable the tracking of transplanted cells through fluorescence, providing insights into their localization, dynamics, and the tissue repair process. This approach is essential for evaluating the efficacy of cell transplantation and optimizing cell therapy strategies.
Initial investigations focused on BMSC transplantation for muscle injury repair. BMSCs derived from GFP labeled Sprague-Dawley (SD) rats were transplanted into partial-thickness lacerations in the tibialis anterior muscle. Histological analysis revealed that BMSCs promoted muscle fiber maturation, and the injured muscle regained near-normal functional strength within 1 month. However, immunohistochemical analysis showed that the transplanted BMSCs neither fused with, nor differentiated into mature skeletal muscle fibers. Instead, they gave rise to muscle precursor cells. These findings indicate that BMSCs facilitate skeletal muscle regeneration through mechanisms that did not involve direct fusion with muscle fibers [35].
The effects of BMSCs on meniscus repair were studied by creating meniscus-like structures using scaffolds derived from normal meniscus tissue combined BMSCs. Meniscus devitalization was induced in thirty SD rats using three freeze-thaw cycles with liquid nitrogen. GFP-SD rat-derived BMSCs were cultured for 2 weeks and seeded onto the scaffolds. Fluorescence microscopy and immunohistochemical staining revealed GFP-positive cells on the surface and in deeper zones of the scaffolds at one and 2 weeks, respectively. By 4 weeks, ECM deposition was observed, alongside messenger RNA (mRNA) expressions of aggrecan and type X collagen. Indentation stiffness testing indicated significant increases in tissue stiffness, comparable to normal meniscus tissue. These results confirmed that BMSCs seeded onto meniscus-derived scaffolds can form structures resembling a normal meniscus [36].
Further investigations assessed tissue co-cultured with BMSCs and meniscus-derived scaffolds transplanted into meniscal defects. At 4 weeks postsurgery, cell proliferation and ECM deposition were evident in the transplanted tissue. By 8 weeks, articular cartilage in the acellular scaffold group exhibited greater damage than in the cell-seeded or meniscectomy groups, highlighting the protective effects of BMSC-seeded scaffolds [37].
The mobilization of BMSCs following intra-articular injections into injury sites was evaluated. Partial injuries were induced in the meniscus and cartilage of the knee joints of SD rats. Following wound closure, 106 or 107 BMSCs isolated from GFP rats were injected into the knee joints. At the 106 BMSC dose, GFP-positive cells were identified at the injured anterior cruciate ligament (ACL) in all knees. At the 107 BMSC dose, GFP-positive cells were also detected at injury sites in the medial meniscus and femoral condyle cartilage, accompanied by signs of ECM deposition around the cells. These findings suggest that BMSCs injected into the joint migrate to damaged tissues, promoting tissue regeneration, and offering a minimally invasive alternative to traditional surgical treatments for such injuries [38].
The effects of intra-articular BMSC injections on partial tears of the ACL were also investigated, using a total of 98 male, 12-week-old SD rats. Histological and biomechanical analyses revealed that the injected MSCs facilitated the healing of partially torn ACLs. This approach highlights intra-articular MSC injections as a promising therapeutic option for treating partial ACL tears [39].
Studies conducted by Agung et al. [38] and Kanaya et al. [39], examined the potential of intra-articular BMSC injections for treating partially damaged intra-articular tissues. However, an increased number of injected cells led to the formation of intra-articular scar tissue, highlighting the need for a minimally invasive method to localize stem cells precisely at the injury site and evaluate its therapeutic efficacy.
In parallel, a research group in the same laboratory explored the accumulation of artificially magnetized liposomes at cancerous sites using external magnetic fields (EMF) [40]. By the early 2000s, technologies for magnetically labeling cells or proteins and separating them using magnetic techniques were already established in biotechnology. Although the concept of magnetic targeting using magnetic fields to direct drugs to target sites was known, its application to cells remained unexplored.
In cartilage repair, cells are typically transplanted as suspensions [41], sheets [42, 43] or incorporated into scaffolds [44, 45]. However, attempts to magnetically label cells for therapeutic use in orthopedics were unsuccessful. If achieved, this approach could enhance the engraftment efficiency of transplanted cells. Conventional methods such as cell suspensions, often encounter challenges, including diffusion from the target site and uneven distribution due to gravity, limiting therapeutic efficacy. Additionally, the limited availability of autologous cells emphasizes the need for efficient cell localization strategies.
To address these challenges, basic research was conducted on minimally invasive treatments using magnetically labeled cells [46].
A method was developed to magnetically label BMSCs by conjugating magnetic beads with antibodies targeting the cluster of differentiation 44 surface antigen, arginine-glycine-aspartic acid-serine peptides, and small carboxyl group-modified beads, Ferri Sphere 100C (diameter: 310 nm). These labeled cells exhibited reduced proliferation compared to nonlabeled cells but demonstrated efficient accumulation at target site under magnetic influence and chondrogenic differentiation in vitro [47]. Further investigations revealed that complexes of magnetic beads and MSCs could undergo osteogenic differentiation [48]. Additionally, magnetic fields were found to regulate chondrogenic differentiation by lowering the concentration of TGF-β, a soluble factor involved in chondrogenesis [49].
This approach was extended to neural progenitor cells, where magnetic targeting successfully localized the cells and promoted axonal growth in vitro [50, 51]. In a rat spinal cord injury model, magnetically labeled BMSCs were effectively directed to the injury site using a magnetic field, highlighting a minimally invasive method for BMSC transplantation in spinal cord injury treatment [52]. In oncology, magnetic accumulation of natural killer cells targeting osteosarcoma was explored, demonstrating its potential as an immunotherapy strategy [53]. A list of applications of magnetically labeled BMSCs in orthopedic research is shown in Table 2.
Applications of magnetically labeled BMSCs or scaffolds in orthopedic research
| Category | Study focus | Reference |
|---|---|---|
| SPIO labeling techniques | Optimization of iron oxide nanoparticle uptake for MRI contrast enhancement | Jasmin et al. [54] 2011 |
| In vivo tracking | High-resolution MRI monitoring of SPIO-labeled BMSCs in orthopedic applications | Lu et al. [55] 2025 |
| Cartilage repair | Evaluation of magnetically guided BMSC transplantation for cartilage repair | Chen et al. [56] 2013 |
| Muscle repair | Evaluation of magnetically guided BMSC transplantation for muscle repair | Oshima et al. [57] 2014 |
| Tendon-bone healing | Investigation of magnetic targeting for enhanced BMSC retention in bone defects | Zhang et al. [58] 2024 |
| Nerve regeneration | Application of SPIO-labeled BMSCs in spinal cord injury model | Zhang et al. [59] 2015 |
| Angiogenesis rromotion | Contribution of SPIO-labeled BMSCs to vascular network formation | Cao et al. [60] 2009 |
| Scaffold integration | Compatibility and differentiation potential of BMSC in SPIO-labeled scaffolds | Chen et al. [61] 2018 |
SPIO: superparamagnetic iron oxide; BMSCs: bone marrow mesenchymal stem cells; MRI: magnetic resonance imaging
Wakitani et al. [62] treated patients with extensive full-thickness articular cartilage defects using bone marrow- or periosteal-derived MSCs combined with type 1 collagen gel, achieving favorable clinical outcomes at 24 weeks post-transplant. Henning et al. [63] used Ferucarbotran-labeled MSCs for tracking purposes. To advance clinical applications, Ferucarbotran, a magnetic resonance imaging (MRI) contrast agent, was used to magnetically label BMSCs for clinical purposes. Studies on osteochondral defects in the central patella of rabbits and pigs demonstrated that magnetically labeled BMSCs could be directed to target sites using an EMF [64]. Furthermore, magnetized human BMSCs were injected into degenerated human cartilage during total knee arthroplasty, and guided by an EMF. These cells were subsequently cultured in chondrogenic medium for 3 weeks. Histological analysis confirmed ECM production, highlighting their potential for osteoarthritis treatment [65].
To advance translational research for clinical application in humans, the safety and quality of magnetically labeled human BMSCs were evaluated in vitro. It was demonstrated that magnetic labeling did not adversely affect the safety or quality of the MSCs [66]. Subsequent clinical trials evaluated the safety and efficacy of this approach in five patients with localized knee cartilage defects. A 1.0 T permanent magnet was precisely positioned on the knee joint, based on the lesion’s location, followed by the injection of magnetized autologous BMSCs. No adverse events were reported during the treatment or follow-up periods. MRI and arthroscopy revealed significant cartilage repair, while clinical outcome scores including the International Knee Documentation Committee subjective knee evaluation and the Knee Injury and Osteoarthritis Outcome Score showed notable improvements in knee-related quality of life at 48 weeks post-treatment. These findings establish magnetic targeting therapy as a promising, minimally invasive method for cartilage repair [67].
BMSCs comprise only 1% of bone marrow cells, necessitating efficient expansion techniques for clinical applications. The study of telomere status in post-differentiated BMSCs is critical to understanding cellular senescence, regenerative capacity, and long-term functional maintenance. Telomeres, DNA sequences at the ends of chromosomes, shorten with each cell division. While BMSCs are pluripotent and capable of differentiating into bone, cartilage, and adipose cells, repeated differentiation and self-renewal lead to telomere shortening, resulting in cellular senescence and functional decline. It is essential for post-differentiation BMSCs used in regenerative medicine to maintain effective functionality within the body. Telomere analysis enables predicting the functional lifespan of differentiated cells, thereby facilitating the design of culture conditions and differentiation protocols that minimize telomere shortening. These advancements have the potential to pave way for personalized medicine in the future through telomere analysis by tailoring BMSC therapies to individual patients, optimizing differentiation processes through novel culture techniques, and standardizing cell quality control using telomere length as an indicator.
The role of fibroblast growth factor-2 (FGF-2) in enhancing MSCs expansion and maintaining their differentiation potential, especially in chondrogenesis, was investigated. Telomere length analysis revealed that MSCs cultured with FGF-2 preserved their differentiation potential and long telomeres, suggesting that telomere length may serve as a valuable marker for chondrogenic progenitor cells [68].
Further analysis focused on the quality of MSCs by profiling the expression of cytokines, including growth factors, mRNAs, and microRNAs, in human BMSCs. The results showed that, regardless of their proliferative and chondrogenic differentiation capacities, these cells exhibited the potential to promote cartilage repair in vivo [69].
Microscopic observation has confirmed the heterogeneity of BMSCs. A diverse population becomes apparent under the microscope displaying various morphologies, including larger, flattened cells, elongated cells, and polygonal cells. Recent advancements in single RNA expression analysis provide innovative approaches to investigating BMSC heterogeneity and their roles in tissue repair [70]. Gene analysis offers the potential to track the effects of transplanted cells in bone-cartilage repair without relying on GFP rats. Understanding the mechanisms underlying cellular aging and differentiation potential is critical in developing more effective BMSC-based therapies.
Tissue engineering research using BMSCs holds a pivotal role in regenerative medicine and bioengineering. The unique capability of BMSCs to differentiate into various tissue types enables their application in addressing a wide range of diseases and injuries, including bone defect repair, articular cartilage regeneration, and muscle restoration. In addition, their flexibility in cell sourcing allows for straightforward harvesting from iliac bone marrow, facilitating both autologous and allogeneic transplantation. Despite their advantages, such as high adaptability post-transplantation and the potential for patient-specific therapies, challenges persist. These include the substantial cost and time required for culture and differentiation, the need for long-term safety and efficacy evaluation, and the risk of immune rejection in allogeneic transplantation.
Tissue engineering was proposed by Langer and Vacanti [71] in 1993. It provides groundbreaking medical treatments and holds immense significance for the following seven reasons. 1) It addresses the organ shortage crisis as the worldwide demand for organ transplants significantly surpasses the available donor supply, enabling the creation of organs and tissues using the patient’s own cells. 2) It advances regenerative medicine by facilitating the repair and regeneration of tissues and organs damaged due to injury or disease. By integrating cells, scaffolds, and bioactive factors, it substantially enhances the scope of tissue restoration. A notable example is cartilage regeneration using J-TECs’ Autologous Cultured Cartilage, JACC, which involves encapsulating autologous chondrocytes in atelocollagen gel [72], resulting in significant improvements in patients’ quality of life. 3) Human tissues developed through tissue engineering serve as valuable models for understanding the pathophysiology of diseases, enabling the reproduction of human-specific reactions that are beyond the scope of animal experiments. In recent years, the use of “organoids” developed from patient-derived cells has gained prominence. 4) Drug development can become significantly more efficient with the use of artificial tissues that closely mimic human tissues, enabling the evaluation of drug safety and efficacy. These tissues serve as a precise alternative to animal testing, providing more accurate data while mitigating ethical concerns. Notably, liver and kidney model tissues are already being used in drug metabolism studies. 5) It contributes to personalized medicine by using the patient’s own cells to create tissue, thereby avoiding rejection and enabling treatments tailored to individual needs. 6) It drives technological innovation by combining disciplines such as biomaterials, nanotechnology, stem cell biology, and bioprinting. The advancements in technologies and materials are being applied beyond the field of medicine. Finally, it contributes to sustainable medicine by reducing costs associated with organ transplantation and chronic disease treatment, while promoting more efficient use of medical resources. This approach supports natural healing processes and enhances the overall sustainability of healthcare systems. For these reasons, advances in tissue engineering hold the promise to overcome the limitations of conventional medicine, offering innovative treatments and groundbreaking technologies. Future research in this field is expected to bring significant benefits to countless patients.
In particular, combining BMSCs with advanced scaffold materials, such as decellularized scaffolds or novel biodegradable materials enriched with ECM derived from allogeneic or autologous tissues, could enhance therapeutic efficacy. Furthermore, the exploration of BMSC-loaded scaffolds for articular cartilage repair, combined with advancements in magnetic targeting methods, is expected to open new horizons for minimally invasive therapies (Figure 1).
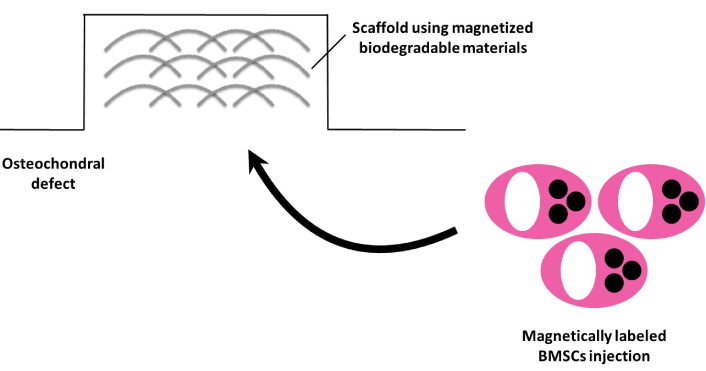
Future directions in magnetic targeting of BMSCs with advanced scaffolding materials for cartilage repair therapy. BMSCs: bone marrow mesenchymal stem cells
The scaffold using magnetized biodegradable materials is arthroscopically placed into the osteochondral defect following the dissection of degenerated cartilage. Subsequently, a suspension of magnetically labeled BMSCs is injected, enabling their accumulation in the affected area.
BMSCs exhibit substantial potential for regenerating bone, cartilage, and muscle tissues due to their multipotency. Their efficacy in muscle, meniscal, and cartilage repair has been well demonstrated. Magnetically labeled BMSCs facilitate minimally invasive and targeted delivery methods, significantly enhancing therapeutic outcomes. Clinical applications, such as intra-articular injections, have shown promising results in cartilage regeneration. Future research should prioritize optimizing cell expansion techniques and improving targeted cellular accumulation. Advancing the understanding of cellular senescence and differentiation will further refine therapeutic strategies. Integration of BMSCs with gene editing technologies, tissue engineering methodologies, and biomaterials offer the potential for more effective and personalized regenerative treatments.
ACL: anterior cruciate ligament
BMSCs: bone marrow mesenchymal stem cells
ECM: extracellular matrix
EMF: external magnetic fields
FGF-2: fibroblast growth factor-2
GFP: green fluorescent protein
HGF: hepatocyte growth factor
MRI: magnetic resonance imaging
mRNA: messenger RNA
MSCs: mesenchymal stem cells
PGE2: prostaglandin E2
SD: Sprague-Dawley
TGF-β: transforming growth factor-beta
The author thanks Editage (www.editage.jp/) for their assistance with English language editing.
SY: Conceptualization, Writing—original draft, Writing—review & editing.
The author declares there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2428
Download: 17
Times Cited: 0
Féaron C. Cassidy ... Cynthia M. Coleman
