Affiliation:
Division of Rheumatology, Allergy and Immunology, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA
ORCID: https://orcid.org/0000-0002-2844-4661
Affiliation:
Division of Rheumatology, Allergy and Immunology, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA
ORCID: https://orcid.org/0000-0002-6390-0941
Affiliation:
Division of Rheumatology, Allergy and Immunology, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA
Email: mkohler@mgh.harvard.edu
ORCID: https://orcid.org/0000-0001-6450-7546
Explor Musculoskeletal Dis. 2025;3:1007106 DOI: https://doi.org/10.37349/emd.2025.1007106
Received: July 01, 2025 Accepted: October 09, 2025 Published: October 22, 2025
Academic Editor: Fernando Pérez-Ruiz, Cruces University Hospital, Spain
The article belongs to the special issue Multifaceted Imaging in Rheumatic and Musculoskeletal Diseases
Immune checkpoint inhibitors (ICIs) have transformed cancer care, but their use is frequently complicated by immune-related adverse events (irAEs), including rheumatic manifestations such as arthritis. Distinguishing between inflammatory and non-inflammatory musculoskeletal symptoms is challenging, yet critical for appropriate management. Musculoskeletal ultrasound (MSKUS) provides unique advantages in this context by enabling the detection of subclinical synovitis, periarticular pathology, and crystal deposition, while also facilitating treatment decisions, including targeted corticosteroid injections. We present four cases that highlight the utility of MSKUS as a frontline tool in the evaluation of musculoskeletal irAEs.
Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of many cancer types, but their use is often complicated by immune-related adverse events (irAEs) [1, 2]. Rheumatic complications are particularly common, occurring in approximately 5–10% of patients. Presentations vary from mild exacerbation of underlying osteoarthritis to florid inflammatory arthritis [3–6].
Musculoskeletal ultrasound (MSKUS) is helpful in the evaluation of many cases of ICI arthritis given its enhanced diagnostic capabilities with the ability to identify clinical and subclinical synovitis by gray scale and power Doppler evaluation, characterize periarticular pathologies (e.g., tendon, soft tissue), visualize crystalline deposition, and define pathology in clinically inaccessible joints such as the shoulder and hip [7, 8]. We have previously published on the utility of MSKUS in distinguishing between rheumatic irAEs [9]. Here, we present several cases that highlight the role of MSKUS in this patient population.
The first case involves a male patient who initially presented at age 73 with new joint symptoms that began approximately 3 weeks after starting treatment for rectal cancer with atezolizumab. He described new pain in his shoulders and right hip as well as aching in the arms and legs. He had a known history of left shoulder osteoarthritis for which he had previously received a corticosteroid injection. On exam, he was noted to have normal shoulder range of motion without evident effusion. No peripheral synovitis was appreciated. Sedimentation rate was mildly elevated at 22 mm/h with normal C-reactive protein. Ultrasound evaluation of the right hip was performed (Figure 1a); he was noted to have advanced degenerative disease without synovitis. Bilateral shoulder and right hip radiographs were obtained, again confirming severe osteoarthritis (Figure 1b).
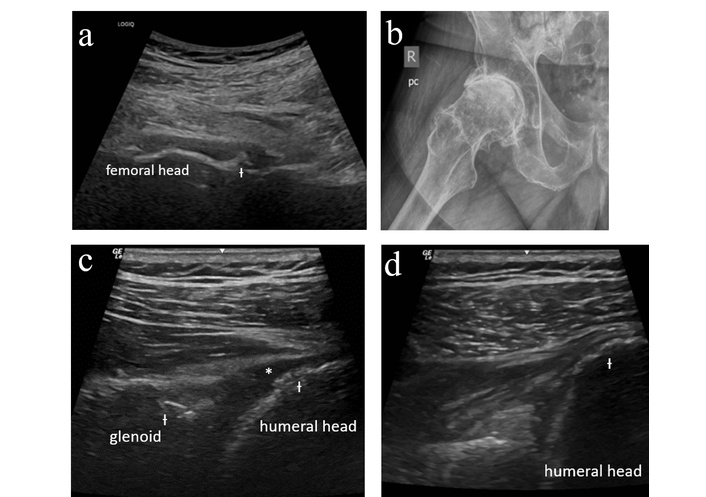
Case 1 ultrasound images. (a) Right hip anterior longitudinal ultrasound demonstrating femoral head flattening and cortical irregularity (Ɨ) along the femoral neck with no synovitis or joint effusion. (b) Right hip radiograph demonstrating severe femoroacetabular osteoarthritis with joint space narrowing, osteophyte formation, subchondral sclerosis, and cyst formation. (c) Left glenohumeral ultrasound (posterior longitudinal with external rotation) demonstrating cortical irregularity (Ɨ) of the humeral head and glenoid with degenerative labral change, minimally compressible joint effusion (*) (not amenable to aspiration) without proliferative synovitis. (d) Right glenohumeral ultrasound (posterior longitudinal with external rotation) demonstrating cortical irregularity (Ɨ) of the humeral head with no synovitis or joint effusion.
An ultrasound-guided right hip steroid injection was performed. He was advised to begin celecoxib and gabapentin with initiation of physical therapy once pain had improved. He later returned for ultrasound-guided bilateral glenohumeral joint injections (Figure 1c–d). At his last follow-up visit, he described significant improvement in pain.
This case demonstrates the utility of MSKUS in excluding inflammatory features and confirming the presence of degenerative change and as such diagnosing “activated osteoarthritis,” which is a condition in which patients develop new or worsening pain in a joint affected by preexisting osteoarthritis following initiation of ICI treatment. Without ultrasound, this patient’s presentation may have been mistaken for a polymyalgia rheumatica-like presentation, resulting in unnecessary exposure to systemic steroids or other immunosuppressive treatment.
Patient 2 is a 53-year-old male who first presented with new joint pain beginning about two weeks after his ninth cycle of pembrolizumab for metastatic melanoma. He reported first developing pain in both knees followed by the onset of pain in his ankles followed by his shoulders, elbows, and hips. He was advised by his oncologist to begin taking ibuprofen, which provided only partial relief. He was referred to rheumatology for further evaluation. At his initial consultation, he described inflammatory symptoms with significant stiffness with inactivity and improvement in pain and stiffness with activity. On exam, he was noted to have tibiotalar fullness without associated tenderness, and no other joints were suspicious for synovitis. Diagnostic ultrasound was performed of the knees and ankles with findings of small knee effusions without synovial hypertrophy, and synovial hypertrophy of both ankles without associated hyperemia (Figure 2a–d). Synovial fluid had 26 white blood cells/μL.
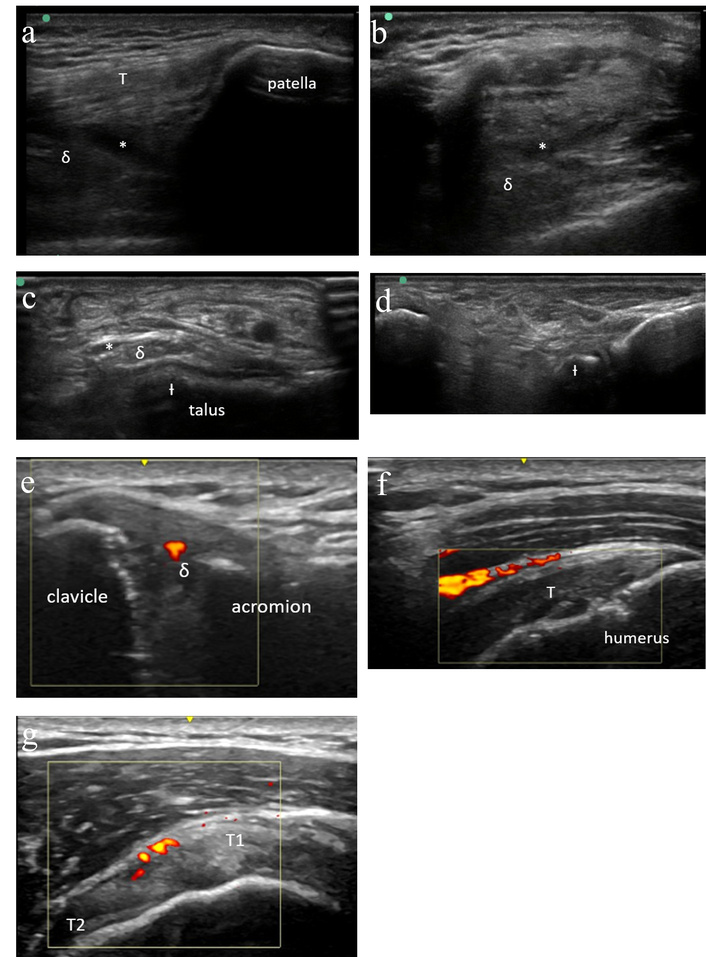
Case 2 ultrasound images. (a) Left knee ultrasound (suprapatellar longitudinal) demonstrating small effusion (*) with minimal synovial hypertrophy (δ) and normal quadriceps tendon (T). (b) Left knee ultrasound (suprapatellar transverse) demonstrating small effusion (*) with minimal synovial hypertrophy (δ). (c) Right ankle (anterior transverse) ultrasound demonstrating grade 2 synovial thickening (δ) and trace effusion (*) as well as moderate cortical irregularity (Ɨ) and scattered calcifications. No tenosynovitis. (d) Right subtalar ultrasound without synovitis. Moderate cortical irregularity (Ɨ) and scattered calcifications noted. (e) Right acromioclavicular joint ultrasound demonstrating grade 2 synovial thickening (δ) of the acromioclavicular joint with positive power Doppler signal. (f) Right shoulder ultrasound demonstrating increased hyperemia at the subdeltoid bursa overlying the supraspinatus tendon (T). (g) Right shoulder ultrasound demonstrating increased hyperemia in the rotator cuff interval between the anterior supraspinatus (T1) and the long head of the biceps tendon (T2).
Given his mixed clinical picture, he was advised to begin a prednisone taper starting at 20 mg daily. Within 24 hours, he noted near-resolution of joint pain, worsening as he tapered. Repeat ultrasound was performed with resolution of previously noted synovial hypertrophy and effusions. He was transitioned to scheduled ibuprofen with ongoing symptom control. A repeat ultrasound then demonstrated only mild re-accumulation of right ankle effusion and synovial fullness but no active synovitis and no recurrent effusions in the knees. He was then advised to begin as-needed ibuprofen, and he resumed pembrolizumab. Unfortunately, his pembrolizumab infusion was followed by worsening pain in his hands, elbows, and shoulders, again improving with reintroduction of prednisone and ibuprofen. Ultrasound was again performed, now demonstrating obvious synovitis involving the right acromioclavicular joint as well as abnormal hyperemia in the rotator cuff interval consistent with tendonitis (Figure 2e–g).
Based on these findings that were consistent with ICI-inflammatory arthritis, he was then started on sulfasalazine as well as increased prednisone dosing. Subsequently, he was able to complete his planned ICI course while slowly tapering off of prednisone. He ultimately tapered off of sulfasalazine the following year without recurrent joint symptoms.
This case illustrates several important concepts in the management of ICI arthritis. First, it demonstrates the utility of MSKUS in distinguishing between activated osteoarthritis and ICI-inflammatory arthritis. This distinction is important as these conditions are treated differently. Additionally, this case demonstrates that patients with ICI-inflammatory arthritis may not initially present with findings such as synovial hyperemia or elevated synovial fluid nucleated cell counts. Finally, this demonstrates the use of MSKUS in evaluating treatment response and its utility in guiding treatment decisions, such as whether to maintain steroid therapy or introduce a disease-modifying antirheumatic drug. While this patient was treated with sulfasalazine with benefit, it is now known that there is a high rate of sulfasalazine intolerance (including hypersensitivity reactions) in ICI patients, and so we avoid this agent when possible [10, 11].
Patient 3 is a 37-year-old female who developed new joint pain after receiving her eighth cycle of pembrolizumab for melanoma. She noted that her pain was most significant in her knees and heels with morning-predominant symptoms. She had been previously diagnosed with ICI colitis for which she had been started on infliximab 5 mg/kg approximately 2 months prior. On exam, she did not have any synovitis, but she was noted to have tenderness localizing to the quadriceps, patellar, and Achilles entheses, and she had postauricular psoriasiform skin changes. MSKUS was performed with demonstration of quadriceps enthesitis with hyperemia, infrapatellar enthesitis with hyperemia, bilateral Achilles tendinitis and retrocalcaneal bursitis with hyperemia, and left plantar fascia thickening (Figure 3a–c). She was also noted to have enthesophytes in multiple locations.
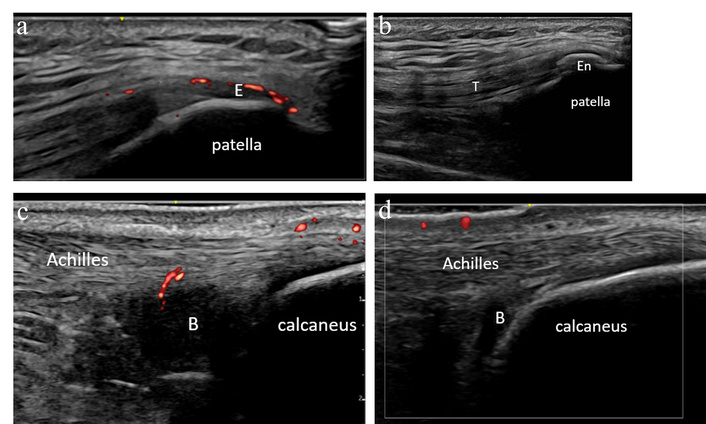
Case 3 ultrasound images. (a) Left knee ultrasound demonstrating a positive power Doppler signal at the quadriceps enthesis (E), consistent with left quadriceps enthesitis with hyperemia. (b) Left knee ultrasound demonstrating a large left patellar enthesophyte (En) near the quadriceps tendon (T) insertion. (c) Right Achilles ultrasound demonstrating mid-body Achilles tendinitis with hyperemia, and large retrocalcaneal bursitis (B). Achilles insertion with normal caliber and normal fibrillar pattern. (d) Right Achilles ultrasound demonstrating improvement from prior evaluation with negative power Doppler, reduced mid-body Achilles tendinitis. Right retrocalcaneal bursa (B) remains enlarged, though less pronounced from prior, now with negative power Doppler.
This patient’s ultrasound evaluation was most consistent with emerging spondyloarthritis. We advised increasing infliximab dosing, which was ultimately effective in controlling her symptoms. A follow-up ultrasound evaluation one month later demonstrated resolution of Achilles tendon hyperemia (Figure 3d).
In this case, MSKUS was helpful in confirming that the patient’s pain was largely due to entheseal inflammation as opposed to synovitis, a distinction that was not discernible by physical exam alone. The presence of active enthesitis on MSKUS as demonstrated by positive color Doppler despite already being on maintenance infliximab was important in guiding the decision to increase infliximab dosing. This case also highlights that patients with comorbid ICI colitis and arthritis often present with a spondyloarthritis pattern of joint and soft tissue involvement.
Patient 4 is a 79-year-old male with a history of melanoma treated with nivolumab, who presented to rheumatology describing worsening bilateral knee pain that began following his first immunotherapy infusion (dual therapy with nivolumab and relatlimab). On exam, he was found to have limited bilateral knee range of motion with mild swelling and small effusions; his exam was otherwise unremarkable. Inflammatory markers had been recently elevated (sedimentation rate 36 mm/h and C-reactive protein 52 mg/L). MSKUS was performed without evidence of synovitis (Figure 4a–b). Both knees were aspirated and injected under ultrasound guidance with a non-inflammatory cell count. A radiograph of the right knee demonstrated moderate medial and patellofemoral compartment osteoarthritis and chondrocalcinosis (Figure 4c).
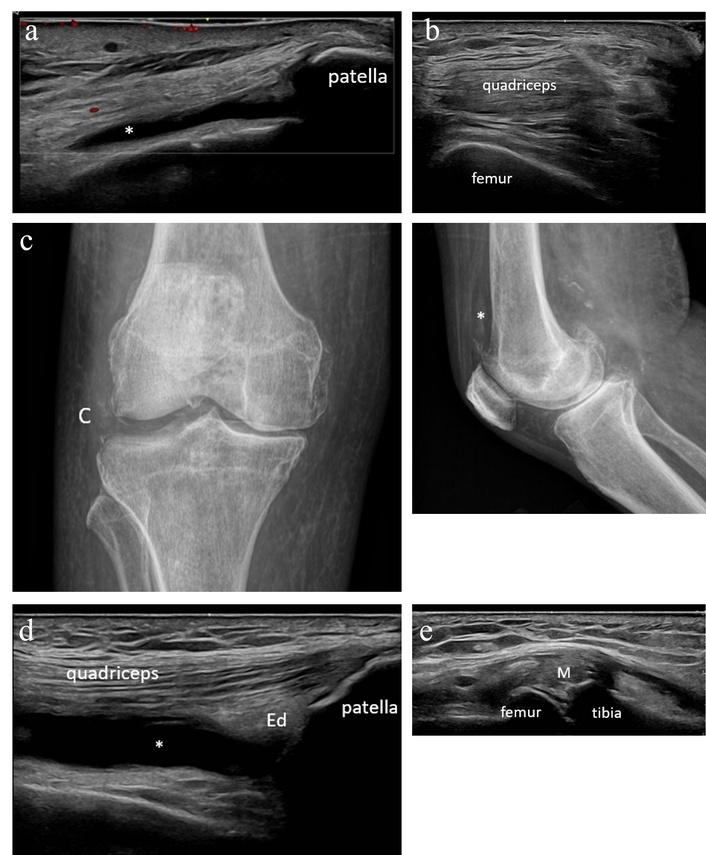
Case 4 ultrasound images. (a) Left knee suprapatellar longitudinal ultrasound with no synovial hypertrophy but a small effusion (*) present in the lateral aspect of the suprapatellar recess. (b) Right knee suprapatellar transverse ultrasound with no synovial hypertrophy or effusion. (c) Radiographs of the right knee demonstrate a small effusion (*), moderate medial and patellofemoral compartment degenerative change, and chondrocalcinosis (C). (d) Right knee suprapatellar longitudinal ultrasound with suprapatellar fat pad edema (Ed) and moderate effusion (*). (e) Right knee medial longitudinal ultrasound with meniscal protrusion (M), osteophytosis, and a few deposits of chondrocalcinosis in fibrocartilage.
Thereafter, the patient had persistent relief in his left knee but recurrence of right knee pain, leading him to again present to the clinic 3 months later. At that time, MSKUS was repeated with demonstration of right knee synovial hypertrophy and hyperemia (Figure 4d–e). The right knee was aspirated and injected with synovial fluid, demonstrating a non-inflammatory cell count but intracellular calcium pyrophosphate crystals. He was diagnosed with pseudogout and started on scheduled colchicine for prophylaxis with benefit.
This case again highlights the ability of MSKUS to detect the presence of inflammatory arthritis even in the setting of a nondiagnostic physical exam and noninflammatory synovial fluid. Without MSKUS, the treating rheumatologist may have easily attributed this patient’s symptoms to activated osteoarthritis and deemed the calcium pyrophosphate crystals as likely consistent with asymptomatic chondrocalcinosis in the presence of noninflammatory synovial fluid.
As demonstrated in each of these cases, MSKUS is helpful in the evaluation of many cases of ICI arthritis. As ICIs can trigger both inflammatory and non-inflammatory musculoskeletal pain, while synovitis and periarticular pathologies are often subclinical, MSKUS provides enhanced diagnostic capability and facilitates timely diagnoses. This point was highlighted by case 4, in which MSKUS demonstrated ICI inflammatory arthritis even in the presence of “non-inflammatory” synovial fluid studies.
Specific MSKUS findings such as enthesitis are helpful in distinguishing between the subsets of patients with ICI inflammatory arthritis with a spondyloarthritis-like presentation or polymyalgia rheumatica-like presentation. As in case 3, these distinguishing features are important in making decisions regarding appropriate steroid-sparing agents. The presence of crystal deposition may also facilitate the diagnosis of an activated crystalline arthritis, as in case 4.
The ability to accurately diagnose pathology in a timely manner is particularly critical in this patient group, as ICI arthritis patients often require arthritis control before resuming ICI treatment, which is lifesaving in many cases. As such, we recommend that MSKUS be integrated into the care of this patient population, particularly in cases in which the presence of inflammatory disease remains uncertain following physical exam and laboratory evaluation.
ICIs: immune checkpoint inhibitors
irAEs: immune-related adverse events
MSKUS: musculoskeletal ultrasound
The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard University, its affiliated academic health care centers, or the National Institutes of Health.
GJC: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. JY: Investigation, Writing—review & editing. MJK: Conceptualization, Investigation, Writing—review & editing, Supervision. All authors read and approved the submitted version.
Dr. Kohler has performed consultancy for Johnson & Johnson (J&J), Novartis, and Pfizer, unrelated to this work. Dr. Challener and Dr. Yinh declare that there are no conflicts of interest.
This study was approved by the Mass General Brigham IRB under protocol #2014P000673. This study complies with the Declaration of Helsinki (2013).
All participants provided their informed consent to be included in the study.
Not applicable.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Dr. Challener is supported by NIH T32 training grant [T32AR007258]. Dr. Kohler has received research support from Setpoint Medical, Sonoma Biotherapeutics, and Johnson & Johnson. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1208
Download: 12
Times Cited: 0
Antje L Greenfield, Riti Kanesa-Thasan
Silvia Magni-Manzoni
Janeth Yinh ... Ali Guermazi
Jürgen Braun, Björn Bühring
