Affiliation:
1Department of Orthopaedics and Traumatology, Medical University of Innsbruck, 6020 Innsbruck, Austria
Affiliation:
1Department of Orthopaedics and Traumatology, Medical University of Innsbruck, 6020 Innsbruck, Austria
ORCID: https://orcid.org/0000-0002-4793-4260
Affiliation:
1Department of Orthopaedics and Traumatology, Medical University of Innsbruck, 6020 Innsbruck, Austria
Affiliation:
2Stabstelle für Patientensicherheit, Qualitäts- und Projektmanagement, Landeskrankenhaus Innsbruck, 6020 Innsbruck, Austria
Affiliation:
3Austria Out-patient service for rheumatology, 6060 Hall, Austria
ORCID: https://orcid.org/0000-0001-9208-7809
Affiliation:
1Department of Orthopaedics and Traumatology, Medical University of Innsbruck, 6020 Innsbruck, Austria
ORCID: https://orcid.org/0000-0001-9439-0051
Affiliation:
1Department of Orthopaedics and Traumatology, Medical University of Innsbruck, 6020 Innsbruck, Austria
ORCID: https://orcid.org/0000-0002-4843-7303
Affiliation:
1Department of Orthopaedics and Traumatology, Medical University of Innsbruck, 6020 Innsbruck, Austria
Email: johannes.pallua@i-med.ac.at
ORCID: https://orcid.org/0000-0003-0203-213X
Explor Musculoskeletal Dis. 2025;3:100794 DOI: https://doi.org/10.37349/emd.2025.100794
Received: February 24, 2025 Accepted: June 05, 2025 Published: June 16, 2025
Academic Editor: Fernando Pérez-Ruiz, Cruces University Hospital, Spain
The article belongs to the special issue Innovation in Orthopedics
Background: The ongoing digital transformation of healthcare has enabled innovative technologies that improve diagnosis, treatment planning, and outcomes. Among these, three-dimensional (3D) printing has gained prominence in surgical disciplines for converting digital imaging data into patient-specific physical models. In orthopedics and traumatology, 3D printing is used to produce anatomical models, surgical guides, and custom implants, thereby enhancing preoperative planning, surgical precision, and interdisciplinary communication. Despite its growing adoption, integrating 3D printing into clinical workflows remains complex and requires stringent quality assurance. Each phase of the production process—from image acquisition and segmentation to material selection and post-processing—affects the safety and performance of the final product. Standardized quality approaches and regulatory frameworks are therefore essential to ensure reproducibility, biocompatibility, and patient safety. This systematic review consolidates current knowledge on quality standards and implementation strategies for 3D printing in orthopedic and traumatological care. It identifies practical pathways for clinical integration while highlighting challenges, opportunities, and areas for future research.
Methods: A systematic literature search was conducted in PubMed, the Cochrane Library, and the Web of Science, following the PRISMA-P (Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols) 2015 checklist and supplemented by manual searches. Reference management was performed using Rayyan QCRI (Qatar Computing Research Institute). Abstracts and full texts were analyzed with Voyant Tools to identify thematic focuses based on collocate analysis.
Results: Nineteen publications met the inclusion criteria. The review highlights a focused but limited body of literature. Key factors influencing product quality include material choice, manufacturing accuracy, and adherence to validated quality control protocols.
Discussion: With increasing digitalization and the integration of artificial intelligence, future quality initiatives will likely emphasize standardized preoperative planning, ethical oversight, and regulatory compliance to support personalized care models in orthopedics and traumatology.
Since 2000, digitalization has integrated into almost all aspects of healthcare, with numerous Industry 4.0-related applications for surgery. Industry 5.0, introduced in 2015, emphasizes the synergy between humans and machines, with artificial intelligence (AI) enabling more personalized and intelligent manufacturing processes, including in healthcare [1, 2]. This development is also reflected in healthcare services. Particularly in surgical specialties, where a person-centered approach is increasingly being pursued, for example, with the personalization of implant production and individualized operation planning [3]. Against this background, additive manufacturing has gained considerable importance in recent decades. This can be attributed to the development of key manufacturing technologies such as stereolithography apparatus (SLA) and fused deposition modeling (FDM), along with the release of new materials and printing techniques [4]. These innovations allow the realization of three-dimensional (3D) physical objects representing externally changed 3D plans. This has revolutionized prototyping and is now established in numerous non-medical fields, such as aviation, industry, automotive, and architecture [5]. Advances in the medical applications of this technology are also promising and are expected to drive further improvements [6, 7]. 3D printing technologies, such as patient-specific implants in maxillofacial surgery and spinal guides for pedicle screw placement, have been successfully implemented in clinical settings [1, 8].
Such applications have already been introduced mainly in orthopedics, spinal surgery, maxillofacial surgery, neurosurgery, and heart surgery [5]. The medical specialty of orthopedic surgery could benefit the most from the advantages of this evolving technology by using custom implants, anatomical training models, and improved preoperative planning tools that enhance surgical accuracy and outcomes [2]. This is particularly relevant in treating complex bone fractures, especially those involving joints. In such cases, 3D-printed anatomical models support training, enhance surgical preparation, and enable the creation of patient-specific implants [9]. Almost all products generated using this method are suitable for the various aspects of this unique field, including preoperative 3D planning of orthopedic interventions, the development of 3D models as patient-centered surgical guides, and customized orthopedic implants as important examples [10]. Physicians often use images from two-dimensional X-rays, computed tomography (CT), or magnetic resonance imaging (MRI) to gain insights into bone pathologies. This requires surgeons to have excellent visualization skills. Introducing 3D representations and combinations of X-rays, CTs, MRIs, and ultrasound images has further improved the visualization of complex pathologies, but visualization alone lacks tactile qualities [5]. Therefore, additive manufacturing further advances the understanding, planning, and execution of reconstructive treatment of complex fractures by precise full-scale extracorporal reproduction of pathological changes, such as 3D-printed pelvic fracture models used for simulation and implant shaping [1, 11]. The virtual models are created by processing digital radiological images of patients using 3D external scanning, computer-aided design (CAD), and reverse engineering techniques. Once the virtual model is made, it can be printed by applying material layer by layer, using different technologies and materials depending on the application [4]. Adding material layer by layer enables the rapid and detailed creation of complex structures that would not be possible with conventional manufacturing techniques. This property and personalized medicine concept has led to its successful application in the medical field [4]. Recent innovations in patient-specific bone prostheses further demonstrate the clinical utility of this approach in complex orthopedic reconstructions [12]. Although trauma stabilization devices are often standardized, 3D printing for preoperative models and guides offers a significant step toward personalized care, especially in anatomically complex regions [1]. It is therefore anticipated that the future of orthopedics and traumatology will be characterized by precision, intelligence, and personalization [13]. However, caution is required as neither the regulations nor the legislation governing the medical use of additive manufacturing is fully adapted to the clinical needs, and there are still numerous legal challenges that require further research and the development of specific medical regulations [4]. Moreover, the current body of research lacks comprehensive insight into how existing quality standards, such as material properties, biocompatibility, mechanical strength, sterilization protocols, and printed product validation, are applied or adapted in clinical orthopedic and traumatological settings. There is also a need to understand better how process reliability, reproducibility, and traceability can be ensured in diverse clinical workflows. These knowledge gaps hinder the safe and standardized implementation of 3D printing in surgical practice and demand focused investigation. Key applications in orthopedics and traumatology include fracture visualization, surgical training, patient-specific cutting guides, and implant customization [14]. Studies report reductions in operative time and improved anatomical fit using 3D-printed guides in joint reconstruction surgeries [1, 6, 15]. Unlike previous reviews that focus on clinical applications or ethical issues alone [1, 2], this is, to our knowledge, the first systematic review addressing quality standards and process implementation of 3D printing specifically in orthopedic and traumatological care.
This review aims to analyze quality approaches and standards associated with using additive manufacturing in the orthopedic and traumatological setting, thus examining whether implementing a 3D printing process is feasible in clinical practice. This systematic review uses an extensive literature search based on existing recommendations. Donabedian’s approach, which categorizes healthcare quality into structural, process, and outcome components [16], serves as a framework for evaluating the integration of 3D printing into clinical practice. This model is further detailed in the methods section.
A systematic literature search was conducted using PubMed, the Cochrane Library, and the Web of Science. The search strategy included the terms: “(orthopedic surgery or orthopedic disease) and (traumatological surgery or trauma or traumatic fracture) and (image processing or designing or computer-aided manufacturing or CAM or computer-aided design or CAD or patient-specific) and (3D printing or three-dimensional printing or additive manufacturing or rapid prototyping or 3D model or model for surgery planning) and (quality or quality indicator) and (process management)”.
Eligible publications were limited to peer-reviewed articles, classic articles, practice guidelines, systematic reviews, and reviews, published between January 1, 2000, and February 2024, in English, German, or Spanish. English was prioritized due to its global relevance in scientific communication, while German and Spanish were included for their regional clinical relevance and the review team’s language proficiency. Non-peer-reviewed articles, case reports, commentaries, and conference abstracts were excluded.
Additional relevant records were identified through manual searches. All references were imported into Endnote X9.1, and duplicates were removed. Following the identification of 18 additional records and the removal of 15 duplicates, 1,353 records were screened. These were imported into Rayyan QCRI (Qatar Computing Research Institute) (https://rayyan.qcri.org), a web-based tool that facilitates blind, independent screening of titles and abstracts, enabling collaborative decision-making and conflict resolution among reviewers [17].
Screening and evaluation followed the PRISMA-P (Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols) 2015 checklist to ensure transparency, replicability, and completeness [18–20]. Titles and abstracts were assessed using the PICO (population, intervention, comparison, outcome) framework, a standard approach in evidence-based medicine. An overview of the search process is provided in Table 1.
Formulation of search terms according to the PICO scheme
| PICO elements | Question | Physician-related | Patient-related |
|---|---|---|---|
| Population | Who is involved or affected? | Physicians and students at all levels of training and experience. | Patients with bone defects require surgical treatment. |
| Intervention | What is being done or implemented? | Studies and use of 3D-printed models for surgical planning and care. | Integration of 3D-printed models into clinical practice. |
| Comparison | What is it being compared to? | Alternative models used for surgical care. | Implementation of alternative models in clinical practice. |
| Outcome | What is the goal or expected change? | Optimized workflow for creating 3D-printed anatomical models in clinical practice. | A process report using graphical modeling language (BPMN) to improve quality. |
3D: three-dimensional; BPMN: business process model and notation; PICO: population, intervention, comparison, outcome
A total of 45 full texts were included for further analysis using Voyant Tools (https://voyant-tools.org/), an open-source text-mining software that enables the visualization and statistical evaluation of textual data [21]. Documents were uploaded from local files, and word frequency analyses and word clouds were generated. For enhanced visualization of high-frequency terms, the Cyrrus tool was additionally employed to display central terms in a consolidated format [21, 22].
To explore deeper linguistic and semantic structures, the following Voyant Tools modules were applied:
Collocates Graph Tool—identifies co-occurrence patterns;
Correlation Tool—performs Pearson correlation of word frequencies;
Mandala Tool—visualizes relationships between terms and documents;
Scatter Plot Tool—applies dimensionality reduction [PCA, t-SNE (t-distributed stochastic neighbor embedding)];
Trend Tool—shows term frequency trajectories across the corpus [21–24].
A relative frequency threshold of 0.0125 was defined for key terms to be included in the qualitative synthesis regarding quality-related themes.
Based on the findings of the literature search on quality approaches and standards in 3D printing for orthopedic and traumatological care, a model of internal and external 3D printing processes was developed using ADONIS (https://www.boc-group.com/de/adonis/). The modeling followed the business process model and notation (BPMN) standard, developed by the Object Management Group and listed under ISO (International Organization for Standardization) 19510 since 2013 [25]. A detailed overview of the underlying process documentation is provided in the supplementary material (Table S1), and the complete visual representation of the workflow is shown in Figure S1 (supplementary material).
BPMN provides a unified graphical language to visualize, document, and analyze complex workflows using standardized symbols and diagrams. This facilitates transparent communication of processes and supports alignment with institutional objectives.
A total of 1,305 articles and 48 book chapters were identified. Records were excluded due to their irrelevance to the predefined PICO questions, while 284 remaining full texts were checked for suitability using the PRISMA-P 2020 checklist. The flowchart of the systematic review is shown in Figure 1.
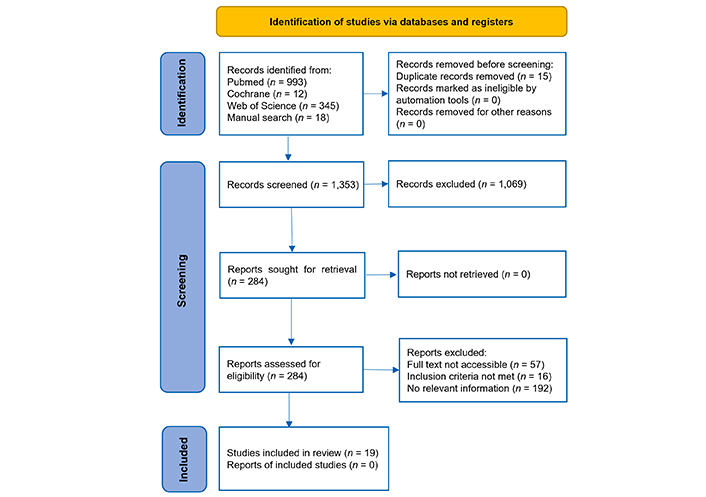
Flow diagram for the PRISMA-P strategy. Source: https://www.prisma-statement.org/. This work is licensed under CC BY 4.0. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/. PRISMA-P: Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols
The analysis of the most frequently used terms in the publications mentioned is shown in Figure 2 using the Cyrrus tool. This analysis makes it clear that the literature search was specifically focused on quality, 3D printing, and indicators.

The Cyrrus plot displays the terms that appear most frequently in the literature search. 3D: three-dimensional
Table 2 shows weak correlations between the word mentions in the publications. According to the search strategy, the strongest correlations were found between words relating to ‘3D’.
List of Pearson correlation coefficients of single words with 3D in the literature search
| Term 1 | Term 2 | Correlation coefficient |
|---|---|---|
| Surgical | 3D | 0.160 |
| Medical | 3D | 0.146 |
| Printing | 3D | 0.126 |
| Models | 3D | 0.122 |
| Studies | 3D | 0.114 |
| Care | 3D | 0.113 |
| Healthcare | 3D | 0.109 |
| Implants | 3D | 0.100 |
When the correlation coefficient approaches 1, it indicates a strong positive correlation between the values. 3D: three-dimensional
The connection between the various terms is visualized in a network graphic depicted in Figure 3 using the Collocate Graph tool. Printing, models, and medical are not interlinked with quality, indicators, and processes. 3D is used in the literature but is not connected with quality, indicators, and process.
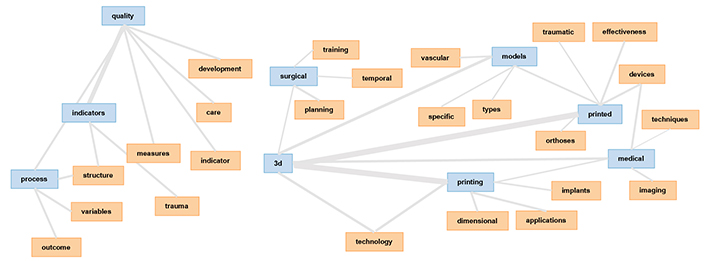
The network graphic generated using the Collocate Graph tool visualizes the connections between various terms. It demonstrates a strong interlinking between “three-dimensional”, “printing”/“print(ed)”, “medical” and “models”. However, terms such as “quality”, “indicators”, and “processes” are not interlinked with “printing”, “models”, and “medical”. This analysis suggests a strong connection between using 3D printing models and technologies in the medical field but highlights a lack of association with quality and quality indicators. 3D: three-dimensional
A strong interlinkage between the terms “3D”, “printing”/“printed”, “medical”, and “models” were identified. Based on this analysis, it can be assumed that there is a strong connection in the selected articles regarding the use of 3D printing models and technologies in the medical field, but that a link to quality and quality indicators and their measurement was not identified. The links are shown in Figure 3.
After examining 1,353 citations according to the selection criteria, 19 publications (consisting of 7 articles and 12 reviews) were systematically analyzed. These publications showed a relative frequency of more than 0.0125 in the trend analysis (Figure 4).
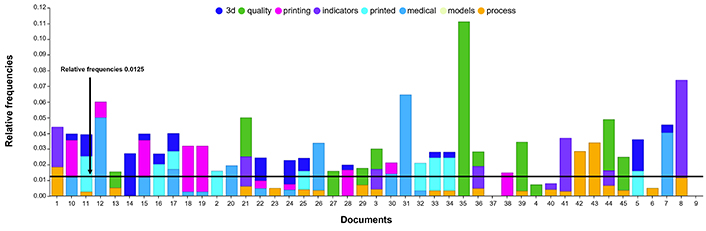
Trend analysis of 45 full texts. Among these, 16 articles are related to 3D printing, 5 discuss the 3D printing process, 7 focus on quality indicators, 2 specifically address quality indicators in the medical field, and 2 are centered on medical 3D printing models. The limit at 0.0125 indicates the relevant relative frequency. 3D: three-dimensional
The analysis of trends in content-related aspects identified five key issues for further content analysis. These issues are outlined in the following paragraphs.
The selection of materials in conjunction with 3D printing technologies critically influences the production of anatomical models, medical devices, and prostheses, particularly regarding accuracy, surface fidelity, flexibility, biocompatibility, and cost efficiency [26]. A wide range of materials—including thermoplastics, thermosets, ceramics, metals, liquids, filaments, and powders—is available, with material choice significantly impacting both production costs and printer requirements. For instance, resin-based models necessitate costly photopolymerization printers, whereas FDM printers can utilize less expensive thermoplastics, sometimes enhanced with ceramic fillers.
Dorweiler et al. [27] demonstrated that both FDM and PolyJet technologies produce vascular models with accuracy within 1 mm, with FDM favored due to lower costs. Shim [28] identified metal 3D printing as particularly advantageous for neurosurgical bone defect reconstruction, facilitating the fabrication of prostheses precisely adapted to patient anatomy and surgical specifications.
Biodegradable polymers such as polylactic acid (PLA), polycaprolactone (PCL), and PCL/β-tricalcium phosphate (β-TCP) composites have shown promise for implantable medical devices, particularly in craniofacial reconstruction and regeneration. Their capacity for in vivo degradation reduces infection risk and eliminates the need for secondary removal surgeries, thereby decreasing patient morbidity [27, 29]. When flexible materials are employed, the incorporation of support structures is essential to mitigate deformation. However, in applications such as ankle-foot orthoses (AFOs), consensus on optimal materials and printing methods remains elusive, with ongoing efforts aimed at improving patient mobility, comfort, and device durability [29].
3D printers are typically categorized by base material compatibility and manufacturing technique. FDM combined with polylactide offers a rapid and cost-effective solution for anatomical modeling. For applications demanding finer resolution, such as surgical guides, SLA with biocompatible photosensitive resins is preferred. Custom implants and reusable surgical instruments are commonly fabricated from steel or titanium alloys, notably Ti6Al4V (Ti64) [30]. While FDM is the most economical and widespread technique, selective laser sintering (SLS) and selective laser melting (SLM) are increasingly employed to produce finished medical-grade implants, prostheses, and orthoses due to their superior mechanical properties and reliability [11].
Standardized processes, effective communication, close collaboration, and documented procedures are critical for quality assurance in additively manufactured medical devices [31]. The integration of medical-technical interaction is essential during the development and regulatory approval of such products. For the precise manufacturing of 3D-printed implants or devices intended for invasive procedures, the purity and performance of raw materials are paramount. Predominantly, metal powders such as medical-grade titanium alloys, tantalum, and nickel-titanium alloys are used. Due to the complex structure of human bone tissue, these implants must meet biomechanical requirements including toughness, strength, biocompatibility, and wear resistance. 3D printing technology enables the production of implants that fulfill these criteria and promote osteointegration.
The selection and assessment of raw materials are vital to ensure a homogeneous and traceable manufacturing substrate. Biocompatible materials such as PCL and hydroxyapatite are recommended for improving print quality [27]. Material quality is maintained through standardized testing procedures, including particle size and polydispersity control. Recycling of printing substrates between print jobs may enhance manufacturing efficiency but poses risks of contamination and degradation [32].
Medical 3D printers must comply with more stringent requirements than non-medical devices, particularly regarding reliability, accuracy, and reproducibility. Strict adherence to manufacturer maintenance protocols is essential, with additional testing recommended to minimize operational failures and ensure system dependability. Routine maintenance is performed after each print, with weekly and monthly checks conducted by local personnel, and preventive maintenance carried out every 3,500 printing hours by manufacturer technicians. Maintenance tasks include the replacement of worn parts, factory calibrations, and functional testing to ensure optimal performance [33].
Nguyen et al. [34] highlight the importance of close collaboration with 3D printing software providers to optimize device operation and maintenance, noting that model accuracy can be significantly influenced by printer upkeep and parameter settings.
The 3D printing workflow comprises five phases: image acquisition, segmentation and processing, production, post-processing, and validation. Each phase presents potential error sources that can impact the accuracy of the final model. CT and MRI are the primary sources of volumetric data for anatomical reconstructions [35]. The precision of medical imaging directly influences the reliability of 3D-printed models, while segmentation and data processing significantly affect model fidelity. Experienced personnel employing validated software are crucial for quality assurance, with manual segmentation generally preferred over automated methods due to higher accuracy [33, 35].
Mesh refinement tools such as MeshLab, Materialise Mimics, and 3D Slicer optimize models by correcting surface errors and smoothing interpolation artifacts resulting from imaging slice thickness. Verification involves overlaying STL (stereolithography) files onto original images, with radiological review ensuring accuracy [33]. Printing quality depends on machine parameters, material properties, and environmental factors. Regular printer maintenance—including UV (ultraviolet) lamp calibration and print head cleaning—combined with standardized protocols, ensures consistency [32, 33].
Post-processing encompasses cleaning, validation, and mechanical testing. Validation methods typically include visual inspection, dimensional measurements (e.g., calipers), and imaging comparisons such as CT or laser scanning [34]. For implantable devices, sterility, biocompatibility, and structural integrity assessments are essential. Cleaning techniques like ultrasonic washing and chemical treatments are validated to prevent alterations of device properties [27]. Quality control is further supported by phantoms, which provide reproducible references for dimensional accuracy and model fidelity over time [33].
Each 3D-printed anatomical model was identified and documented by assigning a unique identifier to each model for traceability purposes. This could be done directly via patient information such as surname or clinic number or by generating a unique identifier linked to the information. Any patient information was protected under the Health Insurance Portability and Accountability Act (HIPAA), similar to other areas of medical practice. Additional labeling, such as left or right or mirror image anatomy, was required and printed on the anatomical models using a CAD program. Long-term storage: the segmentation, STL files, and photos of each model were stored in a dedicated repository for long-term storage. In addition, an image of each model was archived in the patient’s medical record. The STL files can be used at any time to reprint the model without re-segmenting the images or redesigning the parts. All files were stored on secure, high-capacity servers with daily backups [34]. Beyond data protection, ethical considerations such as informed consent for the use of imaging data in model generation and reuse are essential, particularly when used for training or research purposes [2]. Institutions are encouraged to establish governance frameworks that uphold transparency and patient autonomy in the handling of 3D-printed anatomical data [36].
Professionals working in 3D printing were required to maintain a high level of training and competence, and the use of FDA (Food and Drug Administration)-approved software and hardware was recommended [36]. Similarly, Jin et al. [31] emphasized that training and competence were essential for 3D printing professionals. It was recommended that FDA-approved software and hardware be used when necessary to ensure safety and efficacy. Education and training of healthcare 3D printing talent was crucial in meeting the increasing demand for expertise. Universities and vocational schools should establish relevant degree programs to cultivate workforce and research talents. Large manufacturing or technology companies could invest equipment resources in schools to compensate for the lack of training in this field. Another strategy was to train existing talents in related fields, such as doctors in design engineering or biomedical engineers in anatomy and surgical procedures, to use them as coordinators in the development process of 3D-printed medical products. Jin et al. [31] called for more policy measures to ensure the training, employment, and skills enhancement of relevant talents and to promote the development of this field.
Medical 3D-printed products are regulated by various authorities worldwide, including the FDA (USA), EMA (European Medicines Agency) [EU (European Union)], NMPA (National Medical Products Administration) (China), and MFDS (Ministry of Food and Drug Safety) (South Korea). These bodies define which products are medical devices, their approval processes, and the requirements to enter the market.
In the USA, the FDA has advanced regulations since a 2014 workshop titled ‘Additive Manufacturing of Medical Devices’, addressing quality and safety aspects of 3D printing, including bioprinting and metal implants. The 2016 guidance ‘Technical Considerations for Additively Manufactured Medical Devices’ outlines the technical factors throughout the additive manufacturing process, from design to post-processing and testing. The subsequent document ‘Additively Manufactured Medical Devices—The FDA Perspective’ highlights ongoing quality and safety challenges [36]. Most 3D-printed implants fall under Class III, requiring compliance with strict regulatory standards regardless of manufacturing technique [37].
The EU regulates 3D-printed medical devices under Regulation (EU) 2017/745, demanding stringent controls across design, production, marketing, and distribution to ensure patient safety [4]. The 2009 Guideline for Manufacturers of Customized Medical Devices describes regulatory steps for custom devices, while the IMDRF’s (International Medical Device Regulators Forum’s) 2018 Guideline on Terminology and Classification for Personalized Medical Devices fosters international harmonization.
In China, 3D-printed medical devices have been part of national development strategies since 2015. The NMPA issued technical guidelines for the evaluation and registration of customized devices and adopted the Regulation on the Management of Customized Medical Devices in 2019. These regulations cover design, processing, monitoring, and record-keeping, introducing a special regulatory path for 3D printing applications in rare diseases. Additionally, standards focus on risk identification and management over the device lifecycle. Industry associations like the Additive Manufacturing Medical Devices Committee (AMMDC) play a crucial role in developing group standards [31].
South Korea’s MFDS enforces rigorous requirements for 3D-printed medical devices, covering orthopedics, dentistry, and bioprinting. These devices must meet the same safety and performance standards as traditional devices, and manufacturers are required to implement ISO 13485-compliant quality management systems. Recent MFDS guidelines also address good manufacturing practices specific to 3D printing [28].
Regarding device classification, Class I devices—such as anatomical models used for visualization and surgical planning—are considered low risk. Class II devices include digitally enhanced models used for simple surgical planning, often approved via premarket notification. Class III devices, which include complex surgical guides and implants produced via detailed digital planning, are subject to the most stringent controls. The Humanitarian Use Device program offers an alternative approval pathway for devices intended for rare conditions [36].
FDA guidance also includes device surveillance programs and a pre-submission process, although regulatory pathways for 3D printing remain complex and challenging to navigate [32].
Internationally, ISO 13485 establishes quality management system standards essential for manufacturers to ensure safety and regulatory compliance [34]. Complementary standards, such as ISO 10993, assess the biocompatibility of materials used in medical devices and are adopted for Class III device approvals [32].
The ISO/IEC JTC 1 technical committee develops IT standards for 3D printing and scanning, with specific working groups focusing on medical applications. Coordination with ISO technical committees on surgical implants ensures comprehensive coverage of additive manufacturing standards from image processing to implant safety [28].
Prior to formal 2016 guidelines, 3D-printed medical devices in the USA were regulated mainly through premarket notification [510(k)] and new drug application (NDA) processes [31].
Patient data protection is ensured under regulations such as HIPAA, consistent with standards across medical practices [33].
The comprehensive review of quality approaches and standards for 3D printing in orthopedics and traumatology confirms the transformative potential of the technology, while simultaneously revealing several practical implementation barriers.
Our findings verify the broad application of 3D printing in orthopedics and related surgical fields, including cardiovascular and plastic surgery. Benefits range from improved anatomical visualization and surgical planning to the development of patient-specific guides and implants [28, 38–44]. These advantages are well-documented and align with emerging trends in personalized medicine [4, 5]. However, their routine clinical adoption remains limited [4, 26].
Despite its promise of enhancing surgical precision and training, 3D printing faces several barriers hindering widespread clinical integration:
High initial and operating costs: The expense of high-quality printers and biocompatible materials remains a significant obstacle [26, 28].
Regulatory ambiguity: Diverse and often nonspecific regulatory frameworks impede standardization and approval processes [31, 36].
Technical complexity: Critical steps such as image segmentation and STL conversion require skilled personnel and stringent protocols to maintain print accuracy [33, 35].
Insufficient interdisciplinary training: The lack of formal training programs for clinicians and engineers results in workflow inconsistencies and variable quality control [31, 36].
Lack of unified standards: Although some institutions have internal guidelines, no comprehensive consensus on quality assurance metrics and validation exists [32, 34].
Explicitly outlining these barriers clarifies the previously mentioned “challenges and gaps in practical implementation” and addresses the reviewer’s concerns.
Correlation analyses show a strong link between 3D printing and medical applications, yet reveal a disconnect with quality standard adherence. This highlights the urgent need for research bridging technical innovation and clinical quality assurance [27]. Moreover, the sparse reference to quality indicators in the literature points to a deficiency in process-oriented evaluation metrics [21, 22].
To sustainably implement 3D printing in clinical settings, hospitals should:
Establish clear standard operating procedures (SOPs) for image acquisition, segmentation, printing, and post-processing [32, 33].
Adopt international quality management frameworks such as ISO 13485 [34, 36].
Collaborate with regulatory agencies to develop flexible approval pathways for personalized devices [36, 37].
To further advance the field, future research should explore the integration of AI into key stages of the 3D printing workflow. AI-based algorithms offer substantial potential in automating segmentation, optimizing print parameters, and predicting material performance, thereby enhancing process efficiency and reproducibility [28, 45]. In addition, the application of biological 3D printing in orthopedics—particularly for cartilage regeneration, bone scaffolding, and meniscal repair—has shown promise in preclinical studies and may play a pivotal role in the development of implantable, patient-specific tissue constructs [12, 13]. Moreover, to address the evolving complexity of clinical and regulatory environments, innovative quality assessment frameworks are needed. We propose the future development of hybrid quality metrics that combine mechanical testing, biocompatibility profiling, and AI-driven dimensional accuracy validation tools. These could support real-time quality control and regulatory alignment, thereby reinforcing safety, consistency, and clinical translation [31, 33, 36].
This review is based on literature analysis without direct input from manufacturers or regulators, which may underrepresent real-world implementation strategies. Furthermore, the focus on English, German, and Spanish publications might exclude relevant findings in other languages.
3D printing demonstrates significant potential to advance orthopedic and traumatological surgery by enabling personalized and precise interventions. However, realizing this potential requires overcoming systemic challenges, including regulatory ambiguity, insufficient interdisciplinary training, and the absence of standardized quality assurance frameworks. Addressing these critical gaps is imperative to fully integrate 3D printing into routine clinical practice and to unlock the benefits of this rapidly evolving technology for improved patient outcomes.
3D: three-dimensional
AI: artificial intelligence
BPMN: business process model and notation
CAD: computer-aided design
CT: computed tomography
EU: European Union
FDA: Food and Drug Administration
FDM: fused deposition modeling
HIPAA: Health Insurance Portability and Accountability Act
ISO: International Organization for Standardization
MFDS: Ministry of Food and Drug Safety
MRI: magnetic resonance imaging
NMPA: National Medical Products Administration
PCL: polycaprolactone
PICO: population, intervention, comparison, outcome
PRISMA-P: Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols
SLA: stereolithography apparatus
STL: stereolithography
t-SNE: t-distributed stochastic neighbor embedding
The supplementary Table and Figure for this article are available at: https://www.explorationpub.com/uploads/Article/file/100794_sup_1.pdf.
TN: Methodology, Investigation, Data curation, Formal analysis, Visualization, Writing—original draft, Writing—review & editing. RL: Investigation, Writing—review & editing. LK: Data curation, Software, Formal analysis, Visualization, Writing—review & editing. SP: Validation, Software, Visualization, Writing—review & editing. MS: Supervision, Writing—review & editing. DP: Methodology, Supervision, Writing—review & editing. RA: Supervision, Project administration, Writing—review & editing. JDP: Conceptualization, Methodology, Project administration, Supervision, Writing—original draft, Writing—review & editing.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
All data generated or analyzed during this systematic review are included in this article and the supplementary files, and no new raw data were generated in this study.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
