Affiliation:
Sand Consulting, Poway, CA 92064, USA
Email: tsand@biotechexpert.com
ORCID: https://orcid.org/0000-0002-6197-965X
Explor Musculoskeletal Dis. 2025;3:100795 DOI: https://doi.org/10.37349/emd.2025.100795
Received: March 31, 2025 Accepted: June 12, 2025 Published: June 25, 2025
Academic Editor: Philippe Hernigou, University Paris, France
The article belongs to the special issue Innovation in Orthopedics
Commercialized non-autologous biologics are produced from a variety of human tissues and are intended to treat a wide range of musculoskeletal pathologies. This survey focuses on non-autologous biologic products that are delivered via the topical or percutaneous (i.e., injected) routes. The regulatory framework established in the USA will be reviewed, including an assessment of specific categories of non-autologous biologics with their intended uses, since regulatory compliance of a specific composition or physical form of a non-autologous biologic is tightly linked to its advertised use. Guidance is provided on how to manage emerging products whose regulatory status might be unclear. Clinical safety and efficacy for non-autologous biologics for wound and burn care, including minimally processed placental products in sheet form as well as bio-engineered viable cell composite products, are well established, although efficacy tends to be wound type-specific. Micronized placental tissue products have been investigated in treating osteoarthritis of the knee and hip, and for plantar fasciitis, but require large-scale clinical studies and remain to be approved by the United States Food and Drug Administration (USFDA). Several emerging types (secretomes, exosomes) of non-autologous biologics are well documented in pre-clinical studies, but human studies are lacking. There are no Phase 3 studies reported on a secretome-based product, while there is just one Phase 3 clinical trial on-going for a bone marrow progenitor cell derived exosome product that is being used to treat acute respiratory distress syndrome. There has been substantial progress in the commercialization of exosome-based products, with studies in treating musculoskeletal pathologies a priority. Progress has been made in assessing the treatment of osteoarthritic knees and discogenic low back pain with cultured progenitor cells. However, utility and safety of these investigational products remains to be determined.
Therapeutic agents for treating musculoskeletal (MSK) pathologies via surface or percutaneous delivery have expanded greatly in the past decade. Interest in the use of donor-derived biological therapies is driven in part by the serious societal impact due to pain and disability associated with MSK pathologies. For example, the percentages of MSK pathologies toward the total disability-adjusted life years (DALY) index for 2019 were reported to be: lower back pain—42.44%; neck pain—14.71%; osteoarthritis (OA)—12.63%; rheumatoid arthritis—2.17%; and gout—1.12% [1]. Clearly, there is a pressing need for expanded therapeutic options that might mitigate the pain and disability associated with MSK pathologies like neck and back pain. This report will focus on products and future therapeutic product opportunities that are obtained directly from donor-derived tissue (e.g., membranes from the placenta), as well as products that require additional manufacturing (e.g., micronization of placental tissues). This review will focus on those treatments that are performed at point-of-care and are delivered either by topical application (e.g., wound care) or via percutaneous injection for MSK pathologies. However, the survey will not include treatments that involve surgical repair with donor-derived tissues, such as ligaments, tendons and bone (or bone void fillers), nor treatment of hematological cancers with donor cord blood or bone marrow units.
The purpose of this survey is to provide an overview of non-autologous biological (NAB) products currently available in the USA, as well as to review the regulatory framework for NAB products in the USA with guidance on how to practice medicine with the plethora of products. Where available, clinical data on the safety and efficacy of NABs will be reviewed, along with highlighting emerging research on several future potential NABs. Examples of commonly available donor-derived tissues used in treating various human pathologies are shown in Figure 1.
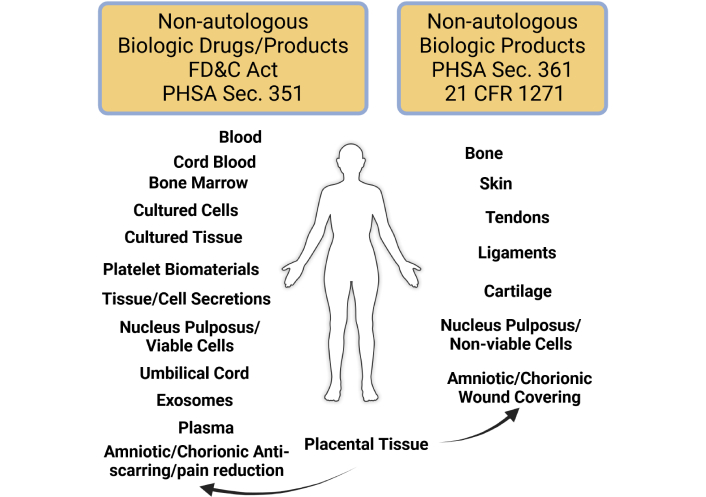
Regulatory classification of non-autologous biologics in the USA. Sources of human tissue-derived products and their regulatory path are described: drugs and biologic products regulated as a Section 351 product require a pre-market approval (PMA) process and a Biologics License prior to sale in the USA. Human tissue-derived products regulated as a Section 361 product are controlled by 21 CFR 1271, but don’t require a PMA prior to sale. FD&C Act: Food, Drug and Cosmetic Act; PHSA: Public Health Service Act; CFR: Code of Federal Regulations. Created in BioRender. Sand, T. (2025) https://BioRender.com/0prp0y3
The regulatory approach for NAB products as established by the United States Food and Drug Administration (USFDA) is based on two laws:
An important regulation was published by the USFDA in January 2001 to deal directly with donor-derived tissues as codified in 21 CFR (Code of Federal Regulations) 1271 Human Cells, Tissues and Cellular and Tissued-Based Products (HCT/Ps) [4]. The HCT/P regulation provides a framework for manufacturers of potential NAB products for navigating the pre-marketing steps required for their product, while also establishing the manufacturing requirements needed to ensure that any product sold in the USA has met appropriate standards (e.g., donor eligibility, good manufacturing practices, etc.) and that the product is safe for use in patients. There are two categories of NAB products identified by the following sections of the PHSA [3]:
Section 351 […No person shall introduce or deliver for introduction into interstate commerce any biological product unless—(A) a Biologics License…is in effect for the biological product…].
Section 361 (…to prevent the introduction, transmission, or spread of communicable diseases).
The key difference between these two sections of the PHSA is that products considered to be biological products requiring a Biologics License (Section 351 category) need to undergo pre-marketing clinical evaluation of safety and efficacy in the form of an Investigational New Drug (IND) or Investigational Device Exemption (IDE) and the materials need to be approved by the USFDA in a pre-market approval (PMA) process similar to that used with small molecule drugs. On the other hand, if the NAB product is determined to be regulated solely as a Section 361 category product, the manufacturer needs to meet the requirements established in 21 CFR 1271, but isn’t required to perform IND/IDE clinical studies prior to marketing and sale of the product. Some human-derived tissues are not considered to be HCT/Ps by definition, including bone marrow, whole blood and blood-derived products (e.g., enriched platelet products, etc.). However, NABs made from these source tissues might be regulated by the FD&C Act and/or Section 351 of the PHSA.
The explicit requirements for an HCT/P to be considered a Section 361 product are described in 21 CFR 1271.10(a). Frequently, manufacturers of NAB products will “self-assess” their product against those requirements to establish the regulatory status of their product vis-à-vis Section 351/361 categorization. It should come as no surprise that the most frequent choice made by most manufacturers is for a Section 361 designation. Where this becomes an issue for the medical provider is in relying on regulatory advice from sales representatives or distributors of the self-assessed NAB products, since incorrect advice means that the medical provider might be treating patients with an experimental product. Treating patients with experimental products outside the safe harbor of a clinical study is not allowed by USA-based state medical boards, so the provider’s medical license might be put at risk, and could impact the provider’s medical malpractice insurance policy. Thus, it is important for a medical professional to be provided by a manufacturer’s representative or a distributor with a copy of the written response from the USFDA to the manufacturer’s Request for Designation (RFD) that would establish how the USFDA views the proposed NAB product. Some companies will offer up a “legal” opinion supporting a Section 361 designation, but if a company didn’t submit a RFD to the USFDA it means that the company can’t provide an unbiased assessment of regulatory status—a situation that could result in a physician treating patients with an experimental product.
One of the first NAB products was obtained from placental tissue for use in skin grafting more than 110 years ago [5], and use of placental-derived tissues remains a prominent therapeutic option in wound care and burns. However, a wide variety of human tissues are used as source tissues for creating NABs with a wide range of indications for use. In the current regulatory framework, the indication for use of a NAB product is inextricably tied to its regulatory status with respect to Section 351 or 361 categorization. As specified in 21 CFR 1271.10(a), a product considered to be an HCT/P must be obtained with minimal manipulation [1271.10(a)(i)] and meet a standard of homologous use [1271.10(a)(ii)] when compared to the in-situ tissue source. For example, amnionic or chorionic membranes from a donor placenta can be processed to make a sheet-type product for use in treating wounds or burns. This combination of product and use is considered to be a Section 361 category HCT/P, because the source tissue acts as a barrier in vivo, and the product is used as a barrier to protect against infection, which fulfills the homologous use criterion. The processing of the source tissue involves separating the placental membranes, cleansing, sizing, packaging and sterilizing the layers to create the wound care sheet-form product, which meets the minimal manipulation criterion. However, if a manufacturer of placental-derived sheet-form products advertised its product for pain relief or scar reduction, the USFDA would consider this as a non-homologous use, since the in vivo use of placental tissue doesn’t include scar reduction or pain relief. Furthermore, if the manufacturer micronized the sheets of placental tissue in order to make an injectable product, micronization of membranes is above the minimal manipulation standard, while also not meeting the homologous use criterion because micronized tissue flecks can’t act as a barrier. Some NABs are excluded by definition from being governed by 21 CFR 1271, including “secretions” like acellular amniotic fluid. However, NABs derived from human tissues that aren’t HCT/Ps might be classified as biological products and regulated by provisions of the FD&C Act and Section 351.
An assessment of a number of NABs and HCT/Ps with specific indications for use with respect to the minimal manipulation standard [1271.10(a)(i)] is listed in Table 1, along with their categorization as Section 351 or 361 products. The application of the homologous use standard [1271.10(a)(ii)] to a variety of NABs and HCT/Ps and indications for use is shown in Table 2, along with their categorization as Section 351 or 361 products. There are several NABs listed in Table 2 that have recently gained attention in the regenerative medicine community:
“Young” plasma is obtained from donors 18 years old or somewhat older, and stored cryopreserved until a “prescription” is received for a volume of “young” plasma.
The recipient usually is older, but in otherwise reasonable health.
Studies are on-going to assess the impact of transfusing “young” plasma on a recipient’s quality of life (QoL) or inflammatory status (so-called inflammaging) [6].
Secretions (in the form of biomolecules) of viable cells present in a living tissue (e.g., placenta) cultured in a bioreactor have been recovered, concentrated, packaged and sold for treating MSK pathologies.
The term “secreted biomolecules” doesn’t appear in 21 CFR 1271, but it clearly is an HCT/P, since the source tissue is human and requires additional processing steps to obtain the secreted biomolecules.
Extracellular vesicles (EVs) are particles released continuously by cells, and exosomes are the smallest type of EVs released by cells in the body [7].
Exosomes can be recovered from cord blood, whole blood and cells cultured in tissue culture containers (e.g., bioreactors).
EV products are considered to be Section 351 products requiring a Biologics License based on the IND pathway to demonstrate safety and efficacy prior to being commercialized.
Acellular amniotic fluid doesn’t contain viable cells and is considered to be a “secretion” by the USFDA.
Secretions are excluded by definition from being an HCT/P [1271.3(d)(3)] [4].
These human tissue-derived secretions are considered a Section 351 product requiring a Biologics License based on the IND pathway to demonstrate safety and efficacy prior to commercialization.
Influence of processing commercial-use HCT/Ps and NABs in meeting the minimal manipulation standard [1271.10(a)(1)] [4]
| Tissue | Property/Function | Processing | Minimally manipulated |
|---|---|---|---|
| Placental tissues | Physical integrity, tensile strength, elasticity; serves as a barrier | Sizing as sheets, preserving | Yes |
| Grinding (micronizing)-packaged as particles | No | ||
| Removing chorion, preserving | Yes | ||
| Fascia lata | Strength, flexibility; cover muscle, aid in movement | Grind into particles | No |
| Preserved as sheets | Yes | ||
| Skin | Flexible, protective covering; water-resistant epidermis; strong connective tissue of the dermis | Sheets of skin are meshed and cryopreserved | Yes |
| Epidermis is removed, dermis is freeze-dried | Yes | ||
| Epidermis is removed, dermis is ground into particles | No | ||
| Adipose tissue | Bulky tissue (adipocytes with lipid content); cushioning and supporting | Decellularize the tissue, leaving the extracellular matrix | No |
| Digest the adipose with enzymes, leaving the stromal vascular fraction (SVF) | No | ||
| Use mechanical means to release and recover the cells | No | ||
| Cartilage | Firmness, smoothness, resistance to deformation; provides load bearing, reduced friction movements | Homogenize cartilage to yield a slurry | No |
| Ligament | Tensile strength; stability and aids in movement | Disaggregate the collagen fibers | No |
| Bone marrow | Ability to differentiate into lymphoid and myeloid cells and self-renew | Collect, store and distribute donor bone marrow units | Yes |
| Peripheral blood (mobilization protocol) | Ability to differentiate into lymphoid and myeloid cells and self-renew | Perform apheresis, concentrate the hematopoietic stem/progenitor cells | Yes |
| Peripheral blood (mobilized), cord blood, bone marrow | Ability to differentiate into lymphoid and myeloid cells and self-renew | Obtain the hematopoietic stem/progenitor cells for culturing and differentiation into terminal (adult) cells | No |
| Cord blood | Ability to differentiate into lymphoid and myeloid cells and self-renew | Collect, store and distribute cord blood units | Yes |
| Placental/Umbilical cord tissue | Structural tissues with stem/progenitor cells | Culturing of selected cells under conditions that maintain their “stemness” | No |
HCT/Ps: Human Cells, Tissues and Cellular and Tissued-Based Products; NAB: non-autologous biological
Homologous use status of NABs, tissues and HCT/Ps with indications for use [1271.10(a)(2)] [4]
| Tissue source/Type | Delivery mode | Indication or treatment site | Regulatory status | Comment |
|---|---|---|---|---|
| Bone marrow, cord blood, mobilized peripheral blood | Infusion | Repair of defective hematopoietic system (acquired, inherited, ablative) | 361 | Homologous use |
| Bone marrow, cord blood, mobilized peripheral blood | Infusion | Treat multiple MSK pathologies | 351 | Non-homologous use |
| “Young” plasma | Infusion | Multiple pathologies targeted | 351 | Plasma isn’t covered by 1271; regulated as a drug under the FD&C Act |
| Biomolecule secretion from whole tissues (in vitro tissue culture) | Implanted; infusion | MSK pathologies | 351 | Non-homologous use |
| Cells (in vitro tissue culture) | Implanted; infusion | MSK pathologies | 351 | Non-homologous use |
| Extracellular vesicles (exosomes) from in vitro tissue culture | Implanted; infusion | MSK pathologies | 351 | Non-homologous use |
| Amniotic fluid—no cells | Implanted | MSK pathologies | 351 | Non-cellular amniotic fluid isn’t an HCT/P; regulated as a drug under the FD&C Act |
| Amniotic fluid—viable cells | Implanted | MSK pathologies | 351 | Non-homologous use |
| Amniotic membrane | Implanted | Bone tissue replacement | 351 | Non-homologous use |
| Amniotic membrane | Applied | Wound healing | 361 | Homologous use |
| Amniotic membrane | Applied | Reduced scarring, mitigating pain | 351 | Non-homologous use |
| Amniotic membrane | Applied | Surface of the eye during in-ocular repair | 361 | Homologous use |
| Acellular dermal product | Applied | Support/covering structures like a tendon | 361 | Homologous use |
| Acellular dermal product | Applied | Tendon repair or replacement | 351 | Non-homologous use |
FD&C Act: Food, Drug and Cosmetic Act; HCT/Ps: Human Cells, Tissues and Cellular and Tissued-Based Products; MSK: musculoskeletal; NAB: non-autologous biological
Homologous use and minimal manipulation are the first two elements of 1271.10(a). The other two elements prohibit the adulteration of the HCT/P with other than preservatives and buffers [1271.10(a)(iii)] and restrict the use of the HCT/P to the donor or first/second degree blood relative if the product contains viable cells or provides a systemic effect [1271.10(a)(iv)]. Most self-assessed NAB products usually fail to be eligible for Section 361 due to exceeding minimal manipulation or are not a homologous use, but NABs with viable donor-derived cells fail the last element of 1271.10(a): not being used to treat the donor or the donor’s first/second degree blood relatives.
It is important to appreciate that the title of 21 CFR 1271 includes the phrase “…cellular and tissue-based products”, which points to the wide scope of the regulation. It isn’t just about tissues themselves, but includes the products derived from those tissues. For example, directly isolating exosomes from cord blood or indirectly obtaining exosomes and/or biomolecular secretions from in vitro culturing of cells isolated from tissue are considered to be HCT/Ps and biological products regulated as Section 351 products. Consequently, if you are a medical provider and have been offered a NAB product to use in treating your patients, the four questions to consider are:
Is the source tissue obtained from a human?
Is the tissue specifically excluded from being regulated as indicated in 1271.3?
Is the product obtained from the human tissue by some process other than simple physical manipulation?
Is the product intended to treat or mitigate a human disease or condition?
In the case of a secretion-type NAB obtained by culturing cells present in or obtained from a human tissue, the answers are:
Yes, the source of the tissue is obtained from a human.
Probably not, so the tissue source used is an HCT/P (e.g., placental tissue).
The secretion-type NAB is obtained from culturing human tissue or cells in a bioreactor and recovering the “secretions” found in the tissue culture fluid, a process that probably exceeds a standard of minimal manipulation.
Yes, the product is intended to treat or mitigate a human disease or condition or why is it being offered to you?
Even though 21 CFR 1271 doesn’t explicitly mention secretions from cells cultured in a bioreactor cited in this example, it is obvious that a secretion-type NAB is at least a biologic product and a Section 351 material (Question #4, FD&C Act). It also could be regulated as an HCT/P, since the “secretion” is not obtained as a fluid directly from a human tissue (e.g., amniotic fluid). Instead, the secretion is created from the viable cell activity of the cultured cells or tissue, which doesn’t meet the Section 361-compliant requirements of 1271.10(a)(i-iv), so most likely it is a Section 351 biologic product. Of course, you could ask to see the USFDA’s response to the manufacturer’s RFD for the product, and if that isn’t provided, the NAB probably is an experimental product, unless it is used in the care of wounds or burns and is in sheet-form—not micronized.
The following sections cover a broad range of NABs, their manufacturing, regulatory status, and published reports on the clinical evaluation of the products, where available. Publications on the clinical use of a particular NAB are listed in Table 3, along with a summary of the format of the clinical study or randomized controlled trial (RCT), enrollment, an assessment of bias (based on randomization and blinding) and the statistical methods used to analyze the data. A summary of serious adverse events (SAEs) and adverse events (AEs) reported in these publications is dealt with in a separate section below.
Description of clinical study or RCT attributes including a bias estimate and statistical methods
| References | Study type | Clinicaltrials.gov identifier | Enrollment | Risk of bias | Statistical methods for analysis |
|---|---|---|---|---|---|
| Gaudilliere et al. [6] | RCT | NCT03981419 | 38 | Randomized with control arm; double blinded; low | Due to the large number of proteomic targets available, the authors used an advanced regression model to identify analytes of interest for comparison between treatment cohorts. Paired comparisons were analyzed with a non-parametric method. |
| Beall et al. [8] | RCT | NCT03709901 | 218 | Randomized with two control arms; patient blinding; moderate | Standard statistical assessment of results was performed. |
| Hunter et al. [9]—a post hoc analysis of the study reported in [8] | RCT | NCT03709901 | 218 | Randomized with two control arms; patient blinding; moderate | Post hoc analysis of the primary dataset reported in [8] was performed with non-parametric methods appropriate for a three-group comparison; significance of the three-group non-parametric analysis was confirmed by a subsequent ranked-based non-parametric analysis. |
| Psathas et al. [13] | Retrospective | NA | 32 | Standardized review conducted; not all patients provided consent; moderate | A standard statistical assessment for significance between categorical variables was performed. |
| Tettelbach et al. [14] | RCT | NCT01693133 | 110 | Randomized with control arm; outcomes validated by blinded adjudicator panel; low | Differences in continuous variables were assessed by standard methods, including a non-parametric analysis of multiple groups. Categorical variables were assessed by standard methods, including regression modeling with fixed effects. |
| Ahuja et al. [15] | Retrospective | NA | 30 | Chart review; high | No comparative statistical analysis was performed. |
| Gomoll et al. [17] | RCT | NCT02318511 | 200 | Randomized with two control arms; single blinded; moderate | Outcomes were assessed for change from baseline with standard statistical assessment of significance between treatment groups. |
| Zelen et al. [18] | RCT | NCT01659827 | 45 | Randomized with control arm and two treatment arms; patient blinded; moderate | Appropriate non-parametric methods were used for comparing two or more groups of continuous data. Parametric methods were used for comparing binary data. |
| Hanselman et al. [19] | RCT | NA | 24 | Randomized with control arm; double blinded; low | Standard methods were used for assessing the significant differences between control and treatment arm outcomes, including a separate assessment of the number of injections on treatment outcomes. |
| Alden et al. [20] | Retrospective | NA | 82 (100 knees) | Chart review; high | Subscores of KOOS were averaged for each timepoint, and an arbitrary cutoff of a change of “10 pts” in any of the subscores compared to baseline was considered to represent a “clinically meaningful improvement”. |
| Natali et al. [21] | Prospective | NA | 25 | Non-randomized; high | Non-parametric analysis was performed for the outcome metrics comparing pre- and post-treatment results, with a specific assessment of age. |
| Meadows et al. [22] | Prospective | NA | 10 | Non-randomized; high | Appropriate non-parametric analysis was performed to assess the significance of all metrics reported post-treatment compared to baseline. |
| Noriega et al. [24] | RCT | NA | 24 (23 at 3.5 year follow-up) | Randomized with control arm; blinding through all phases; low | Appropriate non-parametric and parametric assessments were performed to assess the significant difference in the results. |
| Amirdelfan et al. [25] | RCT | NCT01290367 | 100 | Randomized with two control and two treatment arms; blinding of participants and radiological evaluation; moderate | A complex analysis of outcomes was performed, including the use of appropriate statistical methods for dealing with missing data. The impact of multiple parameters was assessed by repeated measures mixed modelling. |
| Gornet et al. [26] | RCT | NCT03347708 | 60 | Randomized with two control and two treatment arms; double blinded; low | A complex analysis of VAS based on repeated measures mixed-effects linear modelling was performed, adjusting for missing data. Validation of the analysis was performed with a separate statistical assessment with its own null hypothesis. Group differences were assessed by standard methods, including an analysis of co-variance. |
| Abdullah et al. [32] | RCT | NA | 40 | Randomized with control and treatment arms; double blinded; low | Analysis with an appropriate parametric or non-parametric standard method was employed after assessing the distribution of the data as either normal or non-normal. |
| Mazzotta et al. [33] | Retrospective | NA | 96 | Consecutive case review with blinded to treatment matched pair selection; moderate | A stratified approach was used to confirm normality of results, as well as the consistency in variances for datasets. Differences in outcomes over time were assessed in a linear model, along with an ANOVA assessment of between-groups differences when the data was normally distributed and variances were constant. Where datasets didn’t meet these requirements, non-parametric methods were employed. Where appropriate, non-parametric correlative measures were assessed. Standard statistical methods were used for parametric analysis. |
| Zhu et al. [35] | RCT | ChiCTR2100048624 (Chinese Clinical Trial Registration) | 80 | Randomized with treatment and control arms; blinded to cohort; low | Multiple assessments of the normality of the distribution of various datasets were used to perform either parametric or non-parametric analysis. Multiple outcome measures were evaluated in a mixed linear model, with assessments of interactions. Regression models and ANOVA analysis were performed to characterize the influence of secondary outcomes with interactions. Of note, blinding was assessed with the James blinding index. |
| Lightner et al. [40] | RCT | NCT04493242 | 102 | Randomized with two treatment arms and a control arm; double-anonymized; low | Pre-planned standard methods of analysis of the primary outcome of 60-day mortality rate were used. Pre-defined subgroup analyses of mortality data also were performed with standard methods. |
| Gibson et al. [42] | RCT | NCT03005106 | 71 | Randomized with treatment and control (autograft) arms; moderate | Non-parametric methods of analysis were used to assess significant differences in outcome measures of healing between the test article and autograft. Standard methods of analysis were used for parametric assessment of participant-reported outcomes, or for non-participant assessments of healing. |
KOOS: Knee Injury and Osteoarthritis Outcome Score; RCT: randomized controlled trial; VAS: visual analog scale
Bone, ligament, tendon and skin frequently are harvested from cadavers, processed, packaged and sterilized prior to distribution, which is beyond the scope of this review. However, the nucleus pulposus (NP) has been harvested from cadavers, from which NP progenitor cells have been isolated, and expanded in tissue culture to create a treatment for discogenic low back pain [8]. Treatment consists of a lyophilized NP allograft that is rehydrated just prior to injection, which is combined with a cryopreserved aliquot of cultured NP progenitor cells and injected into the disc through a 22-g needle. The presence of viable allogeneic cells in the treatment means it is a Section 351 category HCT/P and requires an IND/IDE and PMA to be completed prior to marketing in the USA. In a Level 1 study (Clinicaltrials.gov Identifier: NCT03709901), the cohort receiving the NP/Cells injection showed a durable decrease in both VAS (visual analog scale) and Oswestry disability index (ODI) at the 12-month milestone [8]. A post-hoc analysis of the data showed that study participants < 42 years old (the median age of the study participants) had a greater decrease in ODI compared to the saline cohort, as well as a higher proportion of responder-level participants (i.e., ≥ 15 points for ODI) compared to the saline cohort. In contrast, the older cohort didn’t show a similar pattern of response with the ODI metric. The incidence of adverse events (AEs) was similar in the two cohorts with rates of 30.9% for the < 42 year-old cohort and 28.8% for the ≥ 42 year-old cohort. The most common AE was back pain associated with either the procedure or treatment [9]. Details of these two studies are provided in Table 3.
As mentioned previously, placental tissue is a major source of NAB products used in treating a variety of pathologies, including wounds, tendons, nerve and bone, and are rich in extracellular matrix, cytokines, growth factors, proteoglycans and proteins [10]. Placental tissue derived products typically are made in various combinations of the amnion, chorion, and placental disk, and are available as fresh (hAM, hCM) or dehydrated (dhAM, dhACM) NABs [11, 12]. Commercially available human tissue derived NABs used for wound and burn care are shown in Table 4.
Human tissue derived wound care NABs
| Product name (reference) | Manufacturer | Composition | Notes |
|---|---|---|---|
| Corplex™ [11] | StimLabs, LLC | Umbilical cord remnant—particulate | USFDA cleared |
| Interfyl® [11] | Cellularity, Inc | Chorionic plate connective tissue matrix—particulate | No amnionic membrane and lacks cells, cell debris, DNA, growth factors and cytokines |
| Dermavest® [11] | AediCell, Inc | Placental disc, amnion/chorion, umbilical cord—sheet form | Tissue is particularized, freeze-dried and pressed into sheet form |
| AXIOFILL® [11] | MIMEDIX Group, Inc | Placental disc derived acellular extracellular matrix—particulate | Can be applied dry or moistened to make a paste |
| NEOX FLO® [11] | Amniox Medical | Amnion with umbilical cord—particulate | Lyophilized, applied dry or as a suspension |
| Amnion Band® [12] | Musculoskeletal Transplant Foundation | Placental amnion/chorion—sheet form | Dehydrated, applied dry of hydrated |
| EPIFIX® [12] | MIMEDIX Group, Inc | Placental amnion/chorion—sheet form | Dehydrated |
NAB: non-autologous biological; USFDA: United States Food and Drug Administration
A recently introduced NAB for use in wound care is composed of the amnion and chorion, but includes the spongy layer that separates these two membranes in situ [dehydrated human amnion chorion membrane/spongy layer (dhACM/SL)] [13]. The spongy layer-containing sheet-form NAB was evaluated in a study of chronic non-healing wounds with various etiologies. The median time to heal was 77 days. Overall, treated wounds had the following levels of healing: 66% of the wounds had 100% healing, 5.7% had 70–99%, 9.4% had 40–70% and 18.9% had poor or no response. Venous leg ulcers, surgical wounds, and traumatic wounds had the highest response to treatment, while ischemic ulcer and pressure injury showed a poorer response. One factor that favored better response was observed for wounds with less than 1 year chronicity [13]. Additional details for this clinical study are provided in Table 3. In a Level 1, multi-clinic study of a dehydrated human amnion chorion membrane (dhACM) product for treating diabetic foot ulcers that were refractory to standard of care, it was found that 70% of the study participants receiving the dhACM product had complete wound closure at 12 weeks compared to just 50% of the participants in the control cohort. The rate of complete closure increased to 95% at the study endpoint of 16 weeks for the dhACM cohort, while just 86% of the control cohort had complete closure [14]. Additional details for this clinical study are provided in Table 3. One area where wound closure is a serious challenge is in treating pediatric burns. A commercially available dhACM graft was used to treat 30 patients with a variety of superficial partial to full thickness burns in place of using a split thickness skin graft (STSG). Healing was enhanced and reported to be faster compared to published reports using STSGs, while pain also was reduced [15]. Additional details for this clinical study are provided in Table 3. Several commercially available dhACM products were compared in a review of published clinical studies in terms of clinical outcomes and use [12]. The sheet form of placental tissue derived NABs also has been used to treat dermatologic conditions that can become chronic wounds. In a review of published case series involving dermatology conditions, dhACM was reported to reduce healing time and pain, and resulted in better cosmetic outcomes [16].
In addition to the sheet form of placental tissue derived NABs discussed above, there are a number of NAB products that are made by micronization of placental tissue, which renders them suitable for injection; no injectable NAB products outside of a single product for use in wound care have been approved by the USFDA so far. Micronized amniotic membrane (AM)-only NAB products are referred to as “amniotic suspension allograft” (ASA) [17], while micronized dehydrated amnion/chorion membrane products are labeled as micronized dehydrated human amnion chorion membrane (mdhACM) [18]. A Level 1 randomized control trial (Clinicaltrials.gov Identifier: NCT01659827) was performed to assess safety and efficacy of a mdhACM product to treat chronic plantar fasciitis [18]. Two doses of the mdhACM were injected, with saline as a control. At the study endpoint of 8 weeks, the control cohort had an average improvement of 12.9 points on the American Orthopedic Foot & Ankle Society Hindfoot metric, while both mdhACM treated cohorts reported an improvement greater than 50 points. No AEs related to the injections were reported [18]. Additional details for this clinical study are provided in Table 3. The use of an injectable, cryopreserved, AM NAB product [cryopreserved human amniotic membrane (c-hAM)] was evaluated in a Level 1 randomized control study for treating plantar fasciitis, with corticosteroid injection as the control. At the 12-week endpoint, there was no meaningful improvement of the c-hAM cohort over the control. However, a small group of participants in each cohort opted for a second injection for which the primary outcome metric of the Foot Health Status Questionnaire was meaningfully improved at the 18-week milestone for the 2-dose c-hAM treated cohort. No AEs associated with the treatments were reported [19]. Additional details for this clinical study are provided in Table 3. A mdhACM product was evaluated for safety and efficacy in treating knee OA in a retrospective case series study of 82 consecutive patients. The primary outcome measure was the Knee Injury and Osteoarthritis Outcome Score (KOOS), whose subscales (QoL and pain) showed a range of improvement from 55% to 118% over baseline at the 6-month endpoint. AEs were reported by 68% of participants, which resolved within 2–7 days post-injection [20]. Additional details for this clinical study are provided in Table 3. An ASA product was evaluated in a randomized clinical study for treating knee OA (Clinicaltrials.gov Identifier: NCT02318511), with saline and hyaluronic acid (HA) as comparators [17]. There were 68 patients treated with ASA, 64 with HA and 68 with saline. The QoL subscore of KOOS was meaningfully improved for the ASA cohort over the HA and saline cohorts out to the 12-month endpoint, but there was no consistent pattern of improvement for the other KOOS subscores. All VAS subscores were meaningfully improved for the ASA cohort over the HA/saline cohorts at the 12-month endpoint. Overall, based on the Outcome Measures in Rheumatology and Osteoarthritis Research Society International (OMERACT-OARSI) criteria for assessing responders, there were 50%, 25% and 25% high responders for the ASA, HA and saline cohorts, respectively. Treatment-emergent AEs were reported by 2.9% and 3.0% of the ASA cohort and the HA cohort, respectively [17]. Additional details for this clinical study are provided in Table 3. A pilot study of the safety and efficacy of treating knee OA (n = 25) with a non-commercial ASA was performed [21]. The in-house ASA agent was prepared by a medical institute with donor screening and microbial sterility testing of the ASA product. No SAEs were reported, although 16% of the study participants reported mild AEs, which resolved within a few days. The International Knee Documentation Committee (IKDC) score and VAS both showed a plateauing of improved scores from the 6- to 12-month endpoints, with both showing a meaningful improvement compared to baseline at the 12-month endpoint [21]. Additional details for this clinical study are provided in Table 3. Finally, a commercially available ASA was used to treat hip OA in a prospective study of ten participants with a 1-year endpoint [22]. One participant left the study at 2 months post-injection and received a total hip replacement. The remaining participants reported meaningful improvements compared to baseline for the modified Harris hip score (HHS; mHHS) and the International Hip Outcome Tool at the 12-month endpoint. Only the “maximal pain over the previous three days” VAS subscore was meaningfully improved compared to baseline at the 12-month endpoint. None of the patients remaining in the study reported any treatment related AEs [22]. Additional details for this clinical study are provided in Table 3.
Cells isolated from a variety of tissues, including bone marrow, adipose tissue (AT) and umbilical cord tissue, have been cultured to create allogeneic cell therapies to treat OA, low back pain and other painful and degenerative tissue pathologies. A recent review of randomized control trials highlighted the variations in using cultured allogeneic mesenchymal stromal cells (MSCs) to treat knee OA [23]. The allogeneic cell trials used cultured MSCs obtained from AT, bone marrow, placental membranes and Wharton’s Jelly. The cell dose injected as well as the number of injections also varied. Despite the variables of dose and OA conditions, five of the six studies reported favorable improvements in pain and disability metrics. In one study, one of the treatment arms involved injecting an allogeneic MSC agent at monthly intervals to treat knee OA, but the study was stopped due to increasing pain at the site of injection [23]. The use of bone marrow derived cultured MSCs to treat discogenic low back pain has been evaluated in two clinical studies. An allogenic dose of 25 × 106 MSCs was injected intradiscally in the cell treatment cohort, while the control group received a sham injection of anesthetic in the paravertebral muscles [24]. At 42-month follow-up, the MSC-treated cohort had VAS and ODI scores that were meaningfully improved compared to baseline, while the control cohort VAS and ODI scores weren’t improved compared to baseline. Importantly, the Pfirrmann grades of the treated discs were assessed periodically (by MRI), which revealed a reduction in the Pfirrmann grade of 0.6 for the MSC-treated cohort, whereas there was an increase in the Pfirrmann grade of 1.0 for the control cohort [24]. Additional details for this clinical study are provided in Table 3. A bone marrow derived allogeneic MSC therapy in the process of commercialization was evaluated in a Phase 1b/2a study (Clinicaltrials.gov Identifier: NCT01290367) to assess safety and efficacy of treating discogenic low back pain with 6 × 106 MSCs suspended in a 1% HA solution compared to saline as a control [25]. At the 36-month milestone, 43% of the participants treated with MSCs and 20% of saline-treated participants showed a 50% decrease in VAS. A total of 20% of the MSC-treated cohort reported AEs, with none being characterized as a SAE. In contrast to the other allogeneic MSC intradiscal study reviewed above, no changes were reported in the modified Pfirrmann grades of any of the participants receiving the allogeneic MSC treatment [25]. Additional details for this clinical study are provided in Table 3.
Not all allogeneic therapeutic cell preparations are obtained from adipose, bone marrow or placental tissues: NP cells were recovered from live donor intervertebral discs and cultured to create a discogenic cell-based therapy. The cultured discogenic cells were evaluated for safety and efficacy in a Phase 1/2 IND randomized control trial (Clinicaltrials.gov Identifier: NCT03347708) for treating discogenic low back pain [26]. A low dose (3 × 106 discogenic cells), a high dose (9 × 106 discogenic cells), hyaluronate (vehicle) and saline (placebo) were used to treat 20, 20, 10 and 10 study participants, respectively. The high dose cohort showed a meaningful difference in VAS and ODI outcomes compared to baseline starting at 1-year and continuing through the 2-year endpoint. The high dose cohort also had the highest number of participants (70%) with a > 30% reduction in VAS compared to 60% for the combined hyaluronic/saline cohorts and 55% for the low dose cohort. The high dose cohort showed a meaningful decrease in ODI that exceeded the minimal clinically important difference (MCID) of –15 points at 12-, 26-, 78- and 104-weeks, while the other three treatment arms failed to reach the MCID. Changes in disc volume assessed by MRI showed a mean increase in disc volume of approximately 400 mm3 for the high dose cohort, while the other three cohorts showed a loss in disc volume (hyaluronic, saline), or no net change in volume (low dose) at the 104-week milestone. The frequency of AEs was lowest for the high dose cohort. There was an incidence of 6.7% for SAEs, which were associated only with study participants in the saline and hyaluronate groups. Overall, the use of an allogeneic cell agent derived from the NP demonstrated efficacy in reducing pain and improving QoL, as well as achieving a durable increase in disc volume out to the 2-year endpoint [26]. Additional details for this clinical study are provided in Table 3.
Conditioned medium recovered from the culturing of a variety of progenitor cells (e.g., MSCs) has been evaluated as a source of therapeutic treatments. Cultured cells release biomolecules and EVs into the tissue culture fluid, which is referred to as the cell’s “secretome” [7, 27, 28]. The excitement surrounding the use of conditioned media as a therapeutic agent is based on the realization that most of the therapeutic benefit attributed to MSCs is associated with the wide variety of biomolecules secreted by the MSCs in vivo, which is referred to as the paracrine effect [29]. Thus, a secretome containing NAB product might offer an opportunity to provide an allogeneic cell-free regenerative therapy, which reduces the risk of immune rejection of the allogeneic cells [27]. However, as is the case with any cultured therapeutic product intended for injection, there is a requirement to produce the secretome under CGMP (current good manufacturing practice) conditions to ensure sterility and safety. The effort to produce a secretome in a lyophilized form under CGMP conditions at a pilot scale has been reviewed [30]. One emerging issue relates to the differences among the secretomes obtained with MSCs isolated from various tissues. In vitro assessments of the secretomes of cells isolated from AT and AM were found to have differential impacts on cultured cell-based models. For example, AT-conditioned medium was shown to induce higher proliferation and better supported neurite outgrowth, while AM-conditioned medium displayed a profile of greater immunomodulatory activity, migration and re-growth of cells in a scratch model assay [31]. In another report, secretomes obtained from umbilical cord MSCs, bone marrow MSCs and AT MSCs were found to have variations in the presence of important biomolecules, as well as in the amount of these molecules in the secretomes studied [28]. While almost all of the therapeutic evaluations of the potential clinical benefits of secretomes are limited to in vitro and pre-clinical studies, there was a recent report on the use of a CGMP-produced secretome to treat severe COVID-19 patients [32]. The Level 1 trial was double blinded, and placebo controlled. Treatment consisted of a single IV infusion of 15 mL of the secretome product in 100 mL of saline, while the control group received the same volume of saline. One of the few clearcut differences reported in the study occurred in the change in the ratio of the level of IL-6 (interleukin-6, pro-inflammatory) to IL-10 (anti-inflammatory) on D14 post-treatment. Control patients showed a meaningful increase in the ratio from D7 to D14, while the ratio of these cytokines for the intervention patients didn’t increase [32]. Additional details for this clinical study are provided in Table 3. An important consideration to keep in mind when thinking about using secretome products is their efficacy compared to the efficacy of the source cultured cell, which is the focus of a very recent study [27]. The secretome dermal stem/stromal cells (seDSCs; adherent cells isolated from human skin) and secretome AT stem/stromal cells [secretome adipose stem/stromal cells (seASCs); adherent AT derived cells isolated from the skin donor] were analyzed and used in a surgical wound murine model. The secretomes were found to share 663 proteins, while 102 proteins each were unique to the dermal stem/stromal cell (DSC) and adipose stem/stromal cell (ASC) derived secretomes. In a pre-clinical study of skin wound healing in a murine model, the secretomes from the ASCs and DSCs were compared with the cultured cells that produced the secretomes, along with a negative control of a commercially available collagen-based scaffold that was used as a scaffold for the treatments. The degree of wound closure on D21 for the treatments was as follows: DSCs (90.3%) > ASCs (86.9%) > seASC (68.1%) ≈ seDSC (67.3%) > Scaffold (55.8%). Wound closure with the DSC and ASC treatments were meaningfully improved over the scaffold, while the seASC and seDSC treatments were not. As noted in the publication, this is the first paper in which both the parent cell and its secretome were assessed for efficacy in a pre-clinical model [27].
Autologous platelet-rich plasma (PRP) is a frequently prescribed therapy for a broad spectrum of MSK pathologies. Since it is an autologous therapy, the patient’s condition might limit the potential therapeutic benefit, which is of concern with diabetics, smokers, the obese, the elderly and those patients with a low platelet count [33]. As an extension of the availability of platelet concentrates (PCs) in blood banks for use in hemostatic support, expired PC units have been used to create platelet lysates (PLs) and are a source of exosomes [34]. There are a few reports that describe the use of allogeneic PRP in treating MSK pathologies. The use of allogeneic PRP to treat knee OA in patients suffering from primary immune thrombocytopenia was evaluated in a randomized control trial [35]. Eighty study participants were randomized into an allogeneic PRP (allo-PRP) treatment cohort or a saline placebo cohort. The allo-PRP was obtained from a single healthy donor. One injection of either the allo-PRP or saline was delivered via the intraarticular route. No hemoanalysis of the allo-PRP injectates was performed, although the device used was cited as producing a leukocyte-poor allo-PRP. The primary outcome metric was Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). Over the course of the 1-year study period, the allo-PRP cohort reported meaningfully improved WOMAC results only at the 3-month milestone compared to the saline cohort. The frequency of short-term AEs in the allo-PRP cohort was 10% and in the saline cohort was 7.5%, which resolved without further intervention within a few days. However, more study participants reported durable pain and swelling in the injected knee through the 12-month endpoint of the study with the allo-PRP treatment compared to the saline treatment. There were no SAEs reported in the study [35]. Additional details for this clinical study are provided in Table 3. The use of cord blood as the source of platelets has been explored to treat hip OA via an intraarticular delivery as reported in a retrospective case review [33]. Patients were treated with either an injection of autologous PRP (A-PRP) or blood-typed/matched allogeneic cord blood derived PRP (C-PRP) once per week for three weeks. Cord blood was obtained from donors with uncomplicated deliveries or C-sections. Homologous cord blood units were pooled and subsequently processed by a manual method to yield three aliquots of C-PRP for use in treating a participant, which was stored frozen. A similar process was used with the autologous blood processing to yield three aliquots of A-PRP, which also were stored frozen. In addition to the use of blood-typed matched C-PRP, the cord blood was processed through a leukocyte-depletion filter, while the A-PRP wasn’t, in order to reduce the risk of immune rejection. The frequency of AEs was not meaningfully different between the two PRP treatment cohorts, and these AEs were of short duration following the injection. There were no meaningfully improved differences in outcomes at any milestone between each type of PRP and baseline. However, if data was stratified according to the patient’s extent of hip OA score (Tonnis metric 1–2 vs. 3), C-PRP patients with low grade hip OA had a meaningful improvement for the HHS at the 12-month endpoint compared to the HHS score for the A-PRP cohort [33]. Additional details for this clinical study are provided in Table 3.
The use of young plasma by humans as a means of combating age-related decline (“anti-aging”) is based on results obtained in a parabiosis rodent model, in which the circulatory systems of young and old mice were surgically connected. Results from a number of parabiosis experiments over the past two decades have demonstrated the benefit of “young plasma factors” on the older mice’s pro-inflammatory status [36], a condition referred to as “inflammaging” [6]. Analysis of the protein contents of “young plasma” have led to the identification of key biomolecules that are thought to play a role in modulating the negative aspects of getting old. For example, apelin is a hormone that is characterized as a “rejuvenating factor”, since it is at higher levels in younger mice and decreases as mice age. β2-Microglobulin is a “pro-aging” factor, which is found at higher levels in older mice, so its reduction would contribute to slower aging [36]. The devil, of course, is in the details in view of the complex feedback loops and interacting metabolic pathways that manage a person’s physiology as they age. Based on the evidence emerging from pre-clinical model studies, it seems like young plasma could be considered as the biological equivalent of a compounding pharmacy. Unfortunately, so far there are virtually no publications on human clinical studies performed at any level to support the potentially beneficial anti-aging effects of young plasma. However, in a very recent publication [6], the influence of a 5% plasma protein fraction derived from young donors (the average age of donors was 35 years old) that is in the process of being commercialized was evaluated in patients undergoing a planned surgery. The Phase 2a IND trial (Clinicaltrials.gov Identifier: NCT 03981419) enrolled patients that would be undergoing knee or hip arthroplasty in order to assess the influence of the 5% plasma agent on the patients’ response to surgical injury. Patients were randomized to receive either plasma or saline infusion, with both cohorts receiving a series of infusions starting the day before surgery, before and after the surgery itself and the day after surgery. Proteomic and cell analyses were performed to assess patterns of up- and down-regulated proteins and types and numbers of cells. Both cellular and protein profiles showed changes that supported anti-inflammatory immune modulation, along with beneficial changes in other supporting pathways. In addition, patients were surveyed for fatigue, impairment of daily function and pain following their surgery. While none of the outcomes were meaningfully different between the two cohorts, the median time to reach “mild” pain in the plasma protein fraction-treated cohort was 12 days, while the same milestone took 18 days for the saline cohort [6]. Additional details for this clinical study are provided in Table 3.
EVs are released by cells in the body in three ranges of diameter: 30–150 nm (exosomes); 150–500 nm (microvesicles); and 500–800 nm (apoptotic bodies). Exosomes contain “cargo” that has been shown to mimic the paracrine effects of the parental MSCs that produce the exosomes. Consequently, MSC derived exosomes have dominated efforts for commercialization, with almost all exosome preparations being derived from bone marrow, AT or umbilical cord tissue [37]. Furthermore, all of the fourteen active clinical studies recently listed on Clinicaltrials.gov that used exosomes as the therapeutic agent were obtained from those same three tissues [37]. The roles played by specific components of exosome cargo, like microRNAs and proteins, in MSK health and regulation have been reviewed [38]. For example, several microRNAs have been found in exosomes released by mineralizing osteoblasts, which were shown to promote differentiation of a murine cell type (ST2) into an osteoblastic lineage [38]. While cultured MSCs release exosomes with cargoes that can act to reduce inflammation, promote resident tissue cell proliferation and enhance angiogenesis (among other effects), a very active area of investigation involves functional modification of exosome cargoes through manipulation of the culture conditions (e.g., hypoxia, additives in the culture medium, etc.) [39]. Improved delivery of exosomes also is being explored based on modifying the surfaces of exosomes to enhance binding to or interacting with hydrogels or other types of carriers [39]. Although the clinical trial doesn’t involve an MSK pathology, clinical outcomes of a significant Phase 2 IND trial (Clinicaltrials.gov Identifier: NCT04493242) of a bone marrow MSC derived exosome-based therapeutic agent recently were published on treating acute respiratory distress syndrome (ARDS) in hospitalized COVID-19 patients [40]. Patients in the active treatment cohort were treated with two doses of the exosome product containing 1.2 × 1012 exosomes/dose, which was diluted in approximately 100 mL of saline and delivered via IV, while the placebo cohort received 100 mL of saline. The exosome product-treated cohort had a shorter time to discharge, and ventilation-free days were meaningfully higher compared to the placebo cohort. As shown in Table 5, while the All-cause mortality in the exosome product-treated cohort was not meaningfully improved compared to placebo for All COVID-related ARDS patients, a post-hoc analysis of the mortality rates for the moderate to severe ARDS patients showed a meaningfully improved outcome of 30.8% for the exosome product-treated cohort compared to 72.7% for the placebo cohort [40]. Additional details for this clinical study are provided in Table 3. Based on the reduction in mortality shown in the Phase 2 IND, the USFDA agreed to allow the Phase 3 trial to start in 2022, and in 2023 agreed to extend the scope of the Phase 3 trial from COVID-19 related ARDS to ARDS of any etiology [41]. The bone marrow MSC derived exosome product under investigation is the first exosome-based therapeutic agent to progress to a Phase 3 IND trial in the USA.
Therapeutic outcomes for treating COVID-19 ARDS patients with a bone marrow MSC derived exosome product [40]
| Patient cohort | All-cause mortality | |
|---|---|---|
| Exosome treated (%) | Placebo (%) | |
| All COVID-related ARDS | 29.4 | 47.1 |
| Moderate to severe ARDS | 30.8 | 72.7 |
ARDS: acute respiratory distress syndrome; MSC: mesenchymal stromal cell
There are two examples of cells being cultured on carriers to create a composite cell-carrier wound care treatment that are USFDA approved. Stratagraft® (Stratagraft Corporation, Madison, WI, USA) is comprised of an allogeneic cellularized scaffold (rat collagen), which is conditioned by human dermal fibroblasts and seeded with a human-derived keratinocyte cell line. Stratagraft® is placed over the debrided burn, which allows the patient’s cells to migrate into and remodel the construct. In a summary of the Phase 3 IND trial (Clinicaltrials.gov Identifier: NCT03005106), Stratagraft® was able to accelerate wound closure in 83% of the study participants by month 3, without the use of an autograft supplemental treatment, which eliminates the risk for donor-site morbidity and wound healing complications in patients with deep partial-thickness burns [42]. Additional details for this clinical study are provided in Table 3. Apligraf® (Organogenesis Inc, Canton, MA, USA) is a composite wound care product that is comprised of a bilayer having human keratinocytes growing as a well-defined epidermal layer, and human fibroblasts in a bovine collagen scaffold on the dermal side [43]. A review of clinical studies in which Apligraf® has been evaluated for treating diabetic foot ulcers and other partial- or full-thickness skin ulcers generally showed slower healing rates compared with other NABs [12].
Regardless of the type of clinical study being performed, the safety of study participants needs to be monitored, which requires that any participant-reported or investigator-observed outcome needs to be documented. These documented events are considered as AEs if there is any untoward or unfavorable medical occurrence in a study participant, including any abnormal sign, symptom, or disease that is temporarily associated with the study participant’s participation in the clinical study, whether or not the event is considered related to the study participant’s participation in the clinical study. If an AE meets any of the criteria below, it is regarded as a SAE:
Results in death.
Leads to a severe deterioration of the health of the subject, with any of the following outcomes:
A life-threatening illness or injury; or
Permanent impairment of a body structure or a body function; or
In-patient or prolonged hospitalization; or
Medical or surgical intervention to prevent life-threatening illness or injury or permanent impairment to a body structure or function
AEs have been reported for most of the studies included in this survey. A distinction should be made between a “treatment”-related AE and one that is “procedure”-related. For example, study participants in a knee OA treatment study might report pain at the site of treatment. If participants in the treatment cohort and the control/placebo cohort report pain at the site of treatment, the AE would be attributed to the protocol and not the therapeutic agent. The treatment site also can play a role in the frequency of AEs. Intervertebral disc injections with NABs frequently are associated with both more numerous and a wide variety of AEs: back pain, site injection pain and muscle spasms, etc. [8, 24–26]. However, SAEs might be related to the specific NAB used as the treatment. For example, disc injection of allogeneic MSCs from one source [24] didn’t result in any reported SAEs, while allogeneic MSCs from a different source [25] were associated with SAEs. The specific protocol used in a study might also contribute to AEs, but investigators will compare the frequency of AEs in the treated versus the control cohorts to assess the possibility that a therapeutic agent is the source of AEs [25]. Since NAB products are by definition derived from unrelated donors, there is a chance that a study participant might have a host-versus-graft reaction. However, only a few clinical studies commented on this specialized type of AE, with one study [21] indicating that there were no immune-mediated AEs observed, while in another study [42], immune-reactivity to components of the NAB was observed, which nonetheless didn’t result in rejection of the graft. In a wound care study with a dhACM product, the investigators indicated that there were three AEs that might be due to the graft itself [14]. Another concern with using allogeneic derived NABs is the possibility that repeated treatments with the same NAB might result in sensitization to the NAB. One such outcome was reported in which repeated injections at monthly intervals into knees resulted in increasing pain at the treatment site, which resulted in an early termination of that arm of the clinical study [23]. Finally, most mild-moderate AEs were reported to resolve within a few days of treatment with the NAB, but durable AEs in knees out to the 1-year milestone were reported in knees treated with the NAB (allo-PRP) but not with the control/placebo [35].
Non-autologous biologics have played and will continue to play an important role in addressing therapeutic challenges as diverse as full thickness wounds, pediatric burns, intervertebral disc degeneration, and osteoarthritic joints. Although there are a mature set of NABs obtained from placental tissue for use in wound and burn care, viable cell-based constructs offer a different approach and an opportunity for continued innovation in treating wounds and burns. Improved cell culture technology, including the development of multicellular spheroids [44, 45], offers the potential for more effective allogeneic cell-based therapies. However, repeated injections of allogeneic cell therapies have a potential for increased AEs, which might limit their use [23]. One option for leveraging the use of MSCs without injecting the cells directly is to collect the secretome found in the conditioned medium of cultured MSCs. Results from pre-clinical models have been supportive of the therapeutic benefits of using MSC-derived secretomes [28], although human studies are lacking. One interesting counterargument against the use of secretomes recently was reported in a pre-clinical study involving a murine skin wound closure model in which the secretomes from source cells were compared with the cultured cells alone. The cell-based therapeutic agents resulted in a faster wound closure rate compared to the matched secretomes [27]. Exosomes, which are found in the secretomes of cultured cells, also offer a cell-free therapeutic approach, with a substantial number of pre-clinical studies demonstrating positive therapeutic benefit with a variety of MSK pathologies. Exosomes are isolated from culture fluid in order to obtain levels of exosomes in the billions or trillions per dose. While the evaluation of exosomes in clinical trials is very active [37], only one Level 1 Phase 3 trial currently is underway in mitigating ARDS in hospitalized patients [40]. Furthermore, the potential to modify cargoes of exosomes is an area of active investigation, which could result in tailored exosome products to maximize therapeutic benefit for specific lesions or pathologies, instead of a one-size-fits-all “generic” exosome product. Allogeneic blood cell products have been studied, but there is a need to leuko-deplete these products in order to minimize immune reactions either by the recipient or donor cells. One alternative that might become a therapy in the future is the use of induced pluripotent stem cells as a source of immortalized megakaryocytes that could produce a virtually unlimited number of human platelets replacing the need for using PCs, an approach that is being investigated in pre-clinical models [46]. Finally, there is a growing interest in the use of young plasma, with the intent to slow the aging process. While evidence in humans is lacking in support of a definitive therapeutic benefit of anti-aging, the potential of young plasma as an anti-aging therapy is an area of active investigation. Given the complexity of aging, it might be overly optimistic to hope that something like young plasma might be universally effective in slowing the aging process. Instead, the extensive research that is on-going to characterize the biomolecular milieu found in young plasma might yield important candidates for further pharmaceutical development, with the potential for fewer off-target activities, like promoting epigenetic changes or proliferation of nascent cancerous cells. Finally, despite the wide variety of NAB products reviewed, no SAEs have been reported that were related to their use as a therapeutic agent. However, therapeutic agent-associated AEs were reported in most clinical studies, with the majority of these AEs resolving within a few days after treatment. Overall, the NAB products reviewed in this report are considered to be safe, while their efficacy isn’t universally or uniformly evident.
AEs: adverse events
Allo-PRP: allogeneic platelet-rich plasma
AM: amniotic membrane
A-PRP: autologous platelet-rich plasma
ARDS: acute respiratory distress syndrome
ASA: amniotic suspension allograft
ASC: adipose stem/stromal cell
AT: adipose tissue
CFR: Code of Federal Regulations
CGMP: current good manufacturing practice
c-hAM: cryopreserved human amniotic membrane
C-PRP: cord blood derived platelet-rich plasma
DALY: disability-adjusted life years
dhACM/SL: dehydrated human amnion chorion membrane/spongy layer
dhACM: dehydrated human amnion chorion membrane
DSC: dermal stem/stromal cell
EVs: extracellular vesicles
FD&C Act: Food, Drug and Cosmetic Act
HA: hyaluronic acid
HCT/Ps: Human Cells, Tissues and Cellular and Tissued-Based Products
HHS: Harris hip score
IDE: Investigational Device Exemption
IKDC: International Knee Documentation Committee
IL-6: interleukin-6
IND: Investigational New Drug
KOOS: Knee Injury and Osteoarthritis Outcome Score
MCID: minimal clinically important difference
mdhACM: micronized dehydrated human amnion chorion membrane
MSCs: mesenchymal stromal cells
MSK: musculoskeletal
NAB: non-autologous biological
NP: nucleus pulposus
OA: osteoarthritis
ODI: Oswestry disability index
OMERACT-OARSI: Outcome Measures in Rheumatology and Osteoarthritis Research Society International
PCs: platelet concentrates
PHSA: Public Health Service Act
PLs: platelet lysates
PMA: pre-market approval
PRP: platelet-rich plasma
QoL: quality of life
RCT: randomized controlled trial
RFD: Request for Designation
SAEs: serious adverse events
seASC: secretome adipose stem/stromal cells
seDSCs: secretome dermal stem/stromal cells
STSG: split thickness skin graft
SVF: stromal vascular fraction
USFDA: United States Food and Drug Administration
VAS: visual analog scale
WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index
TS: Conceptualization, Writing—original draft, Writing—review & editing.
The author declares that he has no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3028
Download: 26
Times Cited: 0
Ali Yüce ... Abdülhamit Misir
Tanja Neussl ... Johannes Dominikus Pallua
