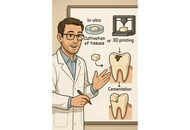




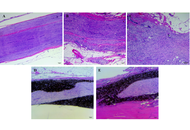

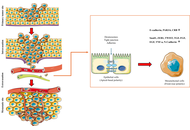

The emergence of stem-cell-derived enamel organoids and dentin-producing dental pulp stem cell constructs presents new possibilities for restoring carious lesions using autologous enamel–dentin inlays. This overview outlines the biological and technological advances supporting this approach and proposes a workflow oriented toward clinical application. The benefits of tissue-based inlays, including inherent biomechanical compatibility, aesthetic accuracy, and potential for biological integration, are contrasted with those of purely artificial materials. Significant regenerative developments include the formation of human enamel organoids and odontoblast-lineage cells in vitro, 3D bioprinting of tooth-shaped constructs with demineralised dentin matrix and poly(ε‑caprolactone) scaffolds, and fibre-guiding periodontal ligament scaffolds that restore Sharpey’s fibres in vivo. The mechanical performance of adhesive resin cements, with bond strengths of approximately 4–7 MPa to enamel and dentin, and their durability in reattaching natural tooth fragments, supports the feasibility of bonding biological inlays. Practical considerations include controlling the slow degradation and hydrophobicity of poly(ε-caprolactone) through the use of ceramic or natural polymer additives, employing multi-material 3D printing to co-print mineralized enamel and cell-laden dentin layers, and achieving the desired shade, microstructure, and mechanical properties, exemplified by a compressive strength of approximately 677 MPa for 3D-printed zirconia crowns. Despite regulatory and translational challenges, the integration of digital dentistry, bioprinting, and stem cell science points toward future “grow and glue” restorations that may replace traditional drill-and-fill methods.
The emergence of stem-cell-derived enamel organoids and dentin-producing dental pulp stem cell constructs presents new possibilities for restoring carious lesions using autologous enamel–dentin inlays. This overview outlines the biological and technological advances supporting this approach and proposes a workflow oriented toward clinical application. The benefits of tissue-based inlays, including inherent biomechanical compatibility, aesthetic accuracy, and potential for biological integration, are contrasted with those of purely artificial materials. Significant regenerative developments include the formation of human enamel organoids and odontoblast-lineage cells in vitro, 3D bioprinting of tooth-shaped constructs with demineralised dentin matrix and poly(ε‑caprolactone) scaffolds, and fibre-guiding periodontal ligament scaffolds that restore Sharpey’s fibres in vivo. The mechanical performance of adhesive resin cements, with bond strengths of approximately 4–7 MPa to enamel and dentin, and their durability in reattaching natural tooth fragments, supports the feasibility of bonding biological inlays. Practical considerations include controlling the slow degradation and hydrophobicity of poly(ε-caprolactone) through the use of ceramic or natural polymer additives, employing multi-material 3D printing to co-print mineralized enamel and cell-laden dentin layers, and achieving the desired shade, microstructure, and mechanical properties, exemplified by a compressive strength of approximately 677 MPa for 3D-printed zirconia crowns. Despite regulatory and translational challenges, the integration of digital dentistry, bioprinting, and stem cell science points toward future “grow and glue” restorations that may replace traditional drill-and-fill methods.
DOI: https://doi.org/10.37349/ebmx.2026.101359
This article belongs to the special issue Innovations in Biomaterials for Dentistry and Oral Surgery

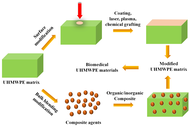
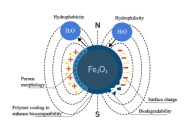
Ultra-high molecular weight polyethylene (UHMWPE) is widely used as a key material in biomedical implants such as artificial joints due to its exceptional wear resistance, high impact strength, and good biocompatibility. However, its inherent bio-inertness, hydrophobicity, risk of osteolysis induced by wear debris, and insufficient mechanical and processing properties severely limit its long-term clinical performance. This review systematically summarizes recent advances in the functional enhancement of UHMWPE via hybrid strategies, including surface modifications (e.g., coatings, chemical grafting, laser processing, plasma treatment) and bulk blending modifications (involving both organic and inorganic composites). These approaches have been shown to significantly improve wear resistance, bioactivity, hydrophilicity, and mechanical properties, while effectively suppressing oxidative degradation and inflammatory responses. The current challenges in modification technologies, such as balancing multiple properties, ensuring long-term biosafety, and achieving clinical translation, are also discussed. Finally, future directions toward multifunctional integration, intelligent responsiveness, and personalized customization of implants are outlined, providing critical insights for the development of next-generation high-performance and long-lasting biomedical materials.
Ultra-high molecular weight polyethylene (UHMWPE) is widely used as a key material in biomedical implants such as artificial joints due to its exceptional wear resistance, high impact strength, and good biocompatibility. However, its inherent bio-inertness, hydrophobicity, risk of osteolysis induced by wear debris, and insufficient mechanical and processing properties severely limit its long-term clinical performance. This review systematically summarizes recent advances in the functional enhancement of UHMWPE via hybrid strategies, including surface modifications (e.g., coatings, chemical grafting, laser processing, plasma treatment) and bulk blending modifications (involving both organic and inorganic composites). These approaches have been shown to significantly improve wear resistance, bioactivity, hydrophilicity, and mechanical properties, while effectively suppressing oxidative degradation and inflammatory responses. The current challenges in modification technologies, such as balancing multiple properties, ensuring long-term biosafety, and achieving clinical translation, are also discussed. Finally, future directions toward multifunctional integration, intelligent responsiveness, and personalized customization of implants are outlined, providing critical insights for the development of next-generation high-performance and long-lasting biomedical materials.
DOI: https://doi.org/10.37349/ebmx.2026.101358

Aim:
Although polytetrafluoroethylene (PTFE) is more hydrophobic than polyvinylidene fluoride (PVDF) in fluorocarbon polymer (FCP) membrane filters, it has been reported that the rate of amyloid fibril formation is faster on PVDF than on PTFE. To clarify whether the effect is due to the membrane’s chemical structure or its hydrophobicity at the membrane interface, studies on amyloid fibril formation were conducted using both hydrophobic and hydrophilic PVDF and PTFE membranes.
Methods:
Heat-treated insulin (INS) was adsorbed onto the FCP membrane filters. Gaussian integrals were employed to determine the amounts of β-sheet and their abundance ratios by curve fitting of attenuated total reflection Fourier transform infrared spectra.
Results:
Adsorbed heat-treated INS onto the FCP membrane filters showed a β-sheet form, with a similar or higher affinity in comparison with that of the β-rich concanavalin A. The adsorption followed a sigmoidal curve with a 2-hour lag time, reaching a plateau after 4–5 hours. The spectral patterns of the adsorbed INS indicated the β-sheet form, demonstrating that INS transformed into β-sheet and then, or simultaneously, adsorbed onto the FCP membrane filters.
Conclusions:
The results regarding the rate and strength of amyloid fibril formation for each FCP membrane filter suggest that, beyond the membrane’s surface hydrophobicity or hydrophilicity, other factors, such as the electron affinity of hydrogen in the PVDF membrane, also influence nucleation. This study provides insight into the role of INS in amyloid fibril formation within FCP membrane filters.
Aim:
Although polytetrafluoroethylene (PTFE) is more hydrophobic than polyvinylidene fluoride (PVDF) in fluorocarbon polymer (FCP) membrane filters, it has been reported that the rate of amyloid fibril formation is faster on PVDF than on PTFE. To clarify whether the effect is due to the membrane’s chemical structure or its hydrophobicity at the membrane interface, studies on amyloid fibril formation were conducted using both hydrophobic and hydrophilic PVDF and PTFE membranes.
Methods:
Heat-treated insulin (INS) was adsorbed onto the FCP membrane filters. Gaussian integrals were employed to determine the amounts of β-sheet and their abundance ratios by curve fitting of attenuated total reflection Fourier transform infrared spectra.
Results:
Adsorbed heat-treated INS onto the FCP membrane filters showed a β-sheet form, with a similar or higher affinity in comparison with that of the β-rich concanavalin A. The adsorption followed a sigmoidal curve with a 2-hour lag time, reaching a plateau after 4–5 hours. The spectral patterns of the adsorbed INS indicated the β-sheet form, demonstrating that INS transformed into β-sheet and then, or simultaneously, adsorbed onto the FCP membrane filters.
Conclusions:
The results regarding the rate and strength of amyloid fibril formation for each FCP membrane filter suggest that, beyond the membrane’s surface hydrophobicity or hydrophilicity, other factors, such as the electron affinity of hydrogen in the PVDF membrane, also influence nucleation. This study provides insight into the role of INS in amyloid fibril formation within FCP membrane filters.
DOI: https://doi.org/10.37349/ebmx.2026.101357

Hydrogels are among the most intensively studied biomaterials for controlled drug delivery, yet translation to routine clinical practice has been limited by rapid diffusion of small molecules and instability of biologics. In their recent report in Nature Nanotechnology (Pogostin et al., 2025, DOI: 10.1038/s41565-025-01981-6), a team from Rice University and collaborators present a nanofibrous supramolecular peptide hydrogel system that addresses these challenges through the incorporation of dynamic covalent chemistry. The SABER (Self-Assembling Boronate Ester Release) platform introduces reversible boronate ester bonds between engineered peptide fibers and boronic acid modified therapeutics, creating a tunable and long-acting drug release system. Proof-of-concept applications included tuberculosis therapy, diabetes management, and prolonged antibody delivery, demonstrating both versatility and clinical relevance. In this Commentary, I situate this advance within the broader trajectory of hydrogel research, highlight the conceptual novelty of dynamic supramolecular interactions, and discuss the opportunities and challenges for clinical translation. I argue that this platform signals a paradigm shift in drug delivery, moving hydrogels from passive depots to dynamic partners in medicine.
Hydrogels are among the most intensively studied biomaterials for controlled drug delivery, yet translation to routine clinical practice has been limited by rapid diffusion of small molecules and instability of biologics. In their recent report in Nature Nanotechnology (Pogostin et al., 2025, DOI: 10.1038/s41565-025-01981-6), a team from Rice University and collaborators present a nanofibrous supramolecular peptide hydrogel system that addresses these challenges through the incorporation of dynamic covalent chemistry. The SABER (Self-Assembling Boronate Ester Release) platform introduces reversible boronate ester bonds between engineered peptide fibers and boronic acid modified therapeutics, creating a tunable and long-acting drug release system. Proof-of-concept applications included tuberculosis therapy, diabetes management, and prolonged antibody delivery, demonstrating both versatility and clinical relevance. In this Commentary, I situate this advance within the broader trajectory of hydrogel research, highlight the conceptual novelty of dynamic supramolecular interactions, and discuss the opportunities and challenges for clinical translation. I argue that this platform signals a paradigm shift in drug delivery, moving hydrogels from passive depots to dynamic partners in medicine.
DOI: https://doi.org/10.37349/ebmx.2026.101356

Luminescent markers have been widely used in medicine, biology, agrotechnology, and for marking nuclear wastes and consumer goods. The high sensitivity and selectivity of the markers/labels allow the detection of various substances and the obtaining of valuable information about the distribution of constituents in specific media. This review describes the state of the art in luminescent marking/labeling of various cellulose forms, including nanosized ones, cellulose derivatives, and cellulose-containing materials. The importance of this consideration is explained by the role of cellulose and its derivatives in human life and their overall impact on mankind’s development. The structure and luminescence properties of cellulose and other related materials and cellulose derivatives are discussed from the viewpoint of cellulose luminescent “self-labeling”. It is shown that dyes, organic molecules, and organic-inorganic complexes, as well as inorganic dielectric and semiconductor micro/nanoparticles, can be effectively applied for the purposes of cellulose luminescent marking/labeling. This review discusses various application examples and explains the performance and mechanisms of various systems labeling (e.g., dye-cellulose, quantum dot-cellulose complex) in these applications. The review not only comprehensively summarizes existing approaches to luminescent labeling of cellulose-containing materials. It also highlights problematic issues that arise for developers of new luminescent markers (quenching of luminescence in an aqueous environment, the need to functionalize the luminescent marker material, etc.). At the same time, this work demonstrates the prospects for luminescent labeling data in modern digital technologies, particularly in the Internet of Things (IoT).
Luminescent markers have been widely used in medicine, biology, agrotechnology, and for marking nuclear wastes and consumer goods. The high sensitivity and selectivity of the markers/labels allow the detection of various substances and the obtaining of valuable information about the distribution of constituents in specific media. This review describes the state of the art in luminescent marking/labeling of various cellulose forms, including nanosized ones, cellulose derivatives, and cellulose-containing materials. The importance of this consideration is explained by the role of cellulose and its derivatives in human life and their overall impact on mankind’s development. The structure and luminescence properties of cellulose and other related materials and cellulose derivatives are discussed from the viewpoint of cellulose luminescent “self-labeling”. It is shown that dyes, organic molecules, and organic-inorganic complexes, as well as inorganic dielectric and semiconductor micro/nanoparticles, can be effectively applied for the purposes of cellulose luminescent marking/labeling. This review discusses various application examples and explains the performance and mechanisms of various systems labeling (e.g., dye-cellulose, quantum dot-cellulose complex) in these applications. The review not only comprehensively summarizes existing approaches to luminescent labeling of cellulose-containing materials. It also highlights problematic issues that arise for developers of new luminescent markers (quenching of luminescence in an aqueous environment, the need to functionalize the luminescent marker material, etc.). At the same time, this work demonstrates the prospects for luminescent labeling data in modern digital technologies, particularly in the Internet of Things (IoT).
DOI: https://doi.org/10.37349/ebmx.2025.101353
This article belongs to the special issue Nature-Based Biomaterials for Biomedical Applications

DOI: https://doi.org/10.37349/ebmx.2025.101354
This article belongs to the special issue Bioinspired Material for Regenerative Medicine

Aim:
Osimertinib’s clinical application is limited by poor aqueous solubility and systemic toxicity. Nano-niosomal formulations can address these challenges by providing controlled release and enhancing delivery. To develop and systematically evaluate nano-niosomal formulations of osimertinib using different surfactants, focusing on physicochemical characteristics, release kinetics, and cytotoxic activity.
Methods:
Four niosomal formulations were prepared using Span 60, Tween 60, Pluronic F-127, and Brij 52 (each at a 1:1 cholesterol-to-surfactant ratio). Particle size, zeta potential, and entrapment efficiency were measured. In vitro drug release was analyzed using Franz diffusion cells and fitted to standard kinetic models. Cytotoxicity was assessed by MTT assay in KAIMRC-2, MDA-MB231, and HCT-116 cell lines. Vesicle morphology was visualized by transmission electron microscopy.
Results:
All nano-niosomal formulations showed nanoscale particle sizes (47–292 nm), negative zeta potentials (−18.7 to −26.5 mV), and high entrapment efficiencies (69.8%–76.2%). Release studies indicated Span 60, Tween 60, and Pluronic F-127 followed diffusion-controlled kinetics (Higuchi/Korsmeyer–Peppas model, R2 up to 0.97), while Brij 52 provided a sustained zero-order release (R2 = 0.98). Compared to free osimertinib, all niosomal systems significantly prolonged release. Cytotoxicity studies demonstrated that all formulations enhanced anti-cancer effects, with Span 60-based niosomes exhibiting the greatest potency across cell lines.
Conclusions:
Optimized nano-niosomal encapsulation of osimertinib enables sustained and controlled drug release, improved cellular uptake, and enhanced cytotoxicity in vitro. Differences in surfactant composition critically influence formulation performance, supporting the further development of niosomal osimertinib as a promising strategy for oncological drug delivery applications.
Aim:
Osimertinib’s clinical application is limited by poor aqueous solubility and systemic toxicity. Nano-niosomal formulations can address these challenges by providing controlled release and enhancing delivery. To develop and systematically evaluate nano-niosomal formulations of osimertinib using different surfactants, focusing on physicochemical characteristics, release kinetics, and cytotoxic activity.
Methods:
Four niosomal formulations were prepared using Span 60, Tween 60, Pluronic F-127, and Brij 52 (each at a 1:1 cholesterol-to-surfactant ratio). Particle size, zeta potential, and entrapment efficiency were measured. In vitro drug release was analyzed using Franz diffusion cells and fitted to standard kinetic models. Cytotoxicity was assessed by MTT assay in KAIMRC-2, MDA-MB231, and HCT-116 cell lines. Vesicle morphology was visualized by transmission electron microscopy.
Results:
All nano-niosomal formulations showed nanoscale particle sizes (47–292 nm), negative zeta potentials (−18.7 to −26.5 mV), and high entrapment efficiencies (69.8%–76.2%). Release studies indicated Span 60, Tween 60, and Pluronic F-127 followed diffusion-controlled kinetics (Higuchi/Korsmeyer–Peppas model, R2 up to 0.97), while Brij 52 provided a sustained zero-order release (R2 = 0.98). Compared to free osimertinib, all niosomal systems significantly prolonged release. Cytotoxicity studies demonstrated that all formulations enhanced anti-cancer effects, with Span 60-based niosomes exhibiting the greatest potency across cell lines.
Conclusions:
Optimized nano-niosomal encapsulation of osimertinib enables sustained and controlled drug release, improved cellular uptake, and enhanced cytotoxicity in vitro. Differences in surfactant composition critically influence formulation performance, supporting the further development of niosomal osimertinib as a promising strategy for oncological drug delivery applications.
DOI: https://doi.org/10.37349/ebmx.2025.101355

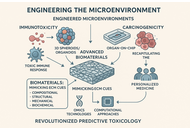
The challenges of conventional animal models and two-dimensional (2D) in vitro cell cultures in effectively forecasting human toxicity have prompted a significant shift towards New Approach Methodologies (NAMs). This development centers on advanced humanized in vitro co-culture models that offer improved physiological relevance for toxicological assessment. This perspective highlights the critical role of biomaterials in the creation of complex microenvironments. This study demonstrates how biomaterials effectively mimic the original extracellular matrix (ECM) through controlled compositional, structural, mechanical, and biochemical signals, thereby enabling the development of sophisticated 3D spheroids, organoids, and Organ-on-Chip systems. These biomaterial-enhanced platforms are essential for precise evaluation of immunotoxicity, as they promote human-specific immune responses and targeted immunomodulation, and for carcinogenicity, as they accurately replicate the tumor microenvironment, affect cancer cell behavior, and enable patient-derived models. Moreover, we underscore the synergistic amalgamation of these biomaterial-based models with omics technologies and computational methodologies (QSAR, AI/ML) for thorough molecular insights and rational design. Despite ongoing challenges in standardization and high-throughput compatibility, the strategic utilization of biomaterials is set to transform predictive toxicology, expedite drug discovery, and promote personalized medicine, thereby diminishing dependence on animal testing and improving human safety.
The challenges of conventional animal models and two-dimensional (2D) in vitro cell cultures in effectively forecasting human toxicity have prompted a significant shift towards New Approach Methodologies (NAMs). This development centers on advanced humanized in vitro co-culture models that offer improved physiological relevance for toxicological assessment. This perspective highlights the critical role of biomaterials in the creation of complex microenvironments. This study demonstrates how biomaterials effectively mimic the original extracellular matrix (ECM) through controlled compositional, structural, mechanical, and biochemical signals, thereby enabling the development of sophisticated 3D spheroids, organoids, and Organ-on-Chip systems. These biomaterial-enhanced platforms are essential for precise evaluation of immunotoxicity, as they promote human-specific immune responses and targeted immunomodulation, and for carcinogenicity, as they accurately replicate the tumor microenvironment, affect cancer cell behavior, and enable patient-derived models. Moreover, we underscore the synergistic amalgamation of these biomaterial-based models with omics technologies and computational methodologies (QSAR, AI/ML) for thorough molecular insights and rational design. Despite ongoing challenges in standardization and high-throughput compatibility, the strategic utilization of biomaterials is set to transform predictive toxicology, expedite drug discovery, and promote personalized medicine, thereby diminishing dependence on animal testing and improving human safety.
DOI: https://doi.org/10.37349/ebmx.2025.101351
This article belongs to the special issue Bioinspired Material for Regenerative Medicine

Aim:
Peripheral nerve injuries (PNIs) often result in a diminished quality of life for those affected and are the most common nervous system injury, with limited treatment options. Regenerative medicine presents novel biomaterial and cell-based therapies to repair the damaged tissue. Graphene oxide (GO), and mesenchymal stem cells (MSCs) have the potential to serve as components to treat PNI. This study evaluates the systemic toxicity of GO and xenogenic human MSCs by analyzing the peripheral blood immune phenotype when a novel nerve guidance conduit (NGC) is implanted in a rat model for six months.
Methods:
A 10-mm long sciatic nerve defect model was created in 8–10-week-old Lewis rats. Four treatment groups were generated: autograft (positive control), poly (lactic-co-glycolic acid) (PLGA) NGC, PLGA NGC with 0.25% GO, and PLGA/GO NGC seeded with 1 × 106 human adipose-derived MSCs. Tail blood was collected before surgery, and at 24 hours, 2 weeks, 2, 3, 5, and 6 months after surgery. Hematological analyses were carried out to evaluate systemic changes, if any, in peripheral immune cell types, namely, T lymphocytes, B lymphocytes, natural killer cells, and macrophages. The treated and contralateral sciatic nerves were excised, paraffin embedded, sectioned, and H&E stained, to identify any local foreign body rejection.
Results:
Treatment groups with GO and MSCs displayed percent total values of peripheral immune cells equivalent to the autograft at each time point. There was no evidence of an inflammatory response in the histological samples.
Conclusions:
The lack of changes in immune phenotype demonstrates a lack of nanotoxicity of the graphene nanoparticles and no evidence of adverse effects due to the MSCs. This was further supported by a lack of local foreign body response at the site of implantation. Overall, the PLGA/GO NGC + MSCs construct is biocompatible for six months in a rat PNI model, exhibiting a potential for clinical translation.
Aim:
Peripheral nerve injuries (PNIs) often result in a diminished quality of life for those affected and are the most common nervous system injury, with limited treatment options. Regenerative medicine presents novel biomaterial and cell-based therapies to repair the damaged tissue. Graphene oxide (GO), and mesenchymal stem cells (MSCs) have the potential to serve as components to treat PNI. This study evaluates the systemic toxicity of GO and xenogenic human MSCs by analyzing the peripheral blood immune phenotype when a novel nerve guidance conduit (NGC) is implanted in a rat model for six months.
Methods:
A 10-mm long sciatic nerve defect model was created in 8–10-week-old Lewis rats. Four treatment groups were generated: autograft (positive control), poly (lactic-co-glycolic acid) (PLGA) NGC, PLGA NGC with 0.25% GO, and PLGA/GO NGC seeded with 1 × 106 human adipose-derived MSCs. Tail blood was collected before surgery, and at 24 hours, 2 weeks, 2, 3, 5, and 6 months after surgery. Hematological analyses were carried out to evaluate systemic changes, if any, in peripheral immune cell types, namely, T lymphocytes, B lymphocytes, natural killer cells, and macrophages. The treated and contralateral sciatic nerves were excised, paraffin embedded, sectioned, and H&E stained, to identify any local foreign body rejection.
Results:
Treatment groups with GO and MSCs displayed percent total values of peripheral immune cells equivalent to the autograft at each time point. There was no evidence of an inflammatory response in the histological samples.
Conclusions:
The lack of changes in immune phenotype demonstrates a lack of nanotoxicity of the graphene nanoparticles and no evidence of adverse effects due to the MSCs. This was further supported by a lack of local foreign body response at the site of implantation. Overall, the PLGA/GO NGC + MSCs construct is biocompatible for six months in a rat PNI model, exhibiting a potential for clinical translation.
DOI: https://doi.org/10.37349/ebmx.2025.101352

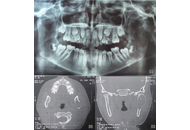
Aim:
To evaluate the precision of computer-assisted surgery simulation in mandibular condyle reconstruction using a costochondral graft.
Methods:
Ten patients (mean age: 14.5 years) with temporomandibular joint (TMJ) pathology and associated pain were included in the study. All patients underwent TMJ reconstruction using costochondral grafts planned through computer-assisted surgical simulation. Preoperative assessment included mouth opening, facial asymmetry, and the differences between planned and actual mandibular positioning.
Results:
Postoperative mouth opening was significantly improved in all patients, and facial profile modifications were enhanced. The site of the costochondral graft relative to the glenoid fossa was found to be satisfactory in postoperative radiographs, computed tomography images, and quantitative analysis.
Conclusions:
The results of this study demonstrate that virtual surgical planning combined with 3D-printed guiding templates enhanced treatment planning, provided precise osteotomy guidance, facilitated accurate repositioning of bony segments, and improved the contouring of mandibular anatomy in the management of TMJ deformities (ClinicalTrials.gov identifier: NCT06811415).
Aim:
To evaluate the precision of computer-assisted surgery simulation in mandibular condyle reconstruction using a costochondral graft.
Methods:
Ten patients (mean age: 14.5 years) with temporomandibular joint (TMJ) pathology and associated pain were included in the study. All patients underwent TMJ reconstruction using costochondral grafts planned through computer-assisted surgical simulation. Preoperative assessment included mouth opening, facial asymmetry, and the differences between planned and actual mandibular positioning.
Results:
Postoperative mouth opening was significantly improved in all patients, and facial profile modifications were enhanced. The site of the costochondral graft relative to the glenoid fossa was found to be satisfactory in postoperative radiographs, computed tomography images, and quantitative analysis.
Conclusions:
The results of this study demonstrate that virtual surgical planning combined with 3D-printed guiding templates enhanced treatment planning, provided precise osteotomy guidance, facilitated accurate repositioning of bony segments, and improved the contouring of mandibular anatomy in the management of TMJ deformities (ClinicalTrials.gov identifier: NCT06811415).
DOI: https://doi.org/10.37349/ebmx.2025.101350

Graphene-based nanomaterials are promising candidates for neuromuscular regeneration due to their electrical conductivity, mechanical strength, and functionalizability. In this perspective, reduced graphene oxide (rGO) nanocomposites decorated with gold nanoparticles (AuNPs) or silver nanoparticles (AgNPs) were synthesized via a one-step green process using Camellia sinensis (tea) extracts. The extracts acted as reducing and stabilizing agents and left bioactive catechins and polyphenols adsorbed on the graphene surface. The resulting nanocomposites combined structural support, electrical conductivity, and bioactive molecular modulation. rGO can provide scaffolding for cell growth, while the retained plant metabolites contributed antioxidant and anti-inflammatory effects. Incorporation of metallic nanoparticles enhanced mechanical strength, surface reactivity, and antimicrobial properties. These multifunctional graphene-metal nanocomposites offer a sustainable and biocompatible platform for guiding neuromuscular regeneration and represent a promising basis for future clinical translation.
Graphene-based nanomaterials are promising candidates for neuromuscular regeneration due to their electrical conductivity, mechanical strength, and functionalizability. In this perspective, reduced graphene oxide (rGO) nanocomposites decorated with gold nanoparticles (AuNPs) or silver nanoparticles (AgNPs) were synthesized via a one-step green process using Camellia sinensis (tea) extracts. The extracts acted as reducing and stabilizing agents and left bioactive catechins and polyphenols adsorbed on the graphene surface. The resulting nanocomposites combined structural support, electrical conductivity, and bioactive molecular modulation. rGO can provide scaffolding for cell growth, while the retained plant metabolites contributed antioxidant and anti-inflammatory effects. Incorporation of metallic nanoparticles enhanced mechanical strength, surface reactivity, and antimicrobial properties. These multifunctional graphene-metal nanocomposites offer a sustainable and biocompatible platform for guiding neuromuscular regeneration and represent a promising basis for future clinical translation.
DOI: https://doi.org/10.37349/ebmx.2025.101349
This article belongs to the special issue Green Nanoparticles for Biomedical Applications

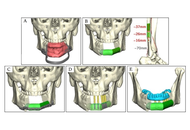
Indications for resection of maxillofacial and mandibular skeletal structures include extirpation of benign and malignant tumors, trauma, and congenital defects. Reconstruction of these structures often demands free tissue transfer incorporating bone and/or soft tissue with placement of rigid titanium implants to span the bony defect and anchor the autologous bone. Historically, such implants were mass-produced in standard formats, requiring manual bending during surgery to the patient’s specific bony anatomy. Recent technological and manufacturing advancements have permitted the use of three-dimensional (3D) printed, patient-specific maxillofacial and mandibular reconstructive prosthetics and implants. Preoperative 3D printing of patient-specific prosthetics and implants composed of titanium has revolutionized maxillofacial and mandibular reconstructive surgery and has been associated with improvements in operative efficiency, enhanced functional outcomes, and reduced complication rates in early studies. Herein, we review the history and current state of metal 3D printing of prosthetics and implants for head and neck oncologic reconstruction and posit future directions for innovation and surgical refinement in this area.
Indications for resection of maxillofacial and mandibular skeletal structures include extirpation of benign and malignant tumors, trauma, and congenital defects. Reconstruction of these structures often demands free tissue transfer incorporating bone and/or soft tissue with placement of rigid titanium implants to span the bony defect and anchor the autologous bone. Historically, such implants were mass-produced in standard formats, requiring manual bending during surgery to the patient’s specific bony anatomy. Recent technological and manufacturing advancements have permitted the use of three-dimensional (3D) printed, patient-specific maxillofacial and mandibular reconstructive prosthetics and implants. Preoperative 3D printing of patient-specific prosthetics and implants composed of titanium has revolutionized maxillofacial and mandibular reconstructive surgery and has been associated with improvements in operative efficiency, enhanced functional outcomes, and reduced complication rates in early studies. Herein, we review the history and current state of metal 3D printing of prosthetics and implants for head and neck oncologic reconstruction and posit future directions for innovation and surgical refinement in this area.
DOI: https://doi.org/10.37349/ebmx.2025.101348
This article belongs to the special issue Metal 3D Printing of Biometals for Prostheses and Implants

Aim:
The high cost and weight of conventional metal pylons used in lower-limb prostheses limit accessibility and increase patient burden. This study evaluated whether a 3D-printed, polylactic acid (PLA) prosthetic pylon, incorporating a biomimetic lattice, meets ISO 10328 mechanical requirements and can serve as a lightweight, cost-effective alternative to metal pylons.
Methods:
A lattice shell inspired by the Euplectella aspergillum sponge architecture was designed to envelop a cylindrical core to mitigate failure under compression and torsion. Pylons were fabricated by fused deposition modeling (FDM) using PLA at 25% infill with a net pylon radius of 6.66 mm. Mechanical testing followed ISO 10328 protocols and included ultimate static compression, torsion, and cyclic compression (dynamic) tests. Performance metrics recorded included ultimate load capacity, cycle endurance, safety factors for compression and torsion, gross mass, and production material usage.
Results:
Optimized PLA pylons passed all ISO 10328 tests with no structural failure or visible defects. The pylons sustained a maximum static compression load of 7,901 N (ISO target: 4,480 N), completed > 3 million cycles under dynamic loading without failure, and achieved safety factors of 2.69 (compression) and 2.15 (torsion). The 3D-printed units weighed ~282 g, approximately 30% lighter than comparable metal pylons (~400 g), and material/geometry optimization reduced material use and manufacturing cost.
Conclusions:
PLA-based, 3D-printed pylons with a biomimetic lattice architecture demonstrate sufficient mechanical integrity to satisfy ISO 10328 requirements and offer a lightweight, lower-cost alternative to traditional metal pylons. These findings support further in-vitro and in-vivo validation and highlight the potential for additive manufacturing to expand prosthetic accessibility—particularly in resource-limited settings.
Aim:
The high cost and weight of conventional metal pylons used in lower-limb prostheses limit accessibility and increase patient burden. This study evaluated whether a 3D-printed, polylactic acid (PLA) prosthetic pylon, incorporating a biomimetic lattice, meets ISO 10328 mechanical requirements and can serve as a lightweight, cost-effective alternative to metal pylons.
Methods:
A lattice shell inspired by the Euplectella aspergillum sponge architecture was designed to envelop a cylindrical core to mitigate failure under compression and torsion. Pylons were fabricated by fused deposition modeling (FDM) using PLA at 25% infill with a net pylon radius of 6.66 mm. Mechanical testing followed ISO 10328 protocols and included ultimate static compression, torsion, and cyclic compression (dynamic) tests. Performance metrics recorded included ultimate load capacity, cycle endurance, safety factors for compression and torsion, gross mass, and production material usage.
Results:
Optimized PLA pylons passed all ISO 10328 tests with no structural failure or visible defects. The pylons sustained a maximum static compression load of 7,901 N (ISO target: 4,480 N), completed > 3 million cycles under dynamic loading without failure, and achieved safety factors of 2.69 (compression) and 2.15 (torsion). The 3D-printed units weighed ~282 g, approximately 30% lighter than comparable metal pylons (~400 g), and material/geometry optimization reduced material use and manufacturing cost.
Conclusions:
PLA-based, 3D-printed pylons with a biomimetic lattice architecture demonstrate sufficient mechanical integrity to satisfy ISO 10328 requirements and offer a lightweight, lower-cost alternative to traditional metal pylons. These findings support further in-vitro and in-vivo validation and highlight the potential for additive manufacturing to expand prosthetic accessibility—particularly in resource-limited settings.
DOI: https://doi.org/10.37349/ebmx.2025.101347
This article belongs to the special issue Metal 3D Printing of Biometals for Prostheses and Implants

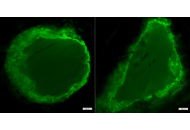
Modeling cancer cell invasion requires physiologically relevant systems, yet traditional 2D/3D assays and animal models fail to capture the biochemical and mechanical complexity of the human extracellular matrix (ECM). The human amniotic membrane (AM) is a clinically approved, abundant, and immunologically privileged tissue with a rich ECM composition and favorable mechanical properties. Despite its extensive use in regenerative medicine, its potential as a cancer invasion scaffold remains underexplored. We propose repurposing decellularized AM (dAM) as a human-derived ECM platform to study tumor invasion. dAM retains structural proteins, growth factor reservoirs, and stiffness gradients that influence epithelial-to-mesenchymal transition (EMT) and invasion pathways. Compared with conventional matrices, it offers improved biochemical fidelity and compatibility with patient-derived organoids. Key challenges, including donor variability, decellularization optimization, and reproducibility, are also addressed. dAM provides a non-invasive, scalable, and physiologically relevant tool for cancer invasion assays, drug screening, and patient-specific models. Its integration into oncology research may enhance translational relevance and accelerate personalized medicine.
Modeling cancer cell invasion requires physiologically relevant systems, yet traditional 2D/3D assays and animal models fail to capture the biochemical and mechanical complexity of the human extracellular matrix (ECM). The human amniotic membrane (AM) is a clinically approved, abundant, and immunologically privileged tissue with a rich ECM composition and favorable mechanical properties. Despite its extensive use in regenerative medicine, its potential as a cancer invasion scaffold remains underexplored. We propose repurposing decellularized AM (dAM) as a human-derived ECM platform to study tumor invasion. dAM retains structural proteins, growth factor reservoirs, and stiffness gradients that influence epithelial-to-mesenchymal transition (EMT) and invasion pathways. Compared with conventional matrices, it offers improved biochemical fidelity and compatibility with patient-derived organoids. Key challenges, including donor variability, decellularization optimization, and reproducibility, are also addressed. dAM provides a non-invasive, scalable, and physiologically relevant tool for cancer invasion assays, drug screening, and patient-specific models. Its integration into oncology research may enhance translational relevance and accelerate personalized medicine.
DOI: https://doi.org/10.37349/ebmx.2025.101346

Aim:
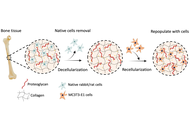
The decellularization process aims to remove cellular components from the tissues while preserving the ultrastructural composition of the extracellular matrix (ECM). Decellularization of bone is gaining attention as a biological scaffold due to its unique histoarchitecture, which consists of both organic and inorganic compounds. This study aims to develop a biological bone ECM using a novel decellularization method for bone regeneration.
Methods:
Rabbit and rat bone tissues were decellularized using a novel process that combines physical, chemical, and enzymatic methods with 0.1% SDS. Bone tissues were evaluated in terms of histology, biochemistry, and biomechanical tests, both before and after decellularization. Additionally, decellularized bone substitutes were recellularized with preosteoblast cells to assess the cytotoxic effect of the decellularization process.
Results:
Our method effectively removes cellular components while preserving both organic and inorganic compounds. We achieved a 95% in DNA content for rabbit bone and 92% for rat bone. The biochemical and biomechanical properties remained unchanged, and mineralization features were preserved after decellularization. The cell culture results revealed that decellularized bone extracellular matrix (dbECM) is biocompatible, bioactive, and provides a suitable environment for cell growth.
Conclusions:
This study demonstrates that our novel decellularization method effectively develops biological bone ECM containing both organic and inorganic compounds while utilizing minimal chemical concentration and incubation time. It is foreseen that the resulting decellularized bone could serve as a biological substitute, providing a favorable microenvironment for bone regeneration.
Aim:
The decellularization process aims to remove cellular components from the tissues while preserving the ultrastructural composition of the extracellular matrix (ECM). Decellularization of bone is gaining attention as a biological scaffold due to its unique histoarchitecture, which consists of both organic and inorganic compounds. This study aims to develop a biological bone ECM using a novel decellularization method for bone regeneration.
Methods:
Rabbit and rat bone tissues were decellularized using a novel process that combines physical, chemical, and enzymatic methods with 0.1% SDS. Bone tissues were evaluated in terms of histology, biochemistry, and biomechanical tests, both before and after decellularization. Additionally, decellularized bone substitutes were recellularized with preosteoblast cells to assess the cytotoxic effect of the decellularization process.
Results:
Our method effectively removes cellular components while preserving both organic and inorganic compounds. We achieved a 95% in DNA content for rabbit bone and 92% for rat bone. The biochemical and biomechanical properties remained unchanged, and mineralization features were preserved after decellularization. The cell culture results revealed that decellularized bone extracellular matrix (dbECM) is biocompatible, bioactive, and provides a suitable environment for cell growth.
Conclusions:
This study demonstrates that our novel decellularization method effectively develops biological bone ECM containing both organic and inorganic compounds while utilizing minimal chemical concentration and incubation time. It is foreseen that the resulting decellularized bone could serve as a biological substitute, providing a favorable microenvironment for bone regeneration.
DOI: https://doi.org/10.37349/ebmx.2025.101345
This article belongs to the special issue Nature-Based Biomaterials for Biomedical Applications

This review presents key molecular biology techniques used to investigate interactions between biomaterials and biological systems, emphasizing their role in evaluating biocompatibility and cellular responses. We focus on methodologies such as recombinant DNA technology, polymerase chain reaction (PCR), in situ hybridization, immunocytochemistry (ICC), and immunohistochemistry (IHC). These tools enable the detection and quantification of gene and protein expression, particularly those involved in inflammation and tissue regeneration, providing molecular-level insights into how cells respond to biomaterial cues. We discuss the relevance of these techniques in identifying inflammatory markers, tracking cell differentiation, and understanding tissue integration processes, as well as how their implementation faces technical challenges, including interference from the physicochemical properties of biomaterials, difficulties in sample preparation, and the standardization of protocols across different platforms. Addressing these limitations is vital to ensure data reliability and reproducibility. Looking ahead, we highlight emerging opportunities involving the integration of 3D imaging technologies and artificial intelligence to manage and interpret high-dimensional biological data. This article also serves as a practical tool for emerging investigators who are entering the field of biomaterials, offering accessible guidance on the selection and application of essential molecular biology techniques. These innovations promise to accelerate the rational design of biomaterials tailored to specific clinical applications and patient needs. In conclusion, molecular biology techniques provide a foundational toolkit for characterizing biological responses to biomaterials, supporting the development of safer and more effective therapeutic materials and empowering emerging investigators to contribute meaningfully to the next generation of biomedical solutions.
This review presents key molecular biology techniques used to investigate interactions between biomaterials and biological systems, emphasizing their role in evaluating biocompatibility and cellular responses. We focus on methodologies such as recombinant DNA technology, polymerase chain reaction (PCR), in situ hybridization, immunocytochemistry (ICC), and immunohistochemistry (IHC). These tools enable the detection and quantification of gene and protein expression, particularly those involved in inflammation and tissue regeneration, providing molecular-level insights into how cells respond to biomaterial cues. We discuss the relevance of these techniques in identifying inflammatory markers, tracking cell differentiation, and understanding tissue integration processes, as well as how their implementation faces technical challenges, including interference from the physicochemical properties of biomaterials, difficulties in sample preparation, and the standardization of protocols across different platforms. Addressing these limitations is vital to ensure data reliability and reproducibility. Looking ahead, we highlight emerging opportunities involving the integration of 3D imaging technologies and artificial intelligence to manage and interpret high-dimensional biological data. This article also serves as a practical tool for emerging investigators who are entering the field of biomaterials, offering accessible guidance on the selection and application of essential molecular biology techniques. These innovations promise to accelerate the rational design of biomaterials tailored to specific clinical applications and patient needs. In conclusion, molecular biology techniques provide a foundational toolkit for characterizing biological responses to biomaterials, supporting the development of safer and more effective therapeutic materials and empowering emerging investigators to contribute meaningfully to the next generation of biomedical solutions.
DOI: https://doi.org/10.37349/ebmx.2025.101344

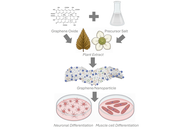
Aim:
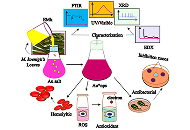
Plants possess tremendous medicinal properties which have been supposed to be promising candidates for biomedical applications, especially in the field of nanobiotechnology. To analyze one such view, the current study was adopted to synthesize gold nanoparticles (Au*nps) by employing the extract of Murraya koenigii (EMk) for the evaluation of phenolics, antioxidant, antimicrobial, hemolytic, and biocompatible activities.
Methods:
The synthesis process was carried out in a single step by mixing EMk and gold salt (Au salt) solution and monitored using UV/Visible spectroscopy. The process was optimized via variation in environmental variables. Characterization techniques such as Fourier transform infrared (FTIR) spectroscopy, transmission electron microscopy (TEM), scanning electron microscopy (SEM), X-ray diffractometer (XRD), and energy dispersive X-rays (EDX) were employed. In vitro biological activities (total phenolic, antioxidant, antimicrobial, and hemolytic) using different concentrations of Au*nps along with EMk were assessed. An in vivo histopathology study on Wistar rats was analyzed.
Results:
The band of Au*nps was observed at 540 nm, which showed successful synthesis. The FTIR spectra of Au*nps indicated the role of different functional groups (alkane, aromatic ester, thiol, nitro, and aldehyde) of EMk during synthesis. The TEM analysis illustrated a 50 nm size of Au*nps; SEM showed the presence of some aggregates; EDX represented elemental nature, and XRD proved the crystalline nature of these Au*nps. The Au*nps possessed significant phenolic content and displayed prominent antioxidant activities by quenching free radicals. Similarly, momentous inhibitory action was observed against microbial strains of Escherichia coli and Bacillus subtilis. The hemolytic study showed the least to non-toxic effect of these nanoparticles on red blood cells (RBCs) even at enhanced concentration. Histopathology study showed fair compatibility without inducing any apparent pathological lesions on the liver tissues of Wistar rats.
Conclusions:
Plausibly, all the above investigations strongly emphasized the use of medicinal plant-based Au*nps for biological applications.
Aim:
Plants possess tremendous medicinal properties which have been supposed to be promising candidates for biomedical applications, especially in the field of nanobiotechnology. To analyze one such view, the current study was adopted to synthesize gold nanoparticles (Au*nps) by employing the extract of Murraya koenigii (EMk) for the evaluation of phenolics, antioxidant, antimicrobial, hemolytic, and biocompatible activities.
Methods:
The synthesis process was carried out in a single step by mixing EMk and gold salt (Au salt) solution and monitored using UV/Visible spectroscopy. The process was optimized via variation in environmental variables. Characterization techniques such as Fourier transform infrared (FTIR) spectroscopy, transmission electron microscopy (TEM), scanning electron microscopy (SEM), X-ray diffractometer (XRD), and energy dispersive X-rays (EDX) were employed. In vitro biological activities (total phenolic, antioxidant, antimicrobial, and hemolytic) using different concentrations of Au*nps along with EMk were assessed. An in vivo histopathology study on Wistar rats was analyzed.
Results:
The band of Au*nps was observed at 540 nm, which showed successful synthesis. The FTIR spectra of Au*nps indicated the role of different functional groups (alkane, aromatic ester, thiol, nitro, and aldehyde) of EMk during synthesis. The TEM analysis illustrated a 50 nm size of Au*nps; SEM showed the presence of some aggregates; EDX represented elemental nature, and XRD proved the crystalline nature of these Au*nps. The Au*nps possessed significant phenolic content and displayed prominent antioxidant activities by quenching free radicals. Similarly, momentous inhibitory action was observed against microbial strains of Escherichia coli and Bacillus subtilis. The hemolytic study showed the least to non-toxic effect of these nanoparticles on red blood cells (RBCs) even at enhanced concentration. Histopathology study showed fair compatibility without inducing any apparent pathological lesions on the liver tissues of Wistar rats.
Conclusions:
Plausibly, all the above investigations strongly emphasized the use of medicinal plant-based Au*nps for biological applications.
DOI: https://doi.org/10.37349/ebmx.2025.101343
This article belongs to the special issue Green Nanoparticles for Biomedical Applications

Polymer-based nanoparticles have emerged as powerful multifunctional platforms in cancer theranostics, offering the ability to integrate diagnostic imaging and targeted therapy within a single system. These nanocarriers enable improved tumor localization, enhanced contrast agent delivery, and controlled therapeutic release, addressing limitations associated with conventional contrast agents such as poor specificity, rapid clearance, and systemic toxicity. Advances in polymer chemistry and nanoparticle fabrication methods, including solvent evaporation, nanoprecipitation, emulsion-diffusion, and emulsion polymerization, have allowed precise control over particle size, surface charge, and drug-loading efficiency, optimizing biodistribution and imaging performance. Hybrid polymer-inorganic nanoparticles further expand functionality by incorporating magnetic, optical, or radiopaque components, enabling multimodal imaging and stimuli-responsive drug release while maintaining biocompatibility. Key factors influencing the efficiency of polymer nanoparticle-based contrast agents include physicochemical properties such as particle size, morphology, surface functionalization, and responsiveness to tumor microenvironmental stimuli. These attributes collectively govern circulation time, cellular uptake, and accumulation in tumor tissues via passive and active targeting strategies. While promising, the clinical translation of these systems faces challenges including immunogenicity, pharmacokinetic variability, long-term safety concerns, and manufacturing scalability. Recent innovations in ligand functionalization, biomimetic coatings, and multifunctional nanoparticle design continue to advance therapeutic specificity and imaging precision, positioning polymer nanoparticles as versatile candidates for personalized oncologic care. This review provides a comprehensive synthesis of current methods for contrast agent integration, the role of physicochemical properties in performance, biological interactions, safety considerations, recent design innovations, translational barriers, and future research directions for polymer nanoparticle-based cancer theranostics.
Polymer-based nanoparticles have emerged as powerful multifunctional platforms in cancer theranostics, offering the ability to integrate diagnostic imaging and targeted therapy within a single system. These nanocarriers enable improved tumor localization, enhanced contrast agent delivery, and controlled therapeutic release, addressing limitations associated with conventional contrast agents such as poor specificity, rapid clearance, and systemic toxicity. Advances in polymer chemistry and nanoparticle fabrication methods, including solvent evaporation, nanoprecipitation, emulsion-diffusion, and emulsion polymerization, have allowed precise control over particle size, surface charge, and drug-loading efficiency, optimizing biodistribution and imaging performance. Hybrid polymer-inorganic nanoparticles further expand functionality by incorporating magnetic, optical, or radiopaque components, enabling multimodal imaging and stimuli-responsive drug release while maintaining biocompatibility. Key factors influencing the efficiency of polymer nanoparticle-based contrast agents include physicochemical properties such as particle size, morphology, surface functionalization, and responsiveness to tumor microenvironmental stimuli. These attributes collectively govern circulation time, cellular uptake, and accumulation in tumor tissues via passive and active targeting strategies. While promising, the clinical translation of these systems faces challenges including immunogenicity, pharmacokinetic variability, long-term safety concerns, and manufacturing scalability. Recent innovations in ligand functionalization, biomimetic coatings, and multifunctional nanoparticle design continue to advance therapeutic specificity and imaging precision, positioning polymer nanoparticles as versatile candidates for personalized oncologic care. This review provides a comprehensive synthesis of current methods for contrast agent integration, the role of physicochemical properties in performance, biological interactions, safety considerations, recent design innovations, translational barriers, and future research directions for polymer nanoparticle-based cancer theranostics.
DOI: https://doi.org/10.37349/ebmx.2025.101342

Aim:
Zinc is essential for normal bone growth and can promote bone regeneration. Processed human bone allograft treated with zinc shows improved bone formation activity. Various factors were tested for effects on zinc binding to bone allograft with the long-term goal of developing methods to enhance the bone formation activity and safety of bone allograft in orthopaedic applications.
Methods:
The amount of zinc bound to allograft was measured using Inductively Coupled Plasma-Mass Spectrometry (ICP-MS). Fluorescent visualization of zinc bound to allograft was accomplished using Zinpyr-1. The potential anti-microbial property of zinc-treated allograft was measured by exposing allograft to Staphylococcus aureus. After washing, the exposed allograft was cultured in bacterial media to measure residual Staphylococcus aureus. Data were analyzed using standard parametric methods.
Results:
Rapid binding of zinc to bone allograft (1–15 min) was relatively insensitive to zinc concentration, incubation time, pH, or divalent cation competition. In contrast, zinc salt counter ions had significant effects, with zinc acetate producing more rapid zinc binding than zinc chloride or zinc picolinate. The ability of Staphylococcus aureus to contaminate bone allograft was also significantly reduced by prior zinc treatment.
Conclusions:
The study results provide guidelines for modifying the processing of bone allograft to enhance bone formation activity while also improving the resistance of the allograft to bacterial contamination.
Aim:
Zinc is essential for normal bone growth and can promote bone regeneration. Processed human bone allograft treated with zinc shows improved bone formation activity. Various factors were tested for effects on zinc binding to bone allograft with the long-term goal of developing methods to enhance the bone formation activity and safety of bone allograft in orthopaedic applications.
Methods:
The amount of zinc bound to allograft was measured using Inductively Coupled Plasma-Mass Spectrometry (ICP-MS). Fluorescent visualization of zinc bound to allograft was accomplished using Zinpyr-1. The potential anti-microbial property of zinc-treated allograft was measured by exposing allograft to Staphylococcus aureus. After washing, the exposed allograft was cultured in bacterial media to measure residual Staphylococcus aureus. Data were analyzed using standard parametric methods.
Results:
Rapid binding of zinc to bone allograft (1–15 min) was relatively insensitive to zinc concentration, incubation time, pH, or divalent cation competition. In contrast, zinc salt counter ions had significant effects, with zinc acetate producing more rapid zinc binding than zinc chloride or zinc picolinate. The ability of Staphylococcus aureus to contaminate bone allograft was also significantly reduced by prior zinc treatment.
Conclusions:
The study results provide guidelines for modifying the processing of bone allograft to enhance bone formation activity while also improving the resistance of the allograft to bacterial contamination.
DOI: https://doi.org/10.37349/ebmx.2025.101341

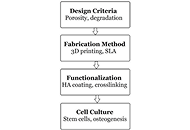
Bone tissue engineering (BTE) represents a cutting-edge approach to treating critical-sized bone defects, complex fractures, and degenerative bone diseases by promoting the regeneration of functional bone tissue. A crucial element in this process is the design and optimization of scaffolds that emulate the natural extracellular matrix (ECM), supporting cell adhesion, proliferation, and differentiation necessary for bone regeneration. Polymers are widely used in scaffold fabrication. They offer versatility, biocompatibility, and tunable properties that are essential for tissue engineering. This paper provides a comprehensive analysis of polymeric scaffolds in BTE, focusing on synthetic and natural polymers, composite scaffold designs, and the fabrication techniques employed to enhance their performance. Key design criteria, such as scaffold porosity, mechanical properties, and biodegradability, are discussed in the context of facilitating optimal bone regeneration. Additionally, we explore functionalization strategies to improve biological interactions, such as the incorporation of growth factors and surface modifications, and evaluate in vivo performance to highlight clinical potential. The paper also addresses current challenges, including the need for enhanced mechanical strength and controlled degradation, while offering insights into future directions for the development of polymeric scaffolds in bone tissue regeneration therapies.
Bone tissue engineering (BTE) represents a cutting-edge approach to treating critical-sized bone defects, complex fractures, and degenerative bone diseases by promoting the regeneration of functional bone tissue. A crucial element in this process is the design and optimization of scaffolds that emulate the natural extracellular matrix (ECM), supporting cell adhesion, proliferation, and differentiation necessary for bone regeneration. Polymers are widely used in scaffold fabrication. They offer versatility, biocompatibility, and tunable properties that are essential for tissue engineering. This paper provides a comprehensive analysis of polymeric scaffolds in BTE, focusing on synthetic and natural polymers, composite scaffold designs, and the fabrication techniques employed to enhance their performance. Key design criteria, such as scaffold porosity, mechanical properties, and biodegradability, are discussed in the context of facilitating optimal bone regeneration. Additionally, we explore functionalization strategies to improve biological interactions, such as the incorporation of growth factors and surface modifications, and evaluate in vivo performance to highlight clinical potential. The paper also addresses current challenges, including the need for enhanced mechanical strength and controlled degradation, while offering insights into future directions for the development of polymeric scaffolds in bone tissue regeneration therapies.
DOI: https://doi.org/10.37349/ebmx.2025.101340
This article belongs to the special issue Bioinspired Material for Regenerative Medicine
 Previous
Previous