Affiliation:
Department of Electrical and Computer Engineering, North Dakota State University, Fargo, ND 58108-6050, USA
Affiliation:
Department of Electrical and Computer Engineering, North Dakota State University, Fargo, ND 58108-6050, USA
ORCID: https://orcid.org/0009-0009-9288-3931
Affiliation:
Department of Electrical and Computer Engineering, North Dakota State University, Fargo, ND 58108-6050, USA
Email: Ivan.Lima@ndsu.edu
ORCID: https://orcid.org/0000-0002-5184-3695
Explor BioMat-X. 2025;2:101333 DOI: https://doi.org/10.37349/ebmx.2025.101333
Received: December 01, 2024 Accepted: March 26, 2025 Published: April 01, 2025
Academic Editor: Feng Chen, Zhejiang University of Technology, China
The article belongs to the special issue Trends in Biomaterials Research for Cardiovascular Applications
Aim: To show that a wireless-powered thrombolytic filter can be used in the treatment of venous thromboembolism (VTE) as an alternative to the existing VTE therapies, which have serious side effects.
Methods: The wireless-powered thrombolytic filter that we propose combines the positive attributes of anticoagulants and thrombolytics, through the capture and dissolution of blood clots, without the associated adverse effects of existing treatments. The filter absorbs radio-frequency energy from a source and converts it into heat at the thrombolytic filter.
Results: We used computer simulations with COMSOL and lab experiments to demonstrate that a wireless-powered thrombolytic filter can be heated up through the absorption of radio-frequency energy from an external source.
Conclusions: We demonstrate that a wireless-powered thrombolytic filter has the potential to be used in the treatment of VTE, since it can be designed to absorb energy from an external radio-frequency source and convert it to heat that is sufficient to dissolve blood clots captured by the thrombolytic filter.
Deep vein thrombosis (DVT) occurs when a blood clot forms in a deep vein, usually in the legs, while pulmonary embolism (PE) happens when a clot travels to the lungs, blocking blood flow. Together, these conditions are known as venous thromboembolism (VTE), which is one of the most serious vascular disorders, second only to heart attacks and strokes [1–3]. The U.S. Surgeon General has recognized VTE as a major public health concern due to its increasing incidence and rising treatment costs [4]. According to the American Heart Association, VTE affects over 900,000 Americans annually, with an incidence rate ranging from 142 to over 300 cases per 100,000 person-years. The average predicted cost of VTE treatment is $62,838, significantly higher than the $24,464 average cost for treating active cancer patients [5, 6].
While anticoagulants, also known as blood thinners, such as warfarin (Coumadin), apixaban (Eliquis), and rivaroxaban (Xarelto) are the standard treatment for VTE, these medications do not dissolve existing thromboembolisms [7, 8]. Instead, they work by preventing new clots from forming and existing ones from growing, by inhibiting various steps in the coagulation cascade [9]. However, these therapies come with significant limitations and side effects, which are tolerated due to the severe risks posed by untreated VTEs. Contraindications, such as pregnancy, active ulcers (open sores in the stomach or intestines that can bleed), aneurysms (weakened, bulging areas in blood vessels that risk rupture), and recent surgery, along with the demanding maintenance required for these medications, contribute to high rates of noncompliance and reduced quality of life for patients [10–12]. These challenges highlight the need for more effective, patient-friendly treatments.
When anticoagulants are contraindicated, alternative treatment options for VTE include catheter-directed thrombolysis (CDT), pharmaco-mechanical thrombectomy (PMT), aspiration thrombectomy, and inferior vena cava (IVC) filters. CDT involves delivering clot-dissolving drugs directly to the site of a thrombus using a catheter, accelerating clot breakdown while minimizing systemic bleeding risks [13]. While effective in acute DVT cases, CDT requires hospitalization, imaging guidance, and carries risks such as bleeding complications and post-thrombotic syndrome [13]. PMT combines mechanical clot disruption with the targeted delivery of thrombolytics, offering faster and more effective clot removal than CDT alone, but it still poses bleeding risks [14]. Aspiration thrombectomy, using suction to remove clots, avoids the need for thrombolytic drugs, making it suitable for patients at high risk of bleeding, though it may be less effective for large clots [15].
Another treatment option for VTE patients is the use of an IVC filter. Typically made from stainless steel or platinum, these wire filters are designed to mechanically catch blood clots that may break loose from a vein before reaching the lungs [16]. Clinically available filters come in various models, with some intended for permanent use. Despite their structural durability, the median time before filter retrieval is just 1.9 months (25th percentile, 1.2 months; 75th percentile, 3.2 months; range: 0–108.3 months) [17]. Initially, these filters are highly effective, reducing the risk of PE by 78% (p value = 0.03). However, over time, they accumulate endothelial cells, scar tissue, foreign body granulomas, and small clots, which can cause turbulence and restrict blood flow [18–20]. This added stress on the filter and the vessel walls raises the risk of complications such as VTE recurrence, filter fracture, failure, and vessel puncture [18]. Notably, after two years, the filters no longer reduce the risk of PE and may even increase the risk of recurrent DVT [21, 22].
Both anticoagulants and IVC filters offer benefits that would be valuable in new treatment approaches, but their limitations must be addressed. A promising solution could be a wirelessly powered IVC filter, as illustrated in Figure 1, which incorporates resistive heating to actively break down blood clots. This approach would combine the clot-prevention advantages of anticoagulants with the mechanical filtration provided by traditional IVC filters. Unlike current treatments, this filter would avoid the side effects associated with anticoagulants and prevent the buildup of debris that often leads to filter failure. By integrating these features, the device could offer a more effective and reliable treatment for VTE.
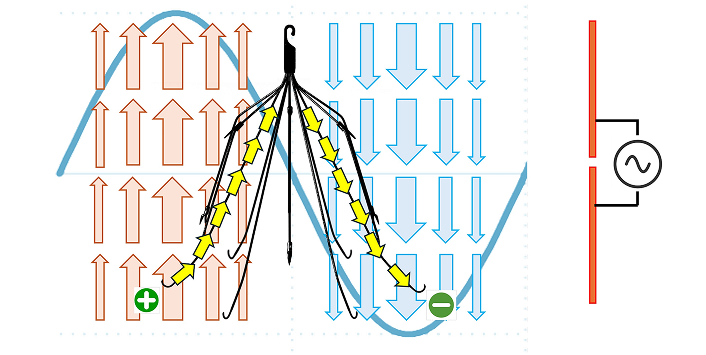
Wirelessly powered IVC Filter. A dipole antenna can generate an electromagnetic wave (blue sine wave) which can be transmitted through the IVC filter (in the middle, shown in black). The corresponding electric field vectors (red arrows and blue arrows), positive and negative charges (shown in green) and the induced current path (yellow arrows) produced by the electromagnetic wave are shown. IVC: inferior vena cava
The wirelessly powered IVC filter operates by harnessing a design in which its width is on the same order of magnitude as the wavelength of the incident electromagnetic wave. This enables the build-up of alternating positive and negative charges on opposite sides of the filter, induced by the incident electromagnetic wave, creating an electric potential difference and an electric current along the filter. As the wave propagates, the direction of current flow alternates, generating resistive heating as the material resists electron flow. This localized heating can be precisely controlled to target captured clots. Research has shown that at 55°C, fibrin—the main protein in blood clots—loses its structural integrity [23, 24]. At this temperature, the D fragments and cross-linking sites within fibrin denature, effectively breaking down the blood clot [23]. This resistive heating approach allows for the controlled degradation of clots without the risks of systemic anticoagulation.
In this study, we propose a novel wirelessly powered IVC filter that combines the mechanical filtration benefits of traditional filters with the clot-dissolution capabilities of resistive heating. Unlike existing treatments, this approach eliminates the systemic side effects of anticoagulants and addresses the structural and functional limitations of conventional IVC filters. The advantage of the use of a wireless-powered IVC filter treatment over the conventional IVC filter treatment is that the use of an external electromagnetic source to heat up a wireless-powered IVC can denature the organic matter captured by the IVC filter using a non-invasive procedure. Through comprehensive simulations and experimental validation, we demonstrate the feasibility of wireless power transfer to achieve localized heating sufficient to denature fibrin and dissolve thrombi. Additionally, we explore advancements in antenna design and energy efficiency to enhance the filter’s therapeutic potential while ensuring safety and compliance with established standard absorption rate (SAR) guidelines. This approach represents a significant step toward developing a more effective, minimally invasive, and patient-friendly treatment for VTE.
The MATLAB R2023a Antenna Toolbox and COMSOL Multi-Physics 5.3a software were used to create a 3D model of the IVC filters, human body, and transmitting antenna to simulate the wireless heating mechanism used for the denaturation of blood clots.
Following the simulations, clinically used IVC filter replicas were fabricated and tested in an anechoic chamber, a specialized room designed to completely absorb sound waves and eliminate echoes, to validate the simulation findings. The filters were constructed from surgical-grade stainless steel wire with a diameter of 0.5 mm. A surface-mount 450 Ω resistor was attached to the filter’s apex to match its impedance with the transmitting antenna and enhance current resistance, thereby increasing the resistive heating effect. Since the simulations were conducted at 4.55 GHz, which exceeded the capacity of the available radio-frequency equipment for the experiments, the modified filter used in the experiments was scaled to 2× its original size, allowing the operating frequency to be reduced to 2.275 GHz.
Previous studies have demonstrated that IVC filters can function as their own antennas, successfully receiving wireless power in an air-only environment [25, 26]. The primary objective of this study was to determine if similar efficiency could be achieved when human anatomy is introduced into the system. To evaluate this, tests were conducted in a manner similar to the air environment experiments, as depicted in Figure 2A, with the addition of anatomical models, as shown in Figure 2B.
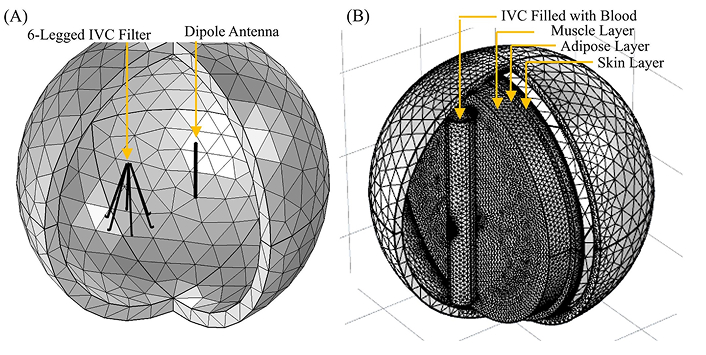
COMSOL simulation models for the IVC filter and dipole antenna at different environmental conditions. A: Mesh rendering of the in-air simulation, showing the full 6-legged IVC filter alongside the dipole transmitting antenna; B: the filter and dipole antenna, at anatomical conditions. The IVC, filled with blood, is represented as the cylinder indicated by the black arrow surrounding the IVC filter. The muscle, adipose, and skin layers, through which the antenna transmits, are shown as disks. IVC: inferior vena cava
The symmetry of the filter results in two possible alignments with the transmitting antenna: alignment A, where the antenna is directly aligned with one of the filter legs, and alignment B, where it is positioned between two legs. Initial studies of alignment A (illustrated in Figure 2A) demonstrated an energy transfer efficiency of 17.86% to the filter. In contrast, the other configuration, alignment B (not depicted), yielded an efficiency of 12.68%. While these devices were not highly efficient, they successfully transferred sufficient energy to heat the apex of the filter to 59.2°C, as shown in Figure 3, exceeding the minimum required temperature of 55°C to denature blood clots. The desired temperature was maintained without significantly heating the filter’s attachment points to the blood vessel; specifically, a temperature increase of 4°C was observed at the connection to the vena cava, while the midpoint of the filter legs averaged an 8°C rise.
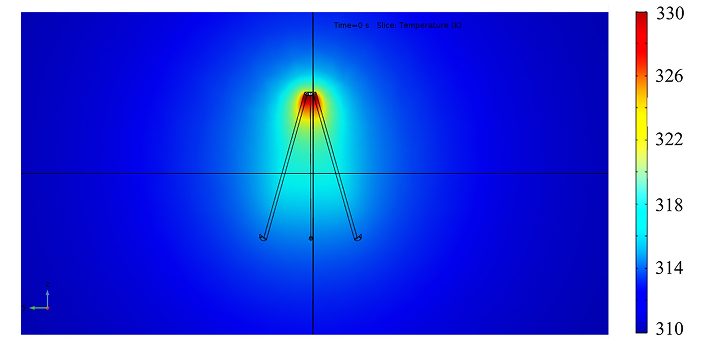
The temperature variation at the IVC filter on a plane that contains in an air-only environment. The color bar shows the temperature in Kelvin. The dipole antenna successfully transferred sufficient energy to heat the apex of the filter to 59.2°C, exceeding the minimum required temperature of 55°C to denature blood clots. IVC: inferior vena cava
However, the initial anatomical simulations produced unsatisfactory results, primarily because the wireless transmission rapidly loses energy as it traverses skeletal muscle. Further analysis revealed that adipose tissue contributes minimal energy loss or signal distortion. In contrast, skeletal and smooth muscles significantly scatter the transmitted wave, allowing only a limited amount of energy to reach the IVC filter, as illustrated in Figure 4. The transmitting dipole antenna used in all previous simulations was selected for its simplicity in calculations and simulations. However, its radiation pattern indicates that the dipole emits energy in a 360° doughnut shape, resulting in inefficient energy coupling with the filter. This inefficiency could be mitigated by employing a more directive antenna, such as a horn or patch antenna. By utilizing a more focused beam, low permittivity tissues like adipose, tendons, and bones could become more effective targets for energy transfer.
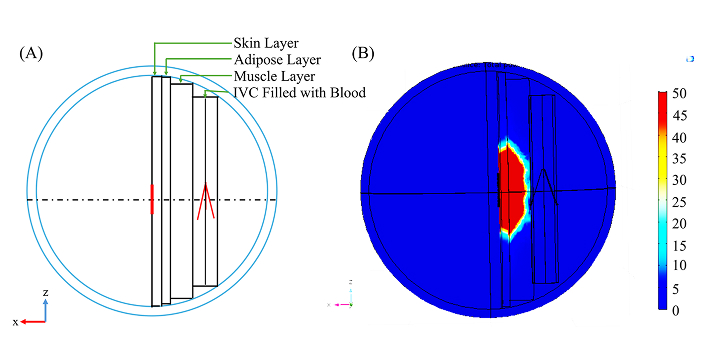
IVC filter and the dipole antenna at the anatomical conditions. A: 2-dimensional view (x-z) of the wire rendering of simulation anatomy; B: energy dissipation from the antenna in W/m3. Adipose tissue contributes minimal energy loss or signal distortion while skeletal and smooth muscles significantly scatter the transmitted wave, reducing the energy incident on the IVC filter. IVC: inferior vena cava
Patch antennas, also known as microstrip antennas, are low-profile designs that can be mounted flush against surfaces, such as the skin in this application. The patch consists of a flat piece of copper placed on top of a larger rectangular copper sheet, separated by a thin insulating layer. The planar design of the antenna results in a more focused, raindrop-like radiation pattern. After several simulations to optimize its dimensions, the new antenna achieved a transfer efficiency of 20.511% in air, marking a significant improvement over the transfer efficiency of the previously studied dipole antenna.
The simulations were then set up in the lab to be tested experimentally (shown in Figure 5). The antenna was radiating 6 cm from the side of the filter. The filter was held in position with an absorbing layer of foam that has negligible electromagnetic reflection. The Fluke Ti10 thermal camera was placed in a stand pointed towards the filter to monitor its temperature.

Anechoic chamber test set up for validation of the simulation results. The antenna was positioned 6 cm away from the side of the filter while emitting radiation. The filter was secured in place using a foam layer with minimal electromagnetic reflection. A Fluke Ti10 thermal camera, mounted on a stand and directed at the filter, was used to monitor its temperature
One limitation of this experiment was the 3 GHz upper frequency limit of the function generator. To address this limitation, a second filter was constructed, scaled up by a factor of two. This 2× scaling doubled the filter’s width, which in turn required a longer wavelength for operation and reduced the operating frequency to 2.27 GHz. When the experiment was repeated with this new configuration, a noticeable heating effect was observed in the filter, as shown in Figure 6. Segments of the filter experienced a temperature increase of 3.4°C from the initial temperature. This heating was achieved using only 2.2% of the simulated source power, effectively reaching and exceeding the 18°C temperature change predicted in the simulations. It is important to note that utilizing a higher power source could yield even greater temperature increases beyond the 18°C observed. Since the experiment used a wireless-powered IVC filter that was twice larger than the one suitable for the treatment of VTE patients, due to the limitation in the lab equipment available for the experimental verification, a scaled-down version of this IVC filter would have absorbed the same amount of heat over a twice smaller filter length, leading to twice-higher heat density than that obtained in this experiment.
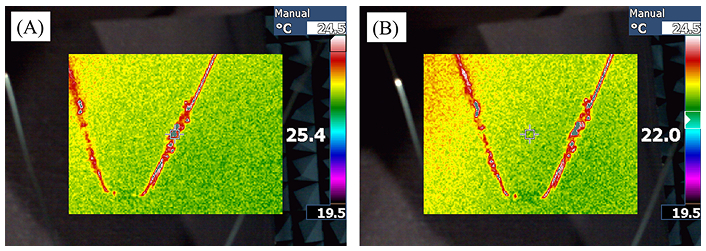
Infrared temperature readings of heated filters. A: the temperature reading of the filter; B: the ambient temperature. Certain sections of the filter exhibited a temperature rise of 3.4°C from the initial value. This heating was achieved using just 2.2% of the simulated source power, successfully reaching and surpassing the 18°C temperature increase predicted in the simulations
The proposed approach to enhance the longevity and efficacy of IVC filters through wireless power transmission has proven to be feasible. However, further research and testing are essential to develop this concept into a practical device. Current success has been observed under specific conditions, utilizing ideal materials, setups, and equipment. For wirelessly powered IVC filters to evolve into an accessible, at-home, long-term treatment option, future work must focus on addressing the energy transfer limitations associated with the filter and overcoming absorption in tissues through the proper choice of antenna configuration and emission wavelength.
A major concern with the wireless power approach is the SAR, which quantifies the radio frequency energy absorbed by human tissue [27, 28]. The Federal Communications Commission (FCC) has established a recommended limit of 0.08 W/kg for whole-body average exposure or 1.6 W/kg for the head or trunk [29, 30]. Similarly, the International Commission on Non-Ionizing Radiation Protection (ICNIRP) has set the same standard for full-body average exposure but a slightly higher recommendation limit of 2.0 W/kg for the head and trunk [31, 32]. Ensuring compliance with these safety standards will be critical in advancing the practical application of wirelessly powered IVC filters.
To achieve therapeutic heating of the filter, an input of approximately 13.99 W is adequate to meet the required power transfer while remaining well within the FCC’s SAR guidelines. This calculation considers that 51% of an average human’s mass—approximately 70 kg—is subject to the energy input [33]. If 100% of the 14 W transmitted toward the body were absorbed by an average torso weighing 35.7 kg, the resulting SAR would be 0.392 W/kg. This value represents only 24.5% of the FCC’s recommended safe level and 19.6% of the ICNIRP’s recommended limits for safe exposure.
To further minimize tissue exposure, a phased array of transmitting antennas could be employed. This approach allows for an increased energy level transmission without raising exposure in areas of tissue beyond the immediate vicinity of the filter, where the energy is specifically required. By concentrating the energy delivery around the filter, this method enhances the efficacy of therapeutic heating while maintaining compliance with safety guidelines.
In summary, this study demonstrates the feasibility of wireless power transmission as a method to enhance the functionality and longevity of IVC filters, marking a significant step toward their development as a safe and effective treatment option. The use of a wireless-powered IVC filter as an alternative to conventional IVC filters has the potential to significantly extend the lifetime of the clinical use of IVC filters in the prevention of blood clots. The procedure proposed consists of periodic preventive application of non-ionizing radiation in the GHz range to denature the organic matter that is captured by the IVC filter. Further studies need to be carried out to determine the frequency in which the patients need to carry out this procedure, which depends on the patient’s diagnosis. Since this procedure is non-invasive and uses non-ionizing radiation, the expected cost of this procedure is expected to a fraction of the cost of surgical procedures. Additional studies need to be carried out during the translation stage to determine the benefits of this technology, including the economic benefits and the improvement in the health of patients.
Even though the proposed approach satisfies current safety standards and achieves the necessary power levels for therapeutic heating, continued refinement is crucial to address energy transfer efficiency and tissue exposure concerns. The energy transferred from the source to the wireless-powered IVC filter depends on several factors, including the amount of body mass of the patient and the position of the IVC filter relative to the location and the direction of the applied non-ionizing radiation emitted by the source. To meet those requirements, this procedure will likely require the use of tomographic imaging. The dose of electromagnetic radiation can be determined based on the amount of the patient’s body mass between the source and the IVC filter, which can be quantified in a deterministic way based on the known principles of electromagnetic wave absorption in organic tissues. Moreover, the amount of energy required to successfully denature the organic matter captured by the IVC filters implanted in VTE patients need to be addressed during the translation studies.
With advancements in antenna technology, material optimization, and rigorous testing under diverse conditions, wirelessly powered IVC filters could transition from conceptual innovation to a transformative solution, offering improved patient outcomes and reducing the need for invasive procedures in the management of thromboembolic conditions.
CDT: catheter-directed thrombolysis
DVT: deep vein thrombosis
FCC: Federal Communications Commission
ICNIRP: International Commission on Non-Ionizing Radiation Protection
IVC: inferior vena cava
PE: pulmonary embolism
PMT: pharmaco-mechanical thrombectomy
SAR: standard absorption rate
VTE: venous thromboembolism
NGS: Conceptualization, Investigation, Writing—original draft. DW: Writing—original draft, Writing—review & editing. ITL: Conceptualization, Validation, Writing—review & editing, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
The authors acknowledge funding from the College of Engineering and from the Department of Electrical and Computer Engineering at North Dakota State University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3035
Download: 35
Times Cited: 0
Betül Gürbüz ... Erkan Türker Baran
