Affiliation:
1Department of Otolaryngology – Head & Neck Surgery, University of Pittsburgh Medical Center, Pittsburgh, PA 15213, USA
ORCID: https://orcid.org/0009-0006-5840-9994
Affiliation:
1Department of Otolaryngology – Head & Neck Surgery, University of Pittsburgh Medical Center, Pittsburgh, PA 15213, USA
Affiliation:
2Department of Radiology, University of Pittsburgh Medical Center, Pittsburgh, PA 15213, USA
Affiliation:
1Department of Otolaryngology – Head & Neck Surgery, University of Pittsburgh Medical Center, Pittsburgh, PA 15213, USA
ORCID: https://orcid.org/0000-0002-2570-1706
Affiliation:
1Department of Otolaryngology – Head & Neck Surgery, University of Pittsburgh Medical Center, Pittsburgh, PA 15213, USA
ORCID: https://orcid.org/0000-0001-6049-1508
Affiliation:
1Department of Otolaryngology – Head & Neck Surgery, University of Pittsburgh Medical Center, Pittsburgh, PA 15213, USA
ORCID: https://orcid.org/0000-0003-4282-0640
Affiliation:
1Department of Otolaryngology – Head & Neck Surgery, University of Pittsburgh Medical Center, Pittsburgh, PA 15213, USA
Email: spectorme@upmc.edu
ORCID: https://orcid.org/0000-0001-7646-6075
Explor BioMat-X. 2025;2:101348 DOI: https://doi.org/10.37349/ebmx.2025.101348
Received: July 22, 2025 Accepted: September 25, 2025 Published: October 12, 2025
Academic Editor: Rupinder Singh, National Institute of Technical Teachers Training and Research, India
The article belongs to the special issue Metal 3D Printing of Biometals for Prostheses and Implants
Indications for resection of maxillofacial and mandibular skeletal structures include extirpation of benign and malignant tumors, trauma, and congenital defects. Reconstruction of these structures often demands free tissue transfer incorporating bone and/or soft tissue with placement of rigid titanium implants to span the bony defect and anchor the autologous bone. Historically, such implants were mass-produced in standard formats, requiring manual bending during surgery to the patient’s specific bony anatomy. Recent technological and manufacturing advancements have permitted the use of three-dimensional (3D) printed, patient-specific maxillofacial and mandibular reconstructive prosthetics and implants. Preoperative 3D printing of patient-specific prosthetics and implants composed of titanium has revolutionized maxillofacial and mandibular reconstructive surgery and has been associated with improvements in operative efficiency, enhanced functional outcomes, and reduced complication rates in early studies. Herein, we review the history and current state of metal 3D printing of prosthetics and implants for head and neck oncologic reconstruction and posit future directions for innovation and surgical refinement in this area.
Common indications for resection of maxillofacial and mandibular skeletal structures include extirpation of benign and malignant tumors, trauma, and congenital defects [1]. Loss of such complex bony structures imparts significant functional and aesthetic sequelae, demanding immediate reconstruction with free tissue transfer incorporating bone and soft tissue [2]. The goals of maxillofacial reconstruction include restoration of oronasal separation and midface projection, maintenance of globe support, and rehabilitation of functional dentition [3]. Mandibular reconstruction prioritizes restoration of masticatory function, maintenance of the airway, support of speech and swallowing, and preservation of lower facial contour [4]. Often, these complex reconstructive goals are best achieved through free tissue transfer of autologous bone harvested from the fibula, scapula, or radial forearm.
Reconstruction of maxillofacial and mandibular skeletal defects requires placement of rigid reconstruction plates to span the bony defect and anchor the autologous bone [5]. Such implants, often titanium, ideally fulfill several important biomechanical criteria. First, their strength must be sufficient to withstand long-term occlusal force loading and plate stresses without weakening or fracture [6]. Second, their material must be durable and capable of promoting bony union and limiting resorption of native and autologous transferred bone [7]. Finally, their profile must be acceptable to minimize risk of overlying soft-tissue irritation and plate exposure, particularly overlying the mandible. Ultimately, optimization of these important biomechanical criteria maximizes the patient’s functional and cosmetic outcome and minimizes risk of dreaded hardware-related complications such as plate fracture, exposure, and fistula [8].
Historically, such implants for maxillofacial and mandibular reconstructions were mass-produced in standard formats via subtractive manufacturing [9]. These “stock plates” necessitate manual bending during surgery to the patient’s specific bony anatomy after the resection is completed. In the past few decades, vast technological and manufacturing improvements have permitted the use of three-dimensional (3D)-printed, patient-specific maxillofacial and mandibular reconstructive implants (PSIs) [10]. Such PSIs are custom, 3D-printed implants utilizing the individual patient’s pre-operative CT images and surgeon-informed resection plan [variably termed virtual surgical planning (VSP) or computer-assisted surgery (CAS) in the literature] [11]. In recent years, preoperative 3D printing of PSIs has revolutionized maxillofacial and mandibular reconstructive surgery and has been associated with improvements in operative efficiency, enhanced functional outcomes, and reduced complication rates in early studies [11, 12].
Herein, we review the history and current state of metal 3D printing of prosthetics and implants for head and neck oncologic reconstruction. Specific advantages of these techniques are highlighted for both maxillofacial and mandibular reconstructions. Finally, we posit future directions for innovation and surgical refinement incorporating 3D printed prosthetics and implants for head and neck oncologic reconstruction.
In the late 1980s and 1990s, free tissue transfers of autologous fibula and scapula bone to reconstruct complex maxillofacial and mandibular defects became popularized [13, 14]. For decades thereafter, the use of mass-produced titanium plates with universal configurations necessitating manual intraoperative bending to the patient’s bony anatomy predominated [15]. However, around the same time as the original descriptions of both the fibula and scapula free flaps, medical applications for 3D printing began to be explored [9]. Borrowing from the aerospace industry, Brix et al. [16] and Mankovich et al. [17] are credited with the first use of 3D printing for head and neck surgical applications. The latter utilized CT-guided stereolithography (SLA) to generate plastic models of patient-specific craniofacial anatomy for surgical planning and medical education. For the first few years after this initial report, surgical planning, medical education, and patient counseling remained the primary applications of 3D printing in head and neck reconstructive surgery [18]. At the turn of the century, 3D printing of osteotomy guides and occlusal splints emerged, improving accuracy, and reducing operative time for bony reconstructions of the maxillofacial and mandibular skeleton [19].
In the 2010s, the additive manufacturing (AM) technique of 3D printing emerged and ushered in a new era of precision reconstruction of maxillofacial and mandibular skeletal defects [20]. AM, in which complex objects are fabricated by the sequential addition of metallic layers, permitted production of metal prostheses and implants with highly complex geometries and microarchitectures and hollow spaces (e.g., screw holes) [21]. AM, supported by reduced production costs and improved design software, facilitated a revolution in head and neck reconstructive surgery [22]. In addition to 3D printed anatomic models for surgical planning and medical education, 3D printing of metal prostheses and implants for customized reconstructive applications became practical. Rapid proliferation of these techniques has prompted the establishment of 3D printing point-of-care (POC) facilities in many academic institutions, independent of industry partners, equipped with expert personnel, 3D printers, and post-processing software [23]. These 3D printing POC facilities permit incorporation of “digitalization and precision surgery” into head and neck reconstructive surgeons’ practice with ease and convenience [10].
Durable reconstruction of the maxillofacial and mandibular skeleton demands surgical implant(s) with favorable biomechanical properties to support long-term form and function of resected bone. Presently, titanium is the gold-standard material for 3D printing of prosthetics and implants for head and neck reconstruction [24]. Titanium plates and screws offer excellent mechanical strength and durability required of load-bearing bony reconstructions. Further, titanium is corrosion resistant, easy to handle, and cost-effective to produce both in bulk quantities and for patient-specific applications [24]. However, titanium prosthetics and implants are associated with occasional temperature sensitivity and palpable sensation of plate(s) and screw(s) [25]. Additionally, their potential interference with postoperative radiation therapy and surveillance imaging (i.e., CT, MRI) for patients with head and neck cancer are important considerations [25]. Ultimately, surgical removal of titanium plate(s) and screw(s) prompted by symptomatic plate infection, exposure, fistula, and/or fracture occurs in as many as 40% of patients undergoing bony reconstruction of the maxillofacial and mandibular skeleton [26].
To obviate the need for eventual explant of plate(s) and screw(s), numerous degradable polymers, including polyglycolic acid (PGA), polylactic acid (PLA), alginate, and chitosan, have been investigated for use in head and neck reconstructive applications [24]. Duration of biodegradation varies depending on the specific polymer, though it generally occurs over several years in vivo [24]. Beyond their eventual biodegradation, purported advantages of such polymers include high biocompatibility, decreased risk of interference with craniofacial growth (e.g., pediatric maxillofacial trauma), and lower production cost compared to metal alloys [27]. However, such polymers are associated with symptomatic foreign body reaction during biodegradation [27]. Ultimately, concerns regarding inadequate strength of degradable polymeric, relative to titanium, prostheses and implants have limited their use in head and neck reconstructive surgical applications outside of pediatric maxillofacial trauma [27]. For instance, a comparative study by Gareb et al. [28] showed titanium to be superior to biodegradable systems with respect to tensile, bending, and torsion forces for all midface and mandibular reconstructive scenarios.
Presently, titanium remains the gold-standard material for metal 3D printing of prosthetics and implants for head and neck oncologic reconstruction [24]. However, hardware-associated complications are highly prevalent and symptomatic for patients, emphasizing the need for study of novel materials and techniques in this space. One promising area for future study is the use of 3D printed prostheses and implants composed of degradable polymeric scaffolds coated with osteogenic growth factors [29]. Such techniques have recently been applied to temporomandibular joint (TMJ) reconstruction, with promising early functional outcomes [29]. However, at present, such techniques are experimental, limited to pre-clinical investigations, and will require thorough vetting and approval by federal regulatory agencies such as the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA). Finally, biodegradable metals such as magnesium (Mg), iron (Fe), and zinc (Zn) alloy are under active investigation as alternatives to titanium with good mechanical stability and osteoconductive properties [27].
Traditional bony reconstructions of extirpative maxillofacial and mandibular defects entail a “free hand” surgical approach in which autologous bone is osteotomized and oriented and titanium plates are cut and shaped based on the surgeon’s expertise [30]. Achieving optimal masticatory, speech, and swallowing outcomes with this approach is largely dependent on surgeon expertise and experience [30]. Intraoperative free hand bending of titanium plates to native and autologous bone can be time consuming and require multiple readjustments, leading to plate stress and weakening [31]. Ultimately, suboptimal plate bending and fixation may impede osseointegration and increase risk of later plate exposure and infection.
PSI’s leveraging computer aided design/computer assisted manufacturing (CAD/CAM) technology were originally conceived as a solution to permit pre-planning and pre-contouring of titanium plates for maxillofacial and mandibular reconstruction [15]. In one such technique, patient-specific, 3D printed models of the patient’s native craniofacial anatomy and custom osteotomy guides are used to facilitate intraoperative plate bending and fixation and precise screw hole placement (Figure 1) [32]. Compared to free hand techniques, this method is associated with decreased operative and free flap ischemia times [33] and theoretical preservation of tensile strength of titanium plates [34].
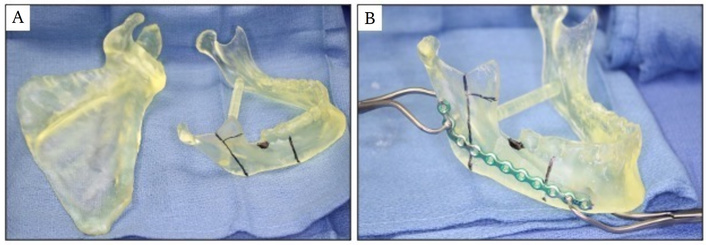
CAD/CAM and 3D printing to manufacture patient specific anatomic models for pre-planning and pre-contouring of titanium plates for maxillofacial and mandibular reconstruction. (A) Patient specific right scapula and mandible models showing planned posterolateral segmental mandibulectomy. (B) Titanium reconstruction plate is precisely pre-contoured to mandible model.
3D printed, pre-bent titanium PSIs may provide additional benefit to surgeon and patient alike in certain reconstructive scenarios. These 3D printed, pre-bent titanium PSIs are most often commercially manufactured (e.g., KLS Martin©) via SLA. SLA is a contemporary 3D printing process with noted advantages in precision, resolution, and speed in comparison to other processes such as electron beam melting (EBM) and selective laser melting (SLM). A detailed comparison of these techniques for medical applications is nicely reviewed in Lakkala et al. [35]. They are particularly useful in cases in which tumor disrupts outer cortical bone of the mandible or necessitates resection of the condyle [36]. In the latter scenario, pre-bent titanium PSIs affixed to a TMJ prosthesis may afford superior range-of-motion and precise occlusion postoperatively [36]. Numerous systematic reviews and meta-analyses have shown consistent and clinically relevant reduction in operative and ischemia times for 3D printed, pre-bent titanium PSIs relative to free hand techniques [37]. Further, use of 3D printed, pre-bent titanium PSIs may improve rates of osseous union [38] and may permit enhanced preservation of perforator vessels to autologous fibula bone [39]. Additionally, some authors have shown improved restoration of preoperative dental occlusion and more rapid return to oral diet with 3D printed, titanium PSIs over free hand techniques [40, 41].
Importantly, VSP techniques utilizing 3D printed, pre-bent titanium PSIs for maxillofacial and mandibular reconstruction have several important disadvantages. First, such techniques require pre-operative estimation of oncologic margins. Thus, intraoperative revision of bony margins to achieve a margin-negative resection may alter planned plate position and screw hole placement [42]. Second, 3D printed, pre-bent titanium PSIs are most often produced by industrial partners, rather than academic POC facilities, contributing to increased costs relative to less sophisticated techniques [43]. Though increased surgical costs are often cited as a major disadvantage of 3D printed, pre-bent titanium PSIs, their immediate costs may ultimately be balanced by reduced need for reoperation for hardware removal [43]. To our knowledge, these longitudinal cost comparisons have not been explored in the literature and thus are an attractive area for further study. Finally, for certain time-sensitive surgical indications such as maxillofacial trauma, 3D printed, pre-bent titanium PSIs produced by industry partners may not be practical. In this situation, academic POC facilities, where available, are invaluable.
Beyond 3D printed, pre-bent titanium PSIs for rigid bony fixation of the reconstructed maxillofacial and mandibular skeleton, some authors have advocated for the use of 3D printed, prefabricated titanium mesh for reconstruction of extirpative maxillectomy defects. Liu et al. [44] noted that contemporary 3D printing technology has permitted shaping of titanium mesh to optimally recreate the orbital floor, hard palate, and vertical buttresses of the maxilla. In their case series of 12 patients undergoing total maxillectomy, reconstruction with 3D printed, prefabricated titanium mesh and soft tissue free flaps nicely restored orbital volume and protrusion to preoperative measurements. Two additional case reports cited high patient satisfaction with facial and orbital symmetry achieved with this reconstructive technique [45, 46].
Restoration of functional dentition is a primary goal of head and neck reconstructive surgery involving the maxillofacial and mandibular skeleton. Traditionally, placement of dental implants into autologous fibula bone reconstructing the maxillary or mandibular alveolus is delayed until several months after the initial oncologic surgery [47]. Besides the significant delay in oral rehabilitation, secondary placement of dental implants poses a tangible risk of implant failure and osteoradionecrosis (ORN) in patients who have undergone adjuvant radiation [48].
Recently, advances in 3D printing and CAD/CAM technology have permitted immediate dental rehabilitation at the time of ablative oncologic surgery, termed “Jaw-in-a-Day” (JIAD) (Figure 2) [49]. Contemporary JIAD approaches encompass four components: 1. digitally planned oncologic resections of the maxilla or mandible, 2. fibular free flap reconstruction with 3D printed titanium plate fixation, 3. primary placement of titanium dental implant(s), and 4. immediate loading of a hybrid dental prosthesis during surgery [50]. Due to the complexity of such approaches, commercial manufacturers (e.g., KLS Martin©) are essential partners for these techniques.
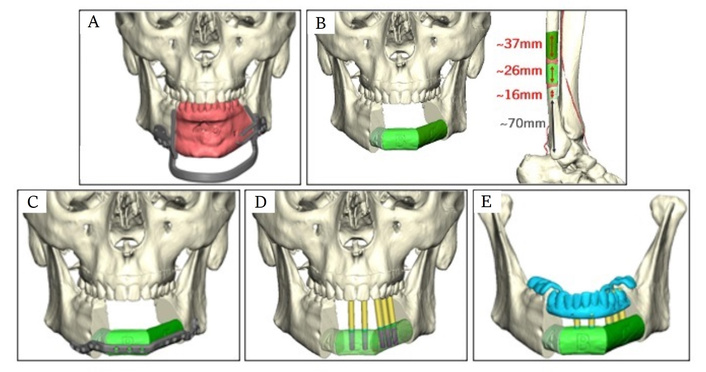
Jaw-in-a-Day reconstructive plan for a patient with a benign ameloblastoma undergoing anterior segmental mandibulectomy. (A) Custom mandibular osteotomy guides permit precise excision of tumor with appropriate oncologic margins (red). (B) Reconstructive plan for a three-segment right fibula free flap. (C) Three-segment fibula free flap is inset into anterior mandibular defect and fixated with custom titanium reconstruction plate. (D) Drill guides (yellow) for precise placement of prosthetic abutments into the fibula. (E) Depiction of custom dental prosthetic affixed to fibula reconstruction.
In 2013, Levine et al. [51] published the first series of four patients undergoing JIAD reconstruction for benign tumors of the maxilla or mandible. CAD/CAM technology utilizing the patients’ preoperative CT scans guided SLA manufacturing of titanium osteotomy guides, rigid fixation plate, and dental prosthetic implants. Using this technique, a mean (range) of 8 (4–12) teeth were immediately restored with rapid return to full oral diet in their four cases [51]. The authors stressed the importance of careful patient selection for success of this novel JIAD approach, such that it should only be offered to compliant, motivated patients with benign tumors not requiring adjuvant radiation.
Presently, JIAD approaches continue to increase in popularity in academic centers across the globe [52]. Early evidence supports rapid rehabilitation of full oral diet, optimal cosmesis, and durable implant viability even in the setting of adjuvant radiation [48, 52]. Incorporation of prosthodontists and/or oral surgeons into preoperative VSP sessions may enhance success and postoperative recovery of JIAD approaches.
Certain extirpative defects of the maxilla (e.g., small, posterior defects) result in loss of functional dentition but are sufficiently reconstructed with a soft tissue free flap, rather than autologous bone, to achieve separation of the nasal and oral cavities [53]. Without autologous fibula bone to accept dental implants, a 3D printed, custom, titanium pre-prosthetic is an excellent option for functional dental rehabilitation in either a primary or delayed fashion [54]. The titanium pre-prosthetic is designed for screw fixation to the patient’s zygomatic arch, nasomaxillary process, and/or the pre-maxillary bone. The soft tissue free flap is then carefully pierced to permit intraoral exposure of implant abutments followed by fixation of a custom denture. 3D printed, titanium pre-prosthetics for maxillary reconstruction are even newer approaches than JIAD approaches. However, initial case series show promising functional outcomes [55]. Like JIAD approaches, incorporation of prosthodontists and/or oral surgeons into pre-prosthetic design, surgery, and postoperative recovery is essential.
The 21st century has seen a rapid evolution in 3D printing technologies and manufacturing capabilities for prosthetics and implants for head and neck oncologic reconstruction. This evolution has led to significantly increased complexity and capabilities for maxillofacial and mandibular reconstruction after extirpative surgery. Importantly, contemporary outcomes data for techniques described herein is encouraging, with consistent improvements shown for surgeon satisfaction, operative time, functional (e.g., speech, swallowing, mastication) and cosmetic outcomes. However, as most published studies are single-institution, retrospective cohort studies, future investigations should prioritize controlled study designs with clearly defined primary and secondary outcome measures. As materials, infrastructure, and technologies continue to grow at an unprecedented pace, the future of metal 3D printing of prosthetics and implants for head and neck oncologic reconstruction is truly exciting.
Titanium implant-associated infection and exposure necessitating surgical removal, most frequently required in patients who have undergone adjuvant radiation, remains a significant clinical problem. Rates of such complications are estimated to occur in as many as 40% of patients undergoing head and neck reconstruction and adjuvant radiation [56]. Investigation of novel metal biomaterials for 3D printed prosthetics and implants, such as Mg, Fe, and Zn alloy, is ongoing. Additionally, 3D printed nickel titanium (nitinol) alloy reconstruction plates may hold superior elastic and osteoconductive properties compared to titanium for mandibular reconstruction [57, 58]. However, concerns remain regarding the tensile strength and durability of novel metal biomaterials such as Mg, Fe, and Zn, and more robust biomechanical studies are needed to support in vivo studies [27].
While currently limited to pre-clinical studies, bioprinting of 3D printed metal scaffolds incorporating osteogenic stem cells or growth factors is an active area of investigation [59]. Ultimately, such novel biomaterials may have exciting applications but will require considerable testing to ensure compatibility, safety, and efficacy in patients undergoing maxillofacial and mandibular reconstruction.
Growth in number and sophistication of 3D printed POC facilities embedded within academic medical centers is another exciting area of growth [60]. Such POC facilities improve surgeon input and collaboration and reduce delivery timeline, particularly important in cases of maxillofacial and mandibular trauma and aggressive cancers [38]. Presently, however, 3D printing capabilities of most POC facilities are limited to non-metal anatomic models and osteotomy guides. As CAD/CAM and manufacturing technologies evolve and become cheaper, POC facilities may become capable of metal 3D printing of titanium PSIs for maxillofacial and mandibular reconstruction. Importantly, expansion in such capabilities of POC facilities will need to be supported by clear regulatory guidance for implementation.
JIAD and maxillary pre-prosthetic approaches are relatively new, first described in 2013 [51]. Their widespread use in head and neck reconstructive surgery will be facilitated by improvements in cost, manufacturing efficiency, and formation of multi-disciplinary teams incorporating head and neck surgeons, prosthodontists, and oral surgeons.
In the past few decades, 3D printing of PSIs has revolutionized maxillofacial and mandibular reconstructive surgery, permitting improvements in operative efficiency, enhanced functional outcomes, and reduced complication rates in early studies. Facilitated by reductions in cost, improvements in manufacturing efficiency, and proliferation of 3D printing capabilities in academic POC facilities, the future of such paradigms is truly exciting.
3D: three-dimensional
AM: additive manufacturing
CAD/CAM: computer aided design/computer assisted manufacturing
CAS: computer-assisted surgery
JIAD: Jaw-in-a-Day
POC: point-of-care
PSI: patient-specific implant
SLA: stereolithography
TMJ: temporomandibular joint
VSP: virtual surgical planning
JDS: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. MKH: Investigation, Writing—review & editing. AG: Conceptualization, Writing—review & editing. KJC: Conceptualization, Investigation, Writing—review & editing. SS: Conceptualization, Investigation, Writing—review & editing. SBC: Conceptualization, Investigation, Writing—review & editing. MES: Conceptualization, Investigation, Writing—original draft, Writing—review & editing, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3183
Download: 31
Times Cited: 0
Fariborz Tavangarian ... Anilchandra Attaluri
Minhaz Husain ... J. P. Davim
Bharat Kalia ... Gurwinder Singh
Antonio Ziranu ... Greta Tanzi Germani
Apurba Das, Pradhyut Rajkumar
