Affiliation:
1Centro Conjunto de Investigación en Química Sustentable (CCIQS), UAEM-UNAM, Toluca, Estado de Mexico 50200, Mexico
2Transdisciplinary Research for Drug Discovery, Sociedad Mexicana de Epigenética y Medicina Regenerativa A. C. (SMEYMER), Mexico City C.P. 07360, Mexico
Email: ravilaa@uaemex.mx
ORCID: https://orcid.org/0000-0002-0683-073X
Explor BioMat-X. 2025;2:101349 DOI: https://doi.org/10.37349/ebmx.2025.101349
Received: July 09, 2025 Accepted: September 30, 2025 Published: October 22, 2025
Academic Editor: Gianni Ciofani, Istituto Italiano di Tecnologia, Italy
The article belongs to the special issue Green Nanoparticles for Biomedical Applications
Graphene-based nanomaterials are promising candidates for neuromuscular regeneration due to their electrical conductivity, mechanical strength, and functionalizability. In this perspective, reduced graphene oxide (rGO) nanocomposites decorated with gold nanoparticles (AuNPs) or silver nanoparticles (AgNPs) were synthesized via a one-step green process using Camellia sinensis (tea) extracts. The extracts acted as reducing and stabilizing agents and left bioactive catechins and polyphenols adsorbed on the graphene surface. The resulting nanocomposites combined structural support, electrical conductivity, and bioactive molecular modulation. rGO can provide scaffolding for cell growth, while the retained plant metabolites contributed antioxidant and anti-inflammatory effects. Incorporation of metallic nanoparticles enhanced mechanical strength, surface reactivity, and antimicrobial properties. These multifunctional graphene-metal nanocomposites offer a sustainable and biocompatible platform for guiding neuromuscular regeneration and represent a promising basis for future clinical translation.
Muscular dystrophy and neuromuscular disorders severely compromise patients’ quality of life, leading to muscle weakness, atrophy, cramping, and respiratory complications [1]. These conditions, often progressive and incurable, affect millions worldwide and present a significant socioeconomic burden. Traditional therapeutic strategies are mostly palliative and unable to fully restore function. Therefore, there is a critical need for regenerative solutions capable of supporting tissue repair and functional recovery.
Nanotechnology has emerged as a powerful ally in this context, enabling the design of biomaterials that interact at the cellular and molecular levels. Among the most promising nanomaterials are those derived from graphene. Due to their exceptional electrical, mechanical, and chemical properties, graphene-based nanomaterials have attracted considerable attention in the field of regenerative medicine. In particular, their potential to support neuromuscular regeneration through both structural and biochemical mechanisms is a compelling area of study.
Graphene is a two-dimensional monolayer composed of sp2-hybridized carbon atoms arranged in a honeycomb lattice. It is the basic structural unit of other carbon allotropes, such as carbon nanotubes and fullerenes. Graphene exhibits remarkable physical properties: It is one of the strongest materials known, with a tensile strength of approximately 130 GPa, and it possesses excellent electrical conductivity (~106 S/m), high thermal conductivity (> 3,000 W/m·K), and a surface area of 2,630 m2/g [2].
These attributes make graphene particularly suitable for biomedical applications, especially in areas where electrical stimulation and mechanical support are essential. In neuromuscular systems, cell signaling and function are closely tied to the extracellular environment. Conductive materials like graphene can support and even modulate these biological processes. Moreover, its high surface area allows for functionalization with biomolecules, drugs, or nanoparticles, enhancing its versatility.
For biomedical purposes, pristine graphene is often modified to enhance its solubility, biocompatibility, and interaction with cells. Two major derivatives include graphene oxide (GO) and reduced graphene oxide (rGO). GO is rich in oxygen-containing functional groups such as hydroxyl, epoxy, and carboxyl, which allow for dispersion in water and further functionalization. However, GO’s insulating nature limits its electrical applications.
rGO partially restores the sp2 carbon network, maintaining functional groups for chemical interaction and improved conductivity, which allows its use in scaffolds interfacing with biological systems [3, 4].
Conventional methods for synthesizing graphene and its derivatives often involve toxic solvents and harsh conditions. Techniques such as chemical vapor deposition (CVD), mechanical exfoliation, and the modified Hummers method are commonly used. However, these approaches may be unsuitable for biomedical applications due to the presence of residual contaminants.
To address these challenges, eco-friendly reduction approaches have been developed. These methods employ natural reducing agents—including ascorbic acid, gellan gum, gelatin, and plant-derived extracts—as non-toxic and environmentally sustainable alternatives to conventional reductants. In this context, our group has employed extracts of Camellia sinensis and Anemopsis californica to reduce GO and simultaneously nucleate metallic nanoparticles in a one-step aqueous process. The polyphenols and flavonoids in these extracts act both as reducing agents and stabilizers, while remaining adsorbed on the graphene surface to impart bioactivity [5–7].
This green synthesis not only simplifies the production process but also enhances the biocompatibility of the resulting material. In addition, the bioactive compounds from the plant extract remain adsorbed on the graphene surface, contributing to the biological functionality of the nanocomposite.
The combination of graphene with metallic nanoparticles such as gold (AuNPs) and silver (AgNPs) creates multifunctional nanocomposites with enhanced properties. Metallic nanoparticles are known for their unique optical and electronic characteristics, as well as their antimicrobial activity. When integrated with graphene, they synergistically improve the composite’s mechanical strength, surface reactivity, and conductivity.
There are two main strategies for forming graphene-metal nanocomposites: (1) in situ synthesis, where metal nanoparticles nucleate directly on the graphene surface, and (2) ex situ blending of pre-synthesized nanoparticles with graphene. The green one-step method using Camellia sinensis falls under the first category, allowing simultaneous reduction of GO and formation of metal nanoparticles in a single reaction medium [5, 8].
These nanocomposites exhibit properties critical for biomedical applications. For instance, the presence of AgNPs enhances the antimicrobial activity of the scaffold, reducing the risk of infection during implantation. AuNPs, on the other hand, improve biocompatibility and can be used for imaging and photothermal applications. Furthermore, their presence creates “hot spots” that can enhance surface-enhanced Raman scattering (SERS), which is useful for biosensing and diagnostics [8, 9].
Compared with conventional reduction methods, such as thermal or chemical approaches, our strategy offers additional advantages beyond sustainability. Traditional reductants (e.g., hydrazine or sodium borohydride) are highly effective but often leave toxic residues, limiting biomedical applicability. In contrast, the use of Camellia sinensis extracts enables a one-step process where reduction of GO and nucleation of metal nanoparticles occur simultaneously in aqueous conditions, without hazardous byproducts (Figure 1). This integration not only streamlines synthesis but also enhances material bioactivity, as phytochemicals remain adsorbed on the graphene surface [5–7, 10, 11]. The resulting nanocomposites therefore combine conductivity, mechanical reinforcement, and molecular modulation—functionalities rarely achieved together with other established methods. These dual structural and biochemical contributions position our approach as particularly impactful for biomedical applications where both safety and multifunctionality are critical.
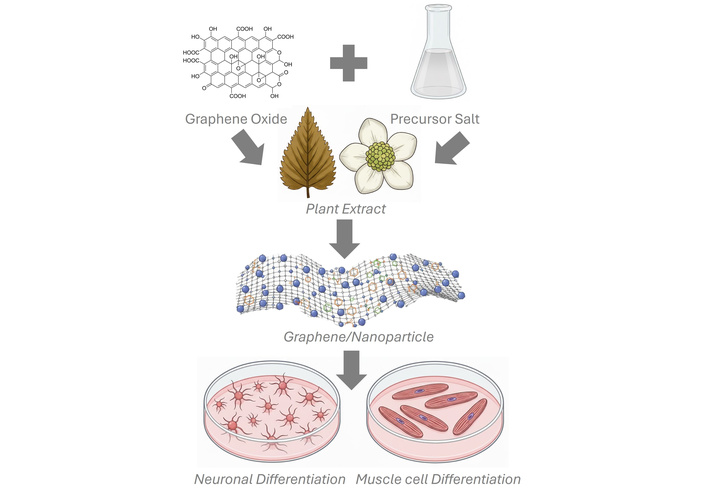
Schematic of rGO and AuNPs or AgNPs nanocomposite synthesis for muscle and neural differentiation. Generated using Gemini.
One of the most distinctive features of these eco-friendly reduction approaches is the retention of bioactive molecules from the plant extract. In traditional synthesis, stabilizers or reducing agents are often removed or rendered inactive. In contrast, green synthesis using Camellia sinensis or Anemopsis californica allows flavonoids, catechins, and polyphenols to remain adsorbed on the graphene surface.
The physicochemical characteristics of the resulting nanocomposites (previously published works [5–7, 10, 11]) corroborate the effectiveness of this strategy. Raman spectroscopy of the reduced materials shows the D band in the expected region (~1,340–1,365 cm–1) and the G band near ~1,580–1,586 cm–1; UV-Vis spectra display the disappearance of the GO π→π* feature at ~230 nm and the emergence of C=C-related peaks at ~270–275 nm, consistent with partial restoration of the sp2 network [6, 7]. FTIR spectra (notable bands at ~3,380 cm–1, 1,710 cm–1, 1,625 cm–1, 1,140 cm–1) and EDS compositional analysis show a clear decrease in oxygen content and a concomitant increase in carbon proportion for the most effective reductions (green and black tea extracts) [6, 7]. Complementary to characterization of GO, reduced density functional theory (DFT) calculations (adsorption energy and quantum-chemical descriptors) indicate stable physisorption of representative phytochemicals on graphene surfaces and support the role of adsorbed bioactives in nanoparticle stabilization and preserved biological function [5, 10]. Taken together, these structural, spectroscopic, compositional and computational descriptors demonstrate that our green, one-step approach achieves effective GO reduction and homogeneous in-situ metal nanoparticle formation while retaining phytochemical adsorption—a multifunctional outcome that contrasts with many established protocols, which either require harsher conditions, multi-step workflows, or which do not preserve bioactive surface moieties.
DFT calculations indicate that these compounds bind via π–π stacking and hydrogen bonding, without altering the electronic structure of graphene [5, 10]. This non-covalent adsorption enables the retained molecules to exert biological effects, such as antioxidant activity, anti-inflammatory responses, or even signaling modulation, when the material is interfaced with living cells.
The integration of electrical, mechanical, and biochemical functions makes graphene-based nanocomposites ideal candidates for neuromuscular regeneration. In this context, conductive scaffolds can facilitate electrical stimulation—a key factor in muscle differentiation and nerve signal propagation.
Experimental evidence shows that rGO-based materials can enhance the adhesion, proliferation, and differentiation of myogenic cells such as C2C12 myoblasts [3, 12]. Graphene foams and hydrogels have been used to support 3D cell cultures, demonstrating increased expression of myogenic markers such as MyoD, myogenin, and myosin heavy chain. These materials mimic the native extracellular matrix, providing not only mechanical support but also topographical and electrochemical cues for tissue regeneration.
Tupone et al. [13] further showed that functionalized graphene substrates can support neural differentiation and guide axonal growth, a property crucial for neuromuscular junction formation. Moreover, the mechanical stiffness and flexibility of graphene-based scaffolds match those of native muscle tissue, promoting integration and functional restoration.
For translational applications, these nanocomposites serve as growth substrates for neuromuscular cells. The adsorbed catechins and polyphenols modulate oxidative stress, inflammatory signaling, and guide muscle and neural differentiation. As substrates, the nanocomposites offer a straightforward, reproducible platform for in vitro studies and for guiding tissue regeneration at the cellular level.
Although various materials have been explored for neuromuscular regeneration, including hydrogels, conductive polymers, and decellularized scaffolds, most provide only a subset of the desired properties, such as mechanical support or biocompatibility. In contrast, our graphene-metal nanocomposites uniquely combine electrical conductivity, mechanical reinforcement, and bioactive signaling through retained catechins and polyphenols from Camellia sinensis. This multifunctional integration enables the material to actively guide both myogenic and neural differentiation, offering a comprehensive approach to neuromuscular tissue regeneration that is rarely matched by alternative scaffolds.
The central hypothesis of our work is that nanocomposites composed of rGO and AuNPs or AgNPs, synthesized via Camellia sinensis extracts, serve as dual-action biomaterials. First, they provide structural and electrochemical support for tissue growth. Second, they modulate molecular signaling pathways through the bioactive compounds retained on their surface.
This dual activity is supported by the retention of catechins and polyphenols, which have been shown to influence cellular oxidative stress, gene expression, and cytokine production. For example, epigallocatechin gallate (EGCG), a major component of green tea, is known to upregulate genes associated with antioxidant response and downregulate pro-inflammatory mediators [14]. When adsorbed onto rGO, these molecules maintain their activity and are available for cell interaction.
The graphene-based nanocomposites developed offer a novel platform for neuromuscular regeneration. Beyond their electrical conductivity, the material is functionalized with catechins from Camellia sinensis, which provide bioactive signals that modulate the cellular environment. Epicatechin gallate (ECG) enhances myogenic differentiation, activates satellite cells, and modulates the Sirtuin, Rho, PPARα/RXRα, and Ephrin signaling pathways, contributing to skeletal muscle repair [15]. EGCG exhibits neuroprotective effects, reducing oxidative stress, promoting neuronal differentiation, supporting neuritogenesis, and regulating transcription factors involved in neural plasticity [16]. The combination of conductive support and catechin-mediated biochemical signaling enables these nanocomposites to actively guide both muscle and neural regeneration, beyond the simple mitigation of oxidative stress.
This approach opens the possibility of engineering scaffolds with not only passive support roles but also active modulation of the cellular microenvironment. In neuromuscular regeneration, where both electrical and biochemical signaling are crucial, such multifunctionality represents a significant advance.
Beyond their functional advantages, these green nanocomposites align with the principles of sustainability and biocompatibility. The synthesis uses non-toxic solvents (water and isopropanol), avoids hazardous reagents, and utilizes renewable biological resources. The one-step method is also scalable and cost-effective, making it suitable for larger-scale production. From a translational perspective, the safety profile of Camellia sinensis—a widely consumed beverage—further supports the use of its extracts in biomedical materials.
Despite the promising properties of graphene-metal nanocomposites, certain translational considerations remain. Plant extract composition can vary depending on harvest, cultivar, and extraction conditions, which may influence reduction efficiency and nanoparticle formation. Our approach mitigates this variability through standardized extraction protocols. Scalability is addressed by the aqueous, one-step synthesis, which is easily adaptable to larger production volumes without the need for hazardous reagents.
The translational potential of the synthesized graphene-based nanocomposite lies in its dual functionality: the incorporation of metallic nanoparticles and the bioactive molecules from Camellia sinensis extracts. Monodispersed gold (AuNPs) and gold-silver nanoparticles (Au-AgNPs) have been shown to support myoblast attachment and proliferation with negligible cytotoxicity, while significantly enhancing myogenic differentiation and in vivo skeletal muscle regeneration through activation of the p38α MAPK signaling pathway [17]. Concurrently, catechins present in the extracts, such as ECG and EGCG, modulate the cellular microenvironment by reducing oxidative stress and promoting both neural and muscular differentiation [15, 16]. This synergistic effect suggests that the nanocomposite can act not only as a biocompatible growth substrate but also as a bioactive scaffold capable of actively guiding neuromuscular regeneration, providing a promising strategy for future therapeutic applications.
Graphene-based nanocomposites synthesized via green chemistry, particularly using Camellia sinensis extracts, represent a new class of multifunctional biomaterials for neuromuscular regeneration. These materials integrate physical properties such as conductivity and stiffness with biochemical capabilities derived from plant-based molecules.
This dual functionality enables both structural support and molecular modulation, providing a more comprehensive approach to tissue regeneration. The synthesis method is sustainable, biocompatible, and scalable, enhancing its potential for clinical translation.
As we move toward more personalized and integrated therapeutic strategies, materials that combine structural, electrical, and biochemical functionalities will be essential. Graphene-based nanocomposites offer a promising path forward, especially when developed through environmentally responsible methods. Further research should focus on in vivo performance, long-term safety, and optimization of molecular loading and release kinetics to fully realize the potential of these innovative materials.
AgNPs: silver nanoparticles
AuNPs: gold nanoparticles
DFT: density functional theory
ECG: epicatechin gallate
EGCG: epigallocatechin gallate
GO: graphene oxide
rGO: reduced graphene oxide
During the preparation of this work, the author used Gemini for creating Figure 1. After using the tool/service, the author reviewed and edited the content as needed and take full responsibility for the content of the publication.
RDAA: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. The author read and approved the submitted version.
The author declares that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
RDAA was financially supported through “Investigadoras e Investigadores COMECYT 2025” program [CAT2024-0077]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1635
Download: 40
Times Cited: 0
Ahmed T. Algahiny ... Fryad Z. Henari
Sumaira Mumtaz ... Muhammad Javid Iqbal
