Affiliation:
Biomedical Sciences Program, University of Science and Technology, Zewail City of Science and Technology, Giza 12578, Egypt
Email: t-ahmd.aboushanab@zewailcity.edu.eg
ORCID: https://orcid.org/0009-0004-3999-4898
Explor BioMat-X. 2025;2:101346 DOI: https://doi.org/10.37349/ebmx.2025.101346
Received: August 05, 2025 Accepted: September 11, 2025 Published: September 17, 2025
Academic Editor: Lucian Baia, ”Babeș-Bolyai” University, Romania
Modeling cancer cell invasion requires physiologically relevant systems, yet traditional 2D/3D assays and animal models fail to capture the biochemical and mechanical complexity of the human extracellular matrix (ECM). The human amniotic membrane (AM) is a clinically approved, abundant, and immunologically privileged tissue with a rich ECM composition and favorable mechanical properties. Despite its extensive use in regenerative medicine, its potential as a cancer invasion scaffold remains underexplored. We propose repurposing decellularized AM (dAM) as a human-derived ECM platform to study tumor invasion. dAM retains structural proteins, growth factor reservoirs, and stiffness gradients that influence epithelial-to-mesenchymal transition (EMT) and invasion pathways. Compared with conventional matrices, it offers improved biochemical fidelity and compatibility with patient-derived organoids. Key challenges, including donor variability, decellularization optimization, and reproducibility, are also addressed. dAM provides a non-invasive, scalable, and physiologically relevant tool for cancer invasion assays, drug screening, and patient-specific models. Its integration into oncology research may enhance translational relevance and accelerate personalized medicine.
Understanding the mechanisms of cancer cell invasion is central to unraveling the metastatic cascade, the primary cause of cancer-related mortality [1]. While two-dimensional (2D) cultures and Transwell invasion assays have provided valuable insights, they fail to replicate the complex three-dimensional (3D) ECM environment encountered by cancer cells in vivo. In recent years, there has been increasing emphasis on developing physiologically relevant in vitro models that better recapitulate the architecture, biochemical cues, and mechanical properties of native tissues [2]. However, widely used matrices such as Matrigel or collagen gels suffer from batch-to-batch variability, non-human origin, and lack of structural fidelity, which limit their reproducibility and translational value [3].
The human amniotic membrane (AM), a clinically approved and widely available biological scaffold, has long been recognized for its regenerative, anti-inflammatory, and anti-scarring properties [4]. It has been successfully applied in a variety of tissue repair and transplantation contexts, including ocular surface reconstruction, chronic wound healing, and soft tissue regeneration [4]. Despite its unique biological composition, rich in collagen, laminin, fibronectin, and bioactive molecules, and its preserved basement membrane architecture, its potential as a scaffold for modeling cancer cell invasion remains largely untapped.
Unlike other human-derived ECM approaches, such as decellularized tumor scaffolds or organotypic tissue slices, which require invasive sampling of diseased tissue, decellularized AM (dAM) can be obtained non-invasively from placental tissue post-delivery, eliminating donor risk and ethical constraints. While tumor-derived matrices offer disease-specific cues, they are limited by heterogeneity, availability, and safety concerns. In contrast, dAM provides a reproducible, ethically accessible, and biochemically rich ECM scaffold, making dAM not only novel but uniquely advantageous among available ECM scaffolds for modeling cancer invasion.
In this perspective, we propose that dAM offers a novel and physiologically relevant platform for assessing the invasive behavior of cancer cells. By leveraging its native ECM features, AM could provide critical insights into cell-ECM interactions, epithelial-to-mesenchymal transition (EMT), and tissue-specific invasion patterns that are difficult to capture in traditional in vitro systems. This article outlines the rationale for repurposing AM in cancer research. It highlights key opportunities, technical considerations, and future directions to establish it as a transformative tool in the study of metastasis.
AM, the innermost layer of the placenta, is a unique and biologically rich tissue that serves critical functions during fetal development. It consists of a thick, collagen-rich ECM and a single epithelial cell layer, providing both structural support and biochemical cues [5]. This composition makes AM a compelling natural scaffold for biomedical applications, particularly in contexts where cellular adhesion, migration, and differentiation are essential.
Structurally, the AM is composed of three major layers: the epithelial layer, the basement membrane, and the stromal matrix [5]. The basement membrane of the AM is one of the thickest in human tissues and is abundant in ECM components, including collagen types I, III, IV, V, and VI, laminin, fibronectin, and nidogen [6]. These components are essential in modulating cell behavior, particularly adhesion, polarity, and migration. Importantly, the AM ECM closely mimics the composition and architecture of native epithelial and mesenchymal tissues, offering a more physiologically relevant alternative to synthetic or animal-derived matrices [7]. Beyond its ECM composition, AM is a reservoir of bioactive molecules. It contains a broad spectrum of growth factors and cytokines such as epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), transforming growth factor-beta (TGF-β), hepatocyte growth factor (HGF), and vascular endothelial growth factor (VEGF) [6]. These molecules play pivotal roles in modulating inflammation, angiogenesis, and tissue regeneration. Furthermore, the AM secretes anti-inflammatory cytokines such as interleukin-10 (IL-10) and interleukin-1 receptor antagonist (IL-1RA), contributing to its well-documented immunomodulatory effects [6].
Biocompatibility is a defining feature of the AM. It is non-immunogenic due to the absence of blood vessels, lymphatics, and expression of major histocompatibility complex (MHC) class II antigens [8]. This property has facilitated its widespread clinical use without significant risk of rejection, even in allogeneic settings. When decellularized using appropriate protocols, the AM retains its structural and biochemical integrity while further minimizing any residual immunogenicity, making it highly suitable for both research and therapeutic applications [8, 9]. Another significant advantage of the AM is its mechanical properties. It is thin, flexible, and semi-transparent, yet it retains considerable tensile strength and elasticity [9, 10]. These mechanical features make it easy to handle, process, and incorporate into experimental designs requiring mechanical stability, such as flow-based invasion assays or live-cell imaging setups. Moreover, its pliability supports conformal integration into various 3D culture systems or microfluidic devices, expanding its versatility as a bioengineering substrate [11].
From a practical standpoint, the AM is a readily accessible and ethically non-controversial material. It is routinely discarded following cesarean deliveries and can be collected under standardized, sterile conditions with informed consent. This makes it an attractive, low-cost, and sustainable source of biological scaffold material [12]. Commercially, cryopreserved and lyophilized AM products are already approved for clinical use in ophthalmology, dermatology, and surgery, further attesting to their safety and translational viability [13].
The mechanical attributes of dAM, particularly its tensile strength and elastic modulus, contribute significantly to its ability to replicate the structural and functional properties of natural ECM. These biomechanical parameters not only provide physical support but also influence cancer cell behavior through mechanotransduction, shaping adhesion, migration, and invasive potential.
Quantitatively, the tensile strength of native human AM has been reported to range from 2 to 10 MPa, depending on hydration status, layer separation, and preparation method [14]. For instance, tensile testing of full-thickness AM samples yielded average tensile strength values of 2.3 ± 0.6 MPa in the longitudinal direction and 1.8 ± 0.4 MPa in the transverse direction [15–17]. These values place the AM within a similar range to other soft connective tissues and well above typical hydrogels used in 3D culture, such as collagen gels or Matrigel, which often exhibit tensile strengths below 1 MPa [18]. This mechanical robustness enables AM to be sutured, stretched, and manipulated without structural failure, making it ideal for prolonged culture and biomechanical stimulation.
The Young’s modulus, a measure of stiffness, of AM varies between 0.15 and 2.5 MPa, depending on hydration and decellularization. For instance, a study by Niknejad et al. [19] reported a modulus of elasticity of 1.5 ± 0.3 MPa for fresh AM and 0.9 ± 0.2 MPa for cryopreserved AM, indicating that preservation slightly reduces stiffness but retains adequate mechanical compliance. These values are well-suited to simulate the compliance of soft stromal tissues, making AM a more realistic ECM analog for studying cancer cell mechanotransduction compared to overly stiff plastic or overly soft gels. In terms of thickness, AM typically ranges between 20 and 500 µm, with regional variability [20]. The basement membrane is the thinnest and most densely packed layer, measuring approximately 50–100 nm, while the stromal matrix contributes most to the overall thickness [21]. These dimensions are compatible with confocal or multiphoton imaging and allow cancer cells to engage with both surface-bound and embedded matrix components in a physiologically meaningful manner.
While direct burst pressure data for human AM are limited, mechanical studies on AM from multiple species have demonstrated high tear resistance. For example, normalized tear strength values of 12.6 ± 3.8 N/mm in bovine AM and 14.8 ± 5.3 N/mm in equine AM have been reported using standardized mechanical testing, indicating robust tissue integrity that likely translates to human-derived membranes as well [14].
These biomechanical properties collectively enhance the AM’s potential as a stable, versatile, and tunable scaffold for dynamic in vitro models. Unlike synthetic matrices, which often need chemical cross-linking to achieve mechanical strength (often reducing biocompatibility), or natural gels, which quickly degrade or collapse under tension, the AM provides an optimal balance of strength and flexibility. This is especially beneficial for cancer invasion assays, as it allows multi-day or even multi-week cultures, co-culture with stromal cells, and exposure to mechanical stressors that replicate tissue microenvironments, without collapsing, detaching, or distorting the ECM.
Taken together, the biological richness, immune privilege, mechanical stability, and accessibility of the AM distinguish it as an ideal candidate for repurposing in cancer research. Its capacity to serve as a biologically active, human-derived scaffold sets the stage for innovative applications beyond regenerative medicine, including, as we propose here, the modeling of cancer cell invasion and tissue-specific metastatic behavior.
AM has been extensively studied and applied as a biologically active scaffold in regenerative medicine and tissue engineering. Its preserved basement membrane and stromal matrix provide a structurally intact, biocompatible substrate that closely resembles native tissue microenvironments. We reported dAM as a suitable platform for 3D culturing mesenchymal stem cells (MSCs), enhancing their regenerative capacities via modulating their mitochondrial oxidation and increasing their glycolytic metabolism [7]. We also developed a novel nanoparticle to facilitate the delivery of AM complex protein composition to MSCs and fibroblasts. The nanoparticles enhanced their proliferation, multi-differentiation potential, and cytoskeleton organization [22]. Elsewhere, we investigated the anti-tumorigenic activities of AM extract [23] to abrogate doxorubicin-induced angiogenesis in neuroblastoma cells [24].
The regenerative properties of AM have been successfully harnessed in numerous clinical settings. In ophthalmology, AM grafts are employed for treating corneal ulcers, chemical burns, and conjunctival reconstruction due to their ability to promote epithelialization and reduce inflammation [25]. In chronic wound management, AM has shown efficacy in enhancing granulation tissue formation and reducing scar formation [26, 27]. Additionally, AM-based scaffolds are used in oral and periodontal surgery, tendon repair, and even nerve regeneration, underscoring their multifaceted utility across tissue types [28, 29].
The basement membrane of AM plays a crucial role in these regenerative outcomes. It is rich in laminin, entactin, and collagen type IV, components known to support epithelial and endothelial cell adhesion, migration, and polarity [6]. Unlike synthetic scaffolds or hydrogels, which often lack the ultrastructural complexity of native ECM, AM retains its hierarchical organization, enabling cells to sense and respond to topographical and biochemical cues in a tissue-specific manner. The stromal matrix, composed of compact and fibroblast layers, provides additional mechanical support and paracrine factors that influence cell phenotype and tissue integration.
Despite its widespread use in regenerative medicine, AM remains virtually unexplored in cancer biology, particularly in the context of cancer cell invasion and metastasis. The intrinsic biological properties of AM make it an exceptional candidate for development as a 3D invasion assay platform. Its preserved ECM, native basement membrane structure, and mechanical integrity allow for dynamic interactions between cancer cells and a human-derived, physiologically relevant microenvironment. These features offer distinct advantages over conventional in vitro systems such as Transwell assays, synthetic gels, and animal-derived matrices like Matrigel (Table 1).
Comparison of the proposed AM-Based 3D Invasion Models vs. Conventional Assays.
| Feature | Proposed AM-Based 3D Model | Matrigel Invasion Assay [31] | Transwell Assay (2D/Pseudo-3D) [32] | In Vivo Tail Vein Injection Model [33] |
|---|---|---|---|---|
| ECM Source | Human-derived amniotic membrane (AM) | Murine tumor ECM (Matrigel) | Synthetic filters + ECM coating | Native mouse tissues (no matrix scaffold used) |
| Structural Integrity | Preserved basement membrane and stroma | Gel-like, lacks defined structure | Rigid, artificial support | Physiological ECM of lung/liver |
| Biochemical Composition | Collagens I/IV, laminin, fibronectin + growth factors | Variable protein concentration of murine origin (9–12 mg/mL) + tumor-derived factors | Essentially absent unless coated (collagen/fibronectin added in µg/cm2) | Full physiological composition, but not human-derived |
| Mechanical Properties | Elastic modulus: 0.1–10 kPa; tensile strength 1–5 MPa | Elastic modulus: 100–500 Pa; lacks tensile integrity | Artificially rigid; tensile strength > 10 MPa; stiffness far above physiological range | Elastic modulus varies widely (e.g., lung ~1 kPa, liver ~0.5–1.5 kPa, bone > 10 MPa) |
| Batch Consistency | Moderate; donor screening required | High batch variability | High | High consistency per mouse strain |
| Real-Time Imaging Compatibility | High (live-cell imaging, confocal, multiphoton) | Limited due to opacity | High (endpoint only) | Limited; requires intravital microscopy or bioluminescence |
| EMT and Invasion Modeling | Supports EMT in 3D with native ECM cues | EMT-inducing, but less structured | Limited EMT modeling | Full EMT and metastasis cascade, but not trackable early |
| Co-culture Capability | Supports stromal, immune, endothelial cells | Limited by gel stability | Low | Possible but complex (e.g., bone marrow transplant) |
| Patient-Derived Organoid Use | Feasible | Difficult | Poor integration | Not directly applicable |
| Translational Relevance | High; human-derived ECM and structure | Moderate; murine origin | Low | High biological control, but low experimental control |
| Cost and Availability | Low-cost; clinically sourced | High cost; proprietary | Inexpensive | High; requires animal facility |
| Ethical & Regulatory Barrier | Minimal (clinical waste) | Moderate | None | High (animal use protocols required) |
| Throughput | Medium (scalable with inserts or chips) | Low | High | Low |
| Quantitative Readouts | Invasion depth, EMT markers, protease activity | Invasion area/number | Cell count or fluorescence | Tumor burden (bioluminescence, histology) |
A critical hallmark of metastatic progression is EMT (Figure 1), a process through which epithelial cancer cells acquire mesenchymal characteristics, enabling migration and invasion through ECM barriers [1]. Traditional 2D cultures fail to recapitulate the spatial, mechanical, and biochemical gradients that influence EMT dynamics [1]. In contrast, an AM-based model could provide a native collagen- and laminin-rich substrate that allows cancer cells to engage with real basement membrane topography and composition, potentially leading to more physiologically relevant EMT activation patterns. This makes AM ideal for studies involving matrix remodeling enzymes such as matrix metalloproteinases (MMPs), which are critical for ECM degradation during invasion.
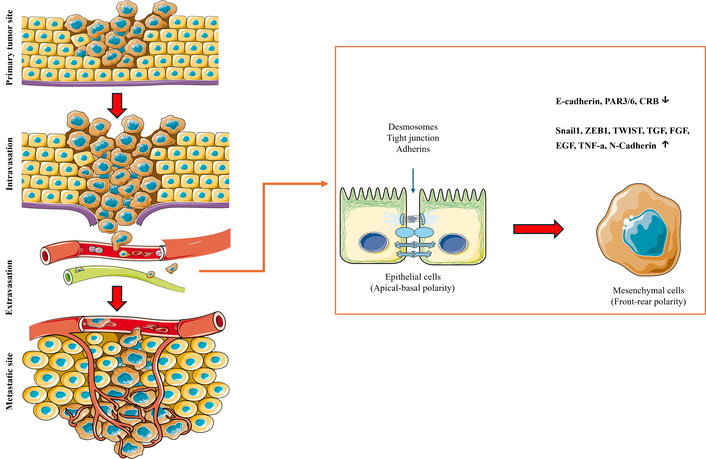
Schematic overview of EMT and its role in cancer metastasis. EMT is a dynamic, reversible cellular program that enables polarized, adherent epithelial cancer cells to acquire mesenchymal properties, facilitating invasion and dissemination. In the early stages of metastasis (left), epithelial cells downregulate intercellular adhesion molecules such as E-cadherin, reorganize their cytoskeleton, and adopt a spindle-shaped, motile phenotype characteristic of mesenchymal cells. This phenotypic plasticity allows tumor cells to breach the basement membrane and degrade surrounding extracellular matrix (ECM), initiating local invasion. Once in proximity to vasculature, mesenchymal-like tumor cells undergo intravasation—entering the circulation through endothelial disruption. Within the bloodstream, circulating tumor cells (CTCs) must resist anoikis and evade immune surveillance to reach distant tissues. Upon arrival at a secondary site, cells extravasate from the vasculature and may undergo mesenchymal–epithelial transition (MET) to reestablish epithelial traits that support colonization, self-renewal, and outgrowth at the metastatic niche. EMT is not binary but exists along a spectrum, with many cancer cells occupying hybrid epithelial/mesenchymal states that enhance plasticity, therapeutic resistance, and stem-like properties. This figure highlights EMT as a key driver of the metastatic cascade, from primary tumor invasion to distant organ colonization. Images are provided by Servier Medical Art (https://smart.servier.com/) and are licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/).
Importantly, AM is conducive to co-culture models. It supports the simultaneous seeding of cancer cells with fibroblasts, endothelial cells, or immune cells, offering an opportunity to study tumor–stroma crosstalk, immune evasion, or angiogenesis in a human-relevant matrix. AM has been used as a scaffold for studying 3D culturing of cancer cells, facilitating the investigation of their cell behavior, drug resistance, and cancer stem cell content [11]. Moreover, its clinical safety and ethical acceptability suggest its potential use as a scaffold for patient-derived organoid seeding or ex vivo invasion assays, expanding opportunities for personalized cancer invasion profiling and drug screening. Additionally, we also used dAM as a natural ECM for developing an HCC organoid [30]. This matrix supported the structural integrity of the organoid model and promoted its proliferation and viability [30]. Moreover, this model exhibited an upregulated glycolytic metabolic signature with downregulated mitochondrial oxidation and the urea cycle [30].
The ECM of dAM is central to its advantage over synthetic or animal-derived scaffolds. Its basement membrane retains collagen types IV and VII, laminin, and fibronectin, providing adhesive and signaling cues that regulate cancer cell polarity, adhesion, and migration. Stromal layers further preserve fibrillar collagens and proteoglycans that reproduce native stiffness gradients and hydration properties, essential for modulating mechanotransduction pathways.
During EMT, epithelial cancer cells downregulate adhesion molecules like E-cadherin, while upregulating mesenchymal markers such as N-cadherin and vimentin, enabling loss of polarity and acquisition of motility. Laminin- and collagen-rich basement membranes, such as those preserved in dAM, provide biochemical and topographical cues that can accelerate EMT by engaging integrins and activating downstream pathways including TGF-β/SMAD, Wnt/β-catenin, and PI3K/AKT. Fibronectin deposition further reinforces EMT by recruiting integrin-linked kinase and focal adhesion signaling, which promote cytoskeletal remodeling and migration. Collectively, these ECM-mediated signals establish gradients of stiffness, adhesion, and growth factor availability that drive phenotypic plasticity, invasion, and eventual dissemination of tumor cells. By retaining a native repertoire of ECM proteins and cytokines, dAM offers a physiologically relevant platform to study the precise regulatory effects of ECM composition on EMT dynamics and invasive behavior.
When compared to standard models, AM offers superior biochemical relevance, reduced batch variability, and better structural fidelity. While Matrigel provides a gel-based 3D matrix, its mouse tumor origin and undefined composition limit its reproducibility and translational accuracy. Transwell invasion assays, though widely used, rely on artificial filters coated with ECM proteins and provide limited spatial and mechanistic information. Table 1 summarizes the key differences between the proposed AM-based 3D invasion models and traditional in vitro invasion assays.
While dAM provides a promising physiologically relevant scaffold, several limitations must be considered. Batch-to-batch variability poses a major challenge, as donor-specific factors such as age, health status, and delivery mode can alter ECM composition and mechanical properties, affecting reproducibility. The decellularization process itself may further compromise scaffold fidelity; detergent-, enzymatic-, or physical-based protocols often deplete or denature key ECM molecules, altering growth factor and cytokine retention that may unpredictably modulate cell behavior. In addition, differences in tensile strength and elasticity between donors complicate standardization across studies. Long-term stability also remains uncertain, as ECM degradation during extended culture may influence cancer cell invasion dynamics and limit prolonged assays. Addressing these challenges will require optimized processing methods, minimal donor screening criteria, and rigorous quality control benchmarks to ensure consistency and reliability in future applications.
The successful implementation of AM as a standardized 3D scaffold for cancer research depends heavily on appropriate processing protocols to preserve its biological integrity while ensuring compatibility with cell culture, imaging, and analytical workflows (Figure 2) [27].
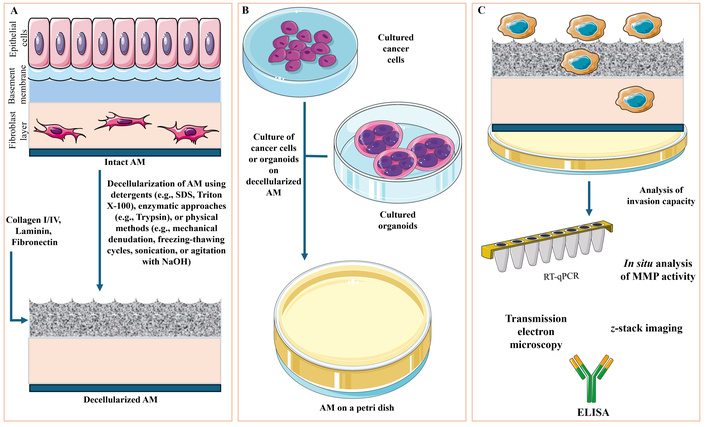
Technical workflow for utilizing dAM as a 3D platform to assess cancer cell invasion. (A) Human AM consists of multiple structurally distinct layers: an epithelial cell layer, a basement membrane rich in ECM proteins (e.g., collagen, laminin, fibronectin), and subepithelial layers including compact, fibroblast, spongy, and chorionic zones. To render AM suitable for in vitro culture, decellularization is performed using chemical (e.g., SDS, Triton X-100), enzymatic (e.g., trypsin), or physical (e.g., mechanical scraping, freeze–thaw cycles, NaOH agitation) methods. This process preserves the ECM architecture while removing immunogenic cellular components. (B) The resulting dAM is then mounted in culture systems such as Petri dishes or Transwell inserts, allowing the seeding of monolayer cancer cell cultures or patient-derived organoids onto its surface. (C) Cancer cell invasion into the scaffold can be assessed by real-time quantitative PCR (RT-qPCR) for EMT marker expression, z-stack confocal imaging for depth of invasion, and in situ detection of proteolytic activity using fluorogenic MMP substrates. Additional characterization includes cytokine profiling via ELISA and ultrastructural imaging via transmission electron microscopy. Together, these approaches enable the detailed, physiologically relevant assessment of cancer invasion dynamics on a biologically active, human-derived matrix. Images are provided by Servier Medical Art (https://smart.servier.com/) and are licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). AM: amniotic membrane; dAM: decellularized amniotic membrane; EMT: epithelial-to-mesenchymal transition; MMPs: matrix metalloproteinases.
Decellularization is a critical step in preparing AM for research applications. It aims to remove all cellular and immunogenic components while retaining the native ECM architecture and bioactive molecules. Three principal categories of decellularization protocols are employed:
Detergent-based methods (e.g., SDS, Triton X-100) are widely used due to their efficiency in lysing cell membranes and solubilizing cellular debris. However, these agents can disrupt collagen fibers and reduce ECM bioactivity if not properly optimized. For example, SDS is effective at removing nucleic acids but may denature ECM proteins and compromise mechanical strength.
Enzymatic approaches, including trypsin or nucleases (DNase/RNase), offer gentler processing and better preservation of ECM composition. However, they may be incomplete without accompanying physical or chemical steps, leading to residual cellular material that can affect downstream applications.
Physical methods such as freeze-thaw cycles, sonication, or agitation are often used in combination with chemical (e.g., NaOH) or enzymatic treatments. While non-toxic, they are typically insufficient on their own and may alter ECM ultrastructure through mechanical shear.
An optimal protocol balances decellularization efficiency with ECM preservation, depending on the intended application. For invasion assays, retaining basement membrane components (e.g., laminin, type IV collagen) is essential, making detergent-enzyme combinations with minimal exposure times preferable (Figure 2).
Sterility is essential for in vitro assays and can be achieved via peracetic acid treatment, gamma irradiation (may degrade ECM proteins), ethanol (requires validation to avoid cytotoxic residues), or antibiotic incubation (requires validation to avoid cytotoxic residues). Processed AM can be cryopreserved, lyophilized, or stored at 4°C in sterile conditions, depending on whether native bioactivity or shelf-life is prioritized. Cryopreservation retains the most native ECM functionality but requires cold-chain logistics.
The thin, semi-transparent nature of AM allows direct compatibility with confocal and multiphoton imaging, enabling real-time tracking of cancer cell morphology, migration, and matrix degradation. The membrane can be mounted onto standard culture inserts or integrated into microfluidic platforms to study invasion under shear stress or chemotactic gradients. These platforms can be customized to control oxygenation, nutrient flow, and mechanical strain, simulating in vivo tumor-stroma interfaces.
AM can be further modified to support advanced applications such as: (1) chemokine gradients, by immobilizing or perfusing cytokines (e.g., CXCL12, EGF) across the membrane to study directed migration. (2) Stiffness modulation, by partial cross-linking (e.g., with genipin or EDC/NHS chemistry), enables studies on mechanotransduction. (3) CRISPR-based screens, where cancer cells with genetic perturbations can be seeded onto AM to identify regulators of invasion, EMT, or matrix remodeling in a high-throughput and physiologically relevant manner. These technical considerations underscore the flexibility of AM as a modular scaffold, capable of supporting both hypothesis-driven and discovery-based studies.
Repurposing dAM as a biologically active scaffold represents a promising frontier in cancer invasion modeling. Its native ECM composition preserved structural fidelity, mechanical resilience, and compatibility with imaging and co-culture systems, making it a uniquely advantageous platform over current in vitro matrices. dAM can enable physiologically relevant studies of EMT, protease-driven ECM remodeling, and tumor–stroma interactions in a human-derived, ethically sourced scaffold. The incorporation of dAM into cancer research could open exciting possibilities for monitoring cell invasion, metastasis, and tumor-microenvironment interactions with higher translational fidelity than current in vitro systems.
Initial proof-of-concept studies can involve seeding multiple cancer cells onto dAM to assess invasion potential. Assays could quantify (1) invasion depth, using z-stack imaging or tissue-clearing techniques, (2) EMT marker expression, by immunofluorescence (e.g., E-cadherin, vimentin) or RT-qPCR, (3) matrix degradation, by in situ detection of MMP activity, (4) cytokine secretion, using multiplexed ELISA to profile secreted inflammatory mediators during invasion. These experiments can be compared against Matrigel-based invasion to establish biological relevance and reproducibility. The AM platform can be integrated with single-cell RNA sequencing, spatial transcriptomics, or mass spectrometry-based proteomics to characterize the dynamic transcriptional and proteolytic changes during invasion. Live imaging coupled with fluorescent reporters (e.g., GFP-tagged EMT markers, FRET-based biosensors for MMPs or Rho GTPases) would allow real-time visualization of cell fate decisions and matrix remodeling events in situ. AM can serve as a foundation for functional ex vivo assays, where patient-derived tumor cells or organoids are seeded onto autologous or standardized dAM. This could enable (1) screening of anti-invasive compounds or EMT inhibitors, cisplatin treatment has been applied to MDA231 cancer cells, where the authors showed their improved chemotherapeutic resistance [11], (2) stratification of patients based on functional invasion phenotypes, and (3) co-culture with immune or stromal cells to test immunotherapy responses. Such platforms would complement genomic profiling with real-time functional outputs, advancing personalized oncology by bridging molecular features with invasive behavior. In the long term, combining AM with high-throughput microscopy and AI-based phenotyping could revolutionize the way we study tumor progression, metastasis, and therapy resistance, transforming AM from a clinical waste product into a critical tool for cancer systems biology.
2D: two-dimensional
3D: three-dimensional
AM: amniotic membrane
dAM: decellularized amniotic membrane
EGF: epidermal growth factor
EMT: epithelial-to-mesenchymal transition
MMPs: matrix metalloproteinases
MSCs: mesenchymal stem cells
TGF-β: transforming growth factor-beta
During the preparation of this work, the author used ChatGPT (OpenAI) for assistance in language editing. The author reviewed and edited the content as needed and takes full responsibility for the content of the publication.
AMAS: Conceptualization, Methodology, Writing—original draft, Writing—review & editing. The author read and approved the manuscript for publication.
The author declares that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
