Affiliation:
1Department of Mechanical Engineering, Stevens Institute of Technology, Hoboken, NJ 07030, USA
Email: afarazin@stevens.edu
ORCID: https://orcid.org/0000-0003-4371-2799
Affiliation:
2Department of Mechanical & Biomedical Engineering, Boise State University, Boise, ID 83725-2085, USA
ORCID: https://orcid.org/0000-0002-6707-6398
Explor BioMat-X. 2025;2:101340 DOI: https://doi.org/10.37349/ebmx.2025.101340
Received: April 07, 2025 Accepted: May 26, 2025 Published: June 25, 2025
Academic Editor: Ajay Vikram Singh, German Federal Institute for Risk Assessment (BfR), Germany
The article belongs to the special issue Bioinspired Material for Regenerative Medicine
Bone tissue engineering (BTE) represents a cutting-edge approach to treating critical-sized bone defects, complex fractures, and degenerative bone diseases by promoting the regeneration of functional bone tissue. A crucial element in this process is the design and optimization of scaffolds that emulate the natural extracellular matrix (ECM), supporting cell adhesion, proliferation, and differentiation necessary for bone regeneration. Polymers are widely used in scaffold fabrication. They offer versatility, biocompatibility, and tunable properties that are essential for tissue engineering. This paper provides a comprehensive analysis of polymeric scaffolds in BTE, focusing on synthetic and natural polymers, composite scaffold designs, and the fabrication techniques employed to enhance their performance. Key design criteria, such as scaffold porosity, mechanical properties, and biodegradability, are discussed in the context of facilitating optimal bone regeneration. Additionally, we explore functionalization strategies to improve biological interactions, such as the incorporation of growth factors and surface modifications, and evaluate in vivo performance to highlight clinical potential. The paper also addresses current challenges, including the need for enhanced mechanical strength and controlled degradation, while offering insights into future directions for the development of polymeric scaffolds in bone tissue regeneration therapies.
Bone is a dynamic and highly vascularized tissue capable of self-regeneration under normal physiological conditions. However, in cases of severe trauma, congenital defects, or diseases such as osteoporosis and bone cancer, the body’s natural healing capacity is insufficient to regenerate large sections of damaged or missing bone [1]. These cases typically require medical intervention in the form of bone grafts, traditionally categorized as autografts, allografts, or xenografts. While autografts (grafts harvested from the patient’s own body) offer the highest success rates due to their osteogenic, osteoconductive, and osteoinductive properties, they are limited by the availability of donor tissue, risk of donor site morbidity, and the necessity for an additional surgical procedure [2, 3]. Allografts, derived from human donors, carry the risk of immune rejection and disease transmission, while xenografts, sourced from animals, face similar limitations in immune compatibility [4].
Bone tissue engineering (BTE) provides a promising alternative to these traditional methods by using bioengineered scaffolds that can facilitate new bone growth [5, 6]. Scaffolds act as a temporary matrix, supporting the regeneration of bone tissue by guiding the growth and differentiation of cells. These scaffolds must mimic the extracellular matrix (ECM) of natural bone tissue in both structural and biochemical terms [7]. They should provide mechanical support while facilitating cellular processes such as adhesion, migration, proliferation, and differentiation. Additionally, scaffolds should promote the formation of a vascular network and degrade at a rate that matches the formation of new bone tissue, ultimately being replaced by the regenerated tissue [8, 9].
Among the various materials used to fabricate scaffolds, polymers have gained significant attention due to their flexibility, biocompatibility, and tunable properties. Polymers can be tailored to degrade over time, are relatively easy to process, and can be modified to carry bioactive molecules that enhance cellular interactions [10]. Polymers used in BTE can be broadly categorized into synthetic, natural, and composite types. Each category has distinct advantages and limitations in terms of mechanical properties, degradation rates, and bioactivity [11–13]. In clinical practice, BTE has shown immense promise in addressing a wide range of skeletal conditions where conventional treatments fall short. These include critical-sized bone defects resulting from high-energy trauma or tumor resection, which surpass the body’s intrinsic regenerative capacity. Osteoporotic fractures, particularly in aging populations, often suffer from impaired healing due to poor bone quality. Furthermore, segmental bone loss caused by chronic osteomyelitis or congenital deformities such as fibrous dysplasia or cleidocranial dysplasia—requires structural and functional restoration that autografts and allografts may not sufficiently provide. Post-resection bone voids following tumor excision, especially in orthopedic oncology, are another major indication for scaffold-based repair. In such cases, BTE offers a compelling alternative by combining biomaterials and regenerative cues to support neotissue formation, vascularization, and integration with host bone.
In this paper, we will delve into the role of polymeric scaffolds in BTE, exploring the various types of polymers, their design criteria, fabrication techniques, and biological interactions. We will also discuss recent advancements in scaffold functionalization and examine the in vivo performance of polymeric scaffolds. Finally, we will address the challenges that remain in this field and propose future directions for the development of more advanced polymeric scaffolds for clinical applications in bone repair.
The hierarchical organization of bone, its anatomical features, and the elemental composition of bone. The choice of polymer plays a critical role in determining the scaffold’s mechanical strength, degradation behavior, and ability to support cell growth and tissue regeneration. Polymers used in BTE can be classified into three broad categories: synthetic polymers, natural polymers, and composite scaffolds [14–16]. To aid in a clearer understanding of the materials discussed, the representative chemical structures of key polymers used in BTE are summarized here. Polylactic acid (PLA) and polyglycolic acid (PGA) are aliphatic polyesters composed of repeating ester-linked units derived from lactic acid and glycolic acid, respectively. Their degradation occurs via hydrolysis of the ester bonds. Polycaprolactone (PCL) consists of a repeating six-carbon monomer unit, offering slower degradation due to its semicrystalline structure and longer aliphatic chains. Poly(lactic-co-glycolic acid) (PLGA) is a copolymer that combines PLA and PGA units in variable ratios, allowing tailored degradation rates. In contrast, natural polymers such as collagen consist of triple-helical polypeptide chains rich in glycine, proline, and hydroxyproline, providing a biomimetic structure. Chitosan, derived from chitin, features β-(1→4)-linked D-glucosamine and N-acetyl-D-glucosamine units with reactive amine groups that contribute to its bioactivity and antibacterial properties. Alginate is composed of blocks of β-D-mannuronic acid (M) and α-L-guluronic acid (G), capable of forming ionic hydrogels in the presence of divalent cations such as Ca2+. These structural differences influence each polymer’s mechanical behavior, degradation kinetics, and interaction with cells and bioactive molecules.
Synthetic polymers are widely used in scaffold fabrication due to their predictable mechanical properties and controlled degradation rates [17]. They can be engineered to have a wide range of physical and chemical properties, making them suitable for various biomedical applications. However, their lack of inherent bioactivity often requires additional surface modifications or the incorporation of bioactive molecules to enhance cellular interactions [18].
PLA is a biodegradable synthetic polymer derived from renewable resources such as corn starch and sugarcane. PLA degrades through hydrolysis into lactic acid, a naturally occurring metabolite that can be safely absorbed by the body. PLA scaffolds are widely used in BTE. They offer good mechanical properties, are biocompatible, and degrade at adjustable rates. The degradation rate of PLA can be controlled by altering its molecular weight, crystallinity, and the ratio of its stereoisomers, L-lactic acid and D-lactic acid. However, PLA’s hydrophobic nature can limit cell adhesion and proliferation, necessitating surface treatments or the addition of bioactive molecules to improve its bioactivity [19].
PGA is another commonly used synthetic polymer in BTE. PGA degrades faster than PLA, making it suitable for applications where rapid scaffold resorption is required. However, the rapid degradation of PGA can lead to a loss of mechanical integrity before new tissue has formed, which limits its use in load-bearing applications. To overcome this limitation, PGA is often combined with PLA to form PLGA, a copolymer with tunable degradation rates and mechanical properties. Like PLA, PGA is also hydrophobic and requires surface modifications to enhance cell attachment [20].
PCL is a semicrystalline polymer with a much slower degradation rate compared to PLA and PGA [21–23]. PCL has excellent mechanical properties and flexibility, making it suitable for load-bearing applications in BTE. PCL scaffolds can be fabricated into a variety of shapes and structures, including nanofibrous meshes through electrospinning, which mimic the fibrous structure of the natural ECM. However, PCL’s hydrophobicity and lack of bioactivity are limitations that must be addressed through surface functionalization or the incorporation of bioactive molecules such as growth factors or nanoparticles to promote osteogenesis [24].
PLGA is a copolymer of PLA and PGA, combining the advantages of both materials. The ratio of lactic acid to glycolic acid in the polymer can be adjusted to control the scaffold’s degradation rate. PLGA has been extensively studied for use in BTE due to its tunable mechanical properties, biocompatibility, and controlled degradation. However, like PLA and PGA, PLGA can produce acidic degradation byproducts, which may lead to localized acidosis and negatively affect the surrounding tissue. To mitigate this, PLGA scaffolds are often functionalized with bioactive molecules or blended with other materials that neutralize the acidic environment [25]. The comparative summary of key synthetic polymers used in BTE is listed in Table 1.
Comparative summary of key synthetic polymers used in bone tissue engineering
| Polymer | Biocompatibility | Degradation rate | Mechanical strength | Processability | Common applications |
|---|---|---|---|---|---|
| PLA | High | Moderate | Good (brittle) | Easy (3D printing, extrusion) | Bone fillers, screws |
| PCL | Excellent | Slow | High (flexible) | Excellent (electrospinning, molding) | Load-bearing scaffolds |
| PLGA | High | Tunable | Moderate | Good | Drug delivery + scaffolds |
| PGA | Good | Fast | Moderate–low | Limited | Fast-degrading implants |
Natural polymers are derived from biological sources and offer several advantages over synthetic polymers, including inherent biocompatibility and the ability to mimic the structure and function of the natural ECM. However, natural polymers typically have weaker mechanical properties and less controllable degradation rates, limiting their use in load-bearing applications [26].
Collagen is the primary structural protein in bone and other connective tissues, making it an ideal material for scaffold fabrication in BTE. Collagen scaffolds provide a biomimetic environment that supports cell adhesion, proliferation, and differentiation. Collagen also possesses natural osteoinductive properties, promoting the differentiation of mesenchymal stem cells (MSCs) into osteoblasts, the cells responsible for bone formation. However, collagen’s poor mechanical strength limits its use in load-bearing applications, and its rapid degradation can result in premature scaffold failure. To address these limitations, collagen is often combined with synthetic polymers or ceramics to improve its mechanical properties and stability [27].
Chitosan is a polysaccharide derived from chitin, found in the exoskeletons of crustaceans. It has gained significant attention in BTE due to its biocompatibility, biodegradability, and antimicrobial properties [28]. Chitosan scaffolds support cell adhesion and proliferation and can be functionalized with growth factors or nanoparticles to enhance their osteoinductive properties [29]. However, like collagen, chitosan’s mechanical properties are insufficient for load-bearing applications, and its degradation rate can vary depending on the degree of deacetylation. To improve its mechanical strength, chitosan is often used in combination with synthetic polymers or ceramics [30].
Alginate is a naturally occurring polysaccharide extracted from brown seaweed [31]. It is widely used in biomedical applications due to its biocompatibility, biodegradability, and ability to form hydrogels. In BTE, alginate scaffolds are often combined with bioactive molecules, such as bone morphogenetic proteins (BMPs) or hydroxyapatite (HA), to enhance their osteoinductive properties. However, alginate’s poor mechanical properties and uncontrolled degradation rate limit its use in load-bearing applications. To address these issues, alginate is frequently combined with synthetic polymers or reinforced with nanoparticles [32].
Composite scaffolds combine the advantages of synthetic and natural polymers to create materials with optimal mechanical and biological properties. These scaffolds can be engineered to possess the mechanical strength necessary for load-bearing applications while also providing a biomimetic environment that supports cell attachment, proliferation, and differentiation. Composite scaffolds are often functionalized with bioactive molecules or nanoparticles to further enhance their osteoinductive and osteoconductive properties [33].
One of the most common composite scaffolds used in BTE is a blend of PCL and HA, a naturally occurring mineral in bone. PCL provides the mechanical strength and stability needed for load-bearing applications, while HA enhances the scaffold’s bioactivity by promoting cell attachment and bone mineralization. Other composite scaffolds combine PLGA with collagen or chitosan to create structures that mimic the natural ECM while providing controlled degradation and mechanical support [34–37]. Composite scaffolds are designed to leverage the mechanical robustness of synthetic polymers and the intrinsic bioactivity of natural polymers. For instance, while PCL and PLGA offer excellent structural integrity and controlled degradation, they lack cellular signaling motifs. By blending these with collagen or chitosan, which contain cell-binding domains and promote osteogenesis, a synergistic effect is achieved—enhancing both scaffold strength and biological functionality [38].
Recent studies have demonstrated that PCL-collagen and PLGA-chitosan composites improve bone regeneration outcomes in both in vitro and in vivo models. Furthermore, alginate-PCL blends have shown promise in load-bearing applications by enhancing elasticity while supporting vascularized bone growth. These examples underscore how specific pairings of polymers can be tailored to address clinical requirements in bone tissue repair [39].
While individual polymers offer distinct advantages, their performance must be weighed critically. For example, PLA offers good mechanical strength but may be too brittle for dynamic environments, whereas PCL provides flexibility and longer degradation but lacks intrinsic osteoinductivity. Likewise, freeze-drying creates highly porous scaffolds but lacks precision control over microarchitecture compared to 3D printing. These trade-offs underscore the importance of application-specific design, where no single polymer or method is universally superior.
The design of polymeric scaffolds for BTE must meet specific criteria to ensure successful bone regeneration. These criteria include biocompatibility, biodegradability, mechanical strength, porosity, and bioactivity as shown in Figure 1 [40].
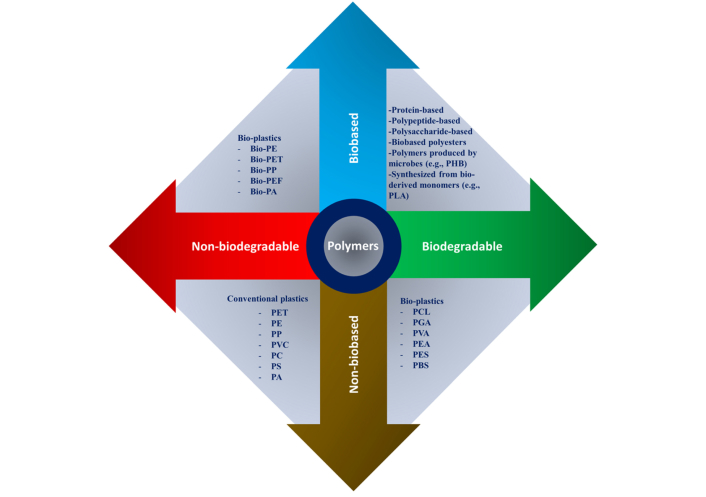
Natural and synthetic polymers were reorganized according to their biological origin (bio-based vs. non-bio-based) and their degradability (biodegradable vs. non-biodegradable). The abbreviations used include: PHB (polyhydroxybutyrate), PLA (polylactic acid), PCL (polycaprolactone), PGA [poly(glycolic acid)], PVA [poly(vinyl alcohol)], PEA [poly(ethylene adipate)], PES (polyethersulfone), PBS (polybutylene succinate), PET (polyethylene terephthalate), PE (polyethylene), PP (polypropylene), PVC (polyvinyl chloride), PC (polycarbonate), PS (polystyrene), PA (polyamide), and PEF (polyethylene furanoate). Reprinted from [40]. CC-BY 4.0
Biocompatibility is a critical factor in the design of polymeric scaffolds. The scaffold must not elicit an immune or inflammatory response upon implantation, and it should promote the adhesion, proliferation, and differentiation of osteogenic cells. Polymers used in scaffold fabrication must be carefully selected to ensure they are non-toxic and do not produce harmful byproducts during degradation. Additionally, the scaffold’s surface properties, such as roughness, hydrophilicity, and chemical composition, play a crucial role in determining its biocompatibility [41].
Biodegradability is another important criterion for scaffold design. The scaffold should degrade at a rate that matches the formation of new bone tissue, gradually transferring mechanical load to the regenerating bone. The byproducts of scaffold degradation should be non-toxic and easily metabolized or excreted by the body. The degradation rate of the scaffold can be controlled by adjusting the polymer’s molecular weight, crystallinity, and composition [42].
Scaffolds used in BTE must possess sufficient mechanical strength to provide structural support in load-bearing applications. The mechanical properties of the scaffold should match those of the surrounding bone tissue to avoid stress shielding, a phenomenon in which the scaffold absorbs too much of the mechanical load, preventing the regenerating bone from experiencing the mechanical stimuli necessary for its growth. The mechanical strength of polymeric scaffolds can be tailored by adjusting the polymer’s molecular structure, processing conditions, and the incorporation of reinforcing materials such as ceramics or nanoparticles [43].
The scaffold’s porosity and architecture are critical factors that influence its ability to support cell infiltration, nutrient diffusion, and vascularization. The scaffold should have an interconnected pore structure with pore sizes large enough to allow cells to migrate and proliferate but small enough to provide sufficient mechanical strength. Optimal pore sizes for BTE are typically in the range of 100 to 500 μm. The scaffold’s architecture can be designed using advanced fabrication techniques such as 3D printing or electrospinning to create highly controlled and reproducible structures [44].
In addition to providing structural support, the scaffold should also exhibit bioactive properties that promote the attachment, proliferation, and differentiation of osteogenic cells. This can be achieved by incorporating bioactive molecules such as growth factors, peptides, or nanoparticles into the scaffold. For example, the incorporation of BMPs into the scaffold can enhance its osteoinductive properties, promoting the differentiation of MSCs into osteoblasts. Surface modifications, such as coating the scaffold with bioactive molecules or creating micro- or nano-scale surface features, can also enhance its bioactivity [45].
The fabrication of polymeric scaffolds for BTE requires precise control over the scaffold’s architecture, porosity, and mechanical properties as shown in Figure 2. Several fabrication techniques are commonly used to create polymeric scaffolds, including 3D printing, electrospinning, freeze-drying, solvent casting, and particulate leaching [46].
3D printing as shown in Figure 3 [48], also known as additive manufacturing, is a highly versatile and precise fabrication technique that allows for the creation of complex scaffold geometries with controlled pore sizes and shapes [49]. In 3D printing, a polymer solution or melt is deposited layer by layer to build the scaffold according to a pre-designed digital model. This technique offers excellent control over the scaffold’s architecture, enabling the creation of scaffolds with tailored porosity and mechanical properties. 3D printing can also be used to incorporate bioactive molecules or cells directly into the scaffold during fabrication, enhancing its bioactivity [50].
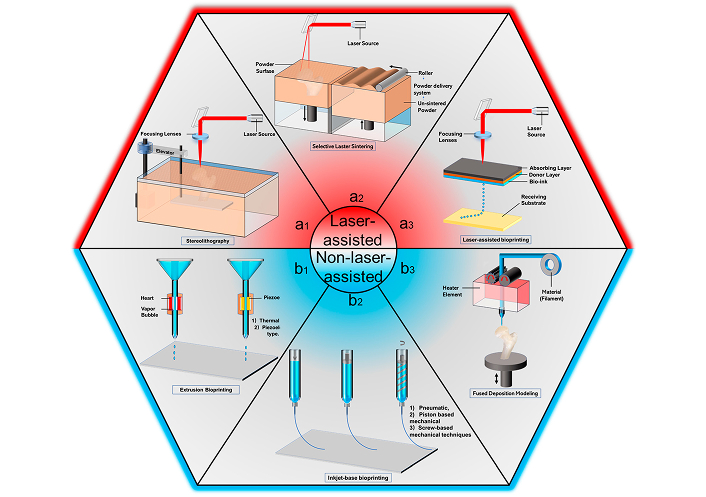
3D printing process diagrams. (a1) Selective laser sintering (SLS); (a2) stereolithography (SLA); (a3) laser-assisted deposition (LAD); (b1) extrusion-based bioprinting; (b2) inkjet bioprinting; (b3) fused deposition modeling (FDM). Reprinted from [48]. CC-BY 4.0
Electrospinning is a technique used to create nanofibrous scaffolds that closely mimic the fibrous structure of the natural ECM. In electrospinning, a polymer solution is charged and extruded through a small nozzle, forming fine fibers that are collected onto a grounded surface. The resulting scaffold has a high surface area-to-volume ratio, which promotes cell attachment and proliferation. Electrospun scaffolds are particularly useful for BTE applications due to their ability to support osteoblast adhesion and differentiation. However, controlling the pore size in electrospun scaffolds can be challenging, limiting cell infiltration and tissue ingrowth [51]. Operational parameters include voltage (10–25 kV), tip-to-collector distance (10–20 cm), and polymer solution flow rate (0.5–2 mL/h), which influence fiber diameter and scaffold porosity.
Freeze-drying is a technique used to create highly porous scaffolds with interconnected pore structures. In freeze-drying, a polymer solution is frozen and then sublimated under vacuum, leaving behind a porous scaffold. The pore size and porosity of the scaffold can be controlled by adjusting the freezing rate and the concentration of the polymer solution. Freeze-dried scaffolds are commonly used in BTE due to their high porosity, which supports cell infiltration and nutrient diffusion. However, freeze-drying can result in scaffolds with weaker mechanical properties compared to other fabrication techniques [52, 53]. Freezing temperature (−20°C to −80°C) and freeze rate determine pore size; slower freezing leads to larger pores. Primary drying is typically done under vacuum at < 0.1 mbar.
Solvent casting and particulate leaching are a widely used technique for fabricating porous scaffolds. In this method, a polymer solution is mixed with a porogen (a particulate material such as salt or sugar) and cast into a mold. After the solvent evaporates, the porogen is leached out, leaving behind a porous scaffold. The pore size and porosity of the scaffold can be controlled by the size and concentration of the porogen. Solvent casting and particulate leaching is a relatively simple and cost-effective method for fabricating scaffolds, but it can be difficult to achieve precise control over the scaffold’s architecture and mechanical properties [54].
The success of polymeric scaffolds in BTE depends not only on their mechanical and structural properties but also on their ability to interact with cells and tissues. The scaffold’s surface properties, such as roughness, hydrophilicity, and chemical composition, play a crucial role in determining how cells attach, proliferate, and differentiate on the scaffold. Additionally, the scaffold’s ability to promote osteogenesis, the process of new bone formation, is critical for successful bone regeneration [55].
Cellular interactions with the scaffold are essential for the regeneration of bone tissue. The scaffold’s surface properties influence how cells adhere to the scaffold, proliferate, and differentiate. Cells such as osteoblasts, which are responsible for bone formation, must be able to attach to the scaffold and produce the ECM necessary for new bone growth. The scaffold’s surface roughness, hydrophilicity, and chemical composition can all affect cell attachment. For example, scaffolds with a hydrophilic surface tend to promote better cell attachment and proliferation compared to hydrophobic scaffolds [56].
Osteoinduction refers to the scaffold’s ability to induce the differentiation of MSCs into osteoblasts, while osteoconduction refers to the scaffold’s ability to support the attachment and proliferation of osteogenic cells. Both of these processes are critical for successful bone regeneration. Osteoinductive scaffolds are often functionalized with bioactive molecules such as BMPs to enhance their ability to induce osteogenesis. Osteoconductive scaffolds provide a surface that promotes the attachment and proliferation of osteogenic cells, facilitating the formation of new bone tissue [57].
The immune response elicited by polymeric scaffolds is an important consideration in scaffold design. An ideal scaffold should evoke a minimal immune response while promoting tissue integration. Certain synthetic polymers, particularly those with acidic degradation byproducts, may trigger an immune response that can interfere with tissue regeneration. To minimize this, scaffold materials must be carefully selected to ensure they are biocompatible and do not elicit an inflammatory response. Additionally, surface modifications, such as coating the scaffold with bioinert materials or incorporating anti-inflammatory agents, can help reduce the immune response [58].
To enhance the biological performance of polymeric scaffolds, various functionalization strategies can be employed. These strategies involve incorporating bioactive molecules, surface modifications, or nanoparticles into the scaffold to improve its osteoinductive and osteoconductive properties [59].
Growth factors, such as BMPs, vascular endothelial growth factor (VEGF), and transforming growth factor-beta (TGF-β), play a critical role in bone regeneration by promoting cell proliferation, differentiation, and angiogenesis. Incorporating growth factors into polymeric scaffolds can significantly enhance their osteoinductive properties, promoting the differentiation of MSCs into osteoblasts and accelerating the formation of new bone tissue. Growth factors can be incorporated into the scaffold through various methods, including surface adsorption, covalent bonding, or encapsulation within the polymer matrix for controlled release [60].
Surface modifications can be used to enhance the scaffold’s bioactivity by improving cell attachment, proliferation, and differentiation. Techniques such as plasma treatment, chemical etching, or coating the scaffold with bioactive molecules can be used to modify the scaffold’s surface properties. For example, plasma treatment can increase the surface roughness and hydrophilicity of the scaffold, promoting better cell attachment and proliferation. Coating the scaffold with bioactive molecules, such as peptides or proteins, can also enhance its ability to support osteogenesis.
The incorporation of nanoparticles into polymeric scaffolds can enhance their mechanical properties and bioactivity. Nanoparticles such as HA, bioactive glass, or carbon nanotubes (CNTs) can be added to the scaffold to improve its osteoconductive properties and promote bone mineralization. HA, a naturally occurring mineral in bone, is commonly used in composite scaffolds to enhance their bioactivity and support the formation of new bone tissue. CNTs, with their excellent mechanical properties, can be used to reinforce the scaffold and improve its mechanical strength for load-bearing applications.
The in vivo performance of polymeric scaffolds is a critical factor in determining their suitability for clinical applications. Preclinical studies using animal models are essential for evaluating the scaffold’s ability to promote bone regeneration, its degradation behavior, and its biocompatibility. Several studies have demonstrated the successful use of polymeric scaffolds in promoting bone healing and regeneration in animal models [60]. For instance, PLGA scaffolds loaded with BMP-2 were implanted into critical-sized femoral defects in sheep and demonstrated significant bone bridging within 12 weeks, along with partial scaffold degradation and minimal inflammatory response [61]. Similarly, PCL-based scaffolds used in rabbit calvarial models showed substantial osteointegration and new bone tissue formation after 8 weeks, although full degradation of PCL was not observed within the study period [62]. These examples highlight both the efficacy and current limitations of polymeric scaffolds in vivo.
Various animal models, such as rats, rabbits, and sheep, are used to evaluate the in vivo performance of polymeric scaffolds. These models allow researchers to study the scaffold’s ability to support bone regeneration, its interaction with surrounding tissues, and its degradation behavior over time. For example, scaffolds made from PLGA or PCL have been implanted into critical-sized bone defects in animal models, showing promising results in promoting new bone formation and integration with surrounding tissues.
Animal models such as rats and rabbits are widely used in preclinical scaffold studies due to their affordability and reproducibility. However, larger animal models like sheep and dogs provide more clinically relevant data for load-bearing bone repair, especially when evaluating scaffold strength and osseointegration. For instance, PCL-HA composite scaffolds have demonstrated significant bone regeneration in rabbit calvarial defects within 8 weeks.
While these studies offer promising insights, translation to clinical practice remains limited. Recent clinical trials have explored the use of 3D-printed PLGA scaffolds combined with growth factors for maxillofacial reconstruction, reporting good integration and minimal adverse effects. However, long-term outcome data and larger patient cohorts are still needed to validate efficacy and safety for broader applications.
While preclinical studies provide valuable insights into the scaffold’s performance, human clinical trials are necessary to determine its efficacy in patients. Several polymeric scaffolds have progressed to clinical trials, where they are used to treat bone defects resulting from trauma, disease, or surgery. These trials assess the scaffold’s ability to promote bone healing, its biocompatibility, and its safety for human use. Early clinical trials have shown promising results, with some scaffolds demonstrating the ability to support bone regeneration and integration with surrounding tissues.
Despite significant advancements in the development of polymeric scaffolds for BTE, several challenges remain. One of the primary challenges is the difficulty in creating scaffolds with mechanical properties that match those of natural bone, particularly in load-bearing applications. Additionally, the degradation behavior of polymeric scaffolds must be carefully controlled to ensure that the scaffold degrades at a rate that matches the formation of new bone tissue.
Another challenge is the clinical translation of polymeric scaffolds. Regulatory barriers, the need for large-scale production techniques, and the lack of standardized testing methods all present obstacles to the widespread adoption of polymeric scaffolds in clinical practice. Moreover, the long-term safety and efficacy of these scaffolds in patients need to be thoroughly evaluated through clinical trials. Despite their promising laboratory results, the clinical translation of polymeric scaffolds faces several challenges. Regulatory hurdles, particularly from agencies like the U.S. FDA, require extensive safety and efficacy data before approval. The high cost of manufacturing, especially for advanced fabrication techniques such as 3D bioprinting, limits scalability. Furthermore, the number of scaffold-related human clinical trials remains limited, highlighting a gap between bench research and bedside implementation. Addressing these challenges requires collaborative efforts in regulatory science, cost-effective fabrication, and the establishment of clinical-grade testing pipelines [63] (Figure 4).
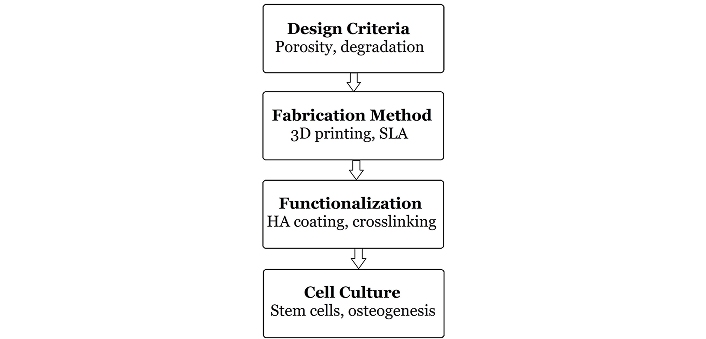
Schematic representation of the ideal scaffold development pathway from material selection to clinical application
Recent advancements in materials science and tissue engineering have led to the development of “smart” scaffolds that can respond to environmental stimuli, such as changes in temperature, pH, or mechanical stress. These scaffolds can release bioactive molecules in a controlled manner or adjust their mechanical properties in response to the surrounding environment. The development of smart scaffolds holds great promise for enhancing the efficacy of BTE.
Another emerging trend is the use of bioprinting, a technique that allows for the precise deposition of cells and biomaterials to create complex tissue constructs. Bioprinting offers the potential to create patient-specific scaffolds that are tailored to the individual’s anatomy and biological needs, further advancing the field of personalized medicine.
Polymeric scaffolds have emerged as a promising solution for BTE, offering a versatile and customizable platform for promoting bone regeneration. Synthetic and natural polymers, as well as composite scaffolds, provide the mechanical strength, biocompatibility, and bioactivity necessary for successful bone healing. Advances in fabrication techniques, such as 3D printing and electrospinning, have enabled the creation of complex scaffold geometries that closely mimic the structure and function of natural bone tissue.
However, challenges remain in optimizing the mechanical properties and degradation behavior of polymeric scaffolds, as well as ensuring their clinical translation. Future research will likely focus on the development of smart scaffolds, bioprinting technologies, and the incorporation of bioactive molecules to enhance the scaffold’s ability to support bone regeneration. With continued advancements in materials science and tissue engineering, polymeric scaffolds hold great potential to revolutionize the treatment of bone defects and improve patient outcomes in regenerative medicine.
BMPs: bone morphogenetic proteins
BTE: bone tissue engineering
CNTs: carbon nanotubes
ECM: extracellular matrix
HA: hydroxyapatite
MSCs: mesenchymal stem cells
PCL: polycaprolactone
PGA: polyglycolic acid
PLA: polylactic acid
PLGA: poly(lactic-co-glycolic acid)
AF, SFD: Conceptualization, Writing—original draft, Writing—review & editing. Both authors discussed the results and reviewed and approved the final version of the manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship and publication of this article.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Sunil Choudhary ... Donato Gemmati
Vladimir Karpiuk ... Olga Ponkina
Zhaoying Ma ... Jan T. Czernuszka
Alexandra C. Dabrowski ... Maribel Vazquez
