Affiliation:
1Department of Physics, Handique Girls’ College, Guwahati 781001, Assam, India
Email: apurba.das@hgcollege.edu.in
ORCID: https://orcid.org/0000-0003-2695-2336
Affiliation:
2Department of Physics, Nabajyoti College, Barpeta 781319, Assam, India
ORCID: https://orcid.org/0000-0003-1896-3262
Explor BioMat-X. 2025;2:101338 DOI: https://doi.org/10.37349/ebmx.2025.101338
Received: February 20, 2025 Accepted: April 25, 2025 Published: May 13, 2025
Academic Editor: Maryam Tabrizian, McGill University, Canada
The article belongs to the special issue Metal 3D Printing of Biometals for Prostheses and Implants
Metal 3D printing has revolutionized the fabrication of biometallic prostheses and implants, offering unprecedented design flexibility, patient-specific customization, and enhanced biomechanical performance. This review explores the current advancements in metal additive manufacturing (AM) techniques, including selective laser melting (SLM), electron beam melting (EBM), fused deposition modeling (FDM), directed energy deposition (DED), sheet lamination, stereolithography (SLA), and binder jetting, for processing biocompatible metals such as titanium, cobalt-chromium, and stainless steel. The article discusses major benefits, such as enhanced osseointegration, complex lattice architectures for weight saving, and optimized mechanical properties. The challenges of residual stresses, surface finish, and regulatory issues are also discussed. The review concludes by defining future research avenues in material design, process development, and clinical translation to increase the efficacy and reliability of 3D-printed biometal implants.
Significant improvements in medical technology have massively increased the standard of living for patients who need prostheses or implants. In the list of vital materials used in various prosthetics, biometals such as titanium, stainless steel, or cobalt-chromium alloys take precedence due to their superior mechanical properties, corrosion resistance, and biocompatibility [1]. Prostheses for implants formulated by traditional manufacturing procedures—casting and machining—often fall short of the optimal geometric features and the personalized fixation modes necessary to achieve best performance and patient-specific solutions [1].
In this context, metal 3D printing was introduced as a technology to address these limitations. Applying this new technology, this method enables one to manufacture structures by depositing one layer over another, allowing the designing of lightweight structures and components with intricate geometries and porous structures that mimic natural bones [2]. Furthermore, cost savings on material and reduced manufacturing timelines have made 3D printing an attractive alternative to the traditional way of doing things [1].
In this review, we explore the application of metal 3D printing in fabricating of biometals for prostheses and implants. The mini-review aims to discuss the different 3D printing technologies, biometal materials, design considerations, current applications, challenges and future prospects. The review will develop an understanding of the potential of metal 3D printing to revolutionize the field of biomedical implants and prostheses, paving the way for development of personalized and high-performance healthcare solutions.
Metal 3D printing technologies are layer-by-layer based, and as such, they can produce complex geometries impossible with the conventional advanced manufacturing process. This process starts with a 3D model in a digital form, which is cut across several layers and transmuted into instructions recognizable by the machine [3]. Metal powders or wires are used as the raw material, while lasers or electron beams heat or join them to form a functional object. These processes provide a level of control that allows for the fabrication of complex designs and patient-specific implants based on an individual’s anatomy [3].
Parts produced using selective laser melting (SLM) are characterized by excellent mechanical properties and high-quality product features due to the typical use of layered metalicious powder, which is melted layer by layer via a high-end laser. Customized acetabular cups for hip replacements are made using SLS, which provides porous materials that promote bone formation. It has been investigated to produce patient-specific spinal cages, which would provide implants complementing the intricate structure of the spine. Thus, SLM is quite favorable for most biomedical applications [3].
Attaining optimal porosity control in SLM for biomedical implants entails overcoming several challenges, especially in designing process parameters like laser power and scan speed. These parameters have a significant effect on the mechanical properties of titanium-based implants fabricated by SLM.
Some challenges associated with porosity control are listed below:
Process Parameter Interdependence: Laser power, scan speed, hatch spacing, and layer thickness are all interconnected. Modifying one can impact others, making it challenging to optimize. For example, an increase in laser power with a fixed scan speed can decrease porosity but could cause overheating and defects such as keyholing [4].
Energy Density Management: Optimizing the correct energy density is essential. Less energy can lead to incomplete melting and porosity, while high energy can result in vaporization and defects. Research has indicated that boosting energy density by modifying laser power and exposure time reduces surface roughness and porosity, thereby improving the material density [4].
Thermal Gradients and Residual Stresses: Steep thermal gradients generated by rapid heating and cooling in SLM cause residual stress and even lead to cracking. They can ultimately affect porosity and overall mechanical performance [4].
Laser power and scan speed significantly influence the mechanical properties of SLM printed products, including:
Tensile Strength and Elongation: The interplay of scan speed and laser power influences tensile properties significantly. Achieving optimal combinations results in higher tensile strength coupled with elongation. In a particular report, it was established that a 170 W laser power and a 900 mm/s scan speed yielded tensile strengths of 1,200–1,265 MPa. However, the elongation varied, depending on scan speed, reaching a maximum of 1,300 mm/s [5].
Microstructural Properties: Changes in scan speed and laser power affect microstructure. Increased scan speeds could sharpen α’ martensite structures, increasing ductility. Lower scan speeds can instead cause microstructures to coarsen, lowering mechanical properties [5].
Surface Roughness and Dimensional Accuracy: Scan speed and laser power also affect the surface properties. Higher laser power may lower the surface roughness, enhancing dimensional accuracy. Too much power can lead to overheating, resulting in surface defects [5].
Therefore, accurate laser power and scanning speed control are critical for maintaining porosity levels and obtaining ideal mechanical properties for titanium-based biomedical implants produced by SLM. A thorough analysis of the dependency relationships between processing parameters is necessary to generate high-performance and longevity implants.
In electron beam melting (EBM), the electron beam acts as a heat source to melt the metal powder in a vacuum. This process is preferably used in titanium and its alloys since the process involves has high efficiency without risking oxidation [1].
EBM involves a vacuum process during additive manufacturing. This type of environment highly impacts the microstructure and mechanical properties of titanium alloys when compared to processing methods such as SLM.
The influence of a vacuum environment on EBM is as follows:
Microstructural Properties: The vacuum produced in EBM reduces oxidation and contamination, resulting in a cleaner microstructure. EBM-processed Ti-6Al-4V shows a predominantly α + β phase, while SLM tends to produce a martensitic α’ phase because of faster cooling rates [6].
Mechanical Properties: The α + β microstructure produced in EBM tends to provide a combination of strength and ductility. Conversely, the α’ martensitic structure produced in SLM tends to produce greater strength but lower ductility. The lower residual stresses produced in EBM can improve fatigue resistance, which is important for load-bearing applications [6].
The following are the limitations of EBM in generating complex geometries in patient-specific implants:
The surface finish of parts made by EBM tends to be coarser than that of SLM. It requires heavy post-processing to obtain smooth surfaces for patient-specific biomedical implants [7].
High dimensional accuracy in EBM can be challenging with factors such as beam focus and powder spreading methods, which could influence the fit of patient-specific implants [4].
It is difficult to achieve high dimensional accuracy in EBM because of beam focus and powder spreading methods, which can influence the fit of patient-specific implants [8].
Complicated geometries risk the trapping of unmelted powder within internal pores, making post-processing more complex and possibly impacting on implant function [8].
To summarise, although EBM’s vacuum environment benefits the production of titanium alloys with desirable microstructures and mechanical properties, difficulties in precision and surface quality for complex patient-specific implants are still present. Solving such limitations entails refining process parameters and executing effective post-processing methods.
This is a process in which a liquid binding agent is deposited on the bed of metal powder and sintered into a solid form. The material has achieved properties that have resulted from sintering. Binder jetting is cheaper and quicker than SLM or EBM, but its mechanical strength is inferior [3]. Custom silicone maxillofacial prostheses with precise anatomical characteristics and a customized fit have been created and reported via binder jetting [9]. Binder jetting makes it possible to include intricate internal features that support osseointegration in hip implants onto patient-specific bone scaffolds [10].
Binder jetting is well known for being cost-effective and design-intensive in additive manufacturing. Its use to create high-load-bearing implants like hip or knee replacements, though, is limited by some mechanical constraints:
Intrinsic Porosity: The binder jetting process tends to create parts with intrinsic porosity because of the process of powder binding, followed by binder removal. Such porosity may cause low density and mechanical strength, reducing the parts’ suitability for high load-bearing applications [11].
Residual Binder Content: Failure to remove the binder during post-processing may result in residuals that negatively impact the mechanical properties of the product [11].
Material Inhomogeneity: Powder packing variations and saturation variability can lead to inhomogeneities in the printed parts. These inhomogeneities are likely to be weak spots, which may become the source of structural failure of biomedical implants [11].
To improve the mechanical properties of binder-jetted metal components, several post-processing methods are utilized:
Sintering: Sintering is a process where the printed part is heated below its melting point to melt metal particles together, thus making it denser and more potent. Sintering, however, causes substantial shrinkage, which needs to be expected during the design stage so that dimensions remain accurate [12, 13].
Infiltration: To decrease porosity further, the sintered component can be infiltrated using a secondary material, for example, bronze. This infiltrating process fills any remaining voids, improving density and mechanical performance. For instance, bronze infiltration of stainless-steel components can produce a final density of up to 95% [12].
Hot Isostatic Pressing (HIP): Uniformly pressurizing a sintered part with high-pressure gas, HIP minimizes internal porosity and enhances strength and fatigue properties. HIP works exceptionally well with parts with high structural integrity [14].
Surface Finishing: Grinding, polishing, and coating are applied to improve surface quality, smoothness, and fatigue resistance. These processes are necessary for attaining the smooth surfaces needed by biomedical implants [15].
Fused deposition modeling (FDM) builds parts layer by layer by extruding thermoplastic material using a heated nozzle. Multi-material FDM has been investigated to produce personalized hand prostheses, emphasizing surface structure change to improve functioning [6]. FDM has been used to facilitate pre-operative planning and the creation of customized prosthetic components to create patient-specific knee joint models.
FDM is an extensively used additive manufacturing technique for creating medical implants from materials such as Polyether Ether Ketone (PEEK). The crystallinity of PEEK plays a critical role in the long-term stability and mechanical behavior of these implants, particularly in load-bearing applications. PEEK is a semi-crystalline thermoplastic, i.e., its structure consists of both amorphous (disordered) and crystalline (ordered) parts. The level of crystallinity influences several important properties that are discussed below:
Mechanical Strength and Stiffness: Greater crystallinity usually increases mechanical strength and stiffness, which is essential in load-bearing implants. However, over-crystallization might make the material more brittle and prone to stress fracture [16, 17].
Thermal Stability: Higher crystalline content enhances thermal stability, which is advantageous in sterilization procedures that include high-temperature processes [17, 18].
Chemical Resistance: Increased crystallinity improves resistance to chemical degradation, helping to maintain the durability of the implant in the physiological environment [18].
It is difficult to control the crystallinity in the FDM process because of high cooling rates, which lead to reduced crystallinity and, as a result, lower mechanical properties. Research has suggested new processes for controlling the crystallinity of 3D-printed carbon fiber-reinforced PEEK composites to overcome these problems.
To improve the precision and resolution of FDM for more complex prosthetic designs, the following steps can be adopted:
Using advanced high-resolution 3D scanning methods to obtain precise anatomical information, along with advanced CAD programs, implant designs can closely replicate patient-specific geometries. This has been found to help improve success rates and outcomes for patients in prosthetics and orthopedics [19].
Optimization of FDM parameters like layer thickness, print speed, and hot end temperature can improve surface finish and dimensional accuracy. Lower layer thicknesses, for example, produce smoother surfaces and more accurate details [20].
Using nozzles of lower diameters provides more delicate extrusion, enhancing the definition of fine details in complicated prosthetic structures [20].
Post-processing techniques such as polishing, annealing, or chemical smoothing can further improve the surface quality and dimensional accuracy of FDM-printed implants [19, 20].
In this process, metal powder or wire is deposited directly onto a substrate and melted by a laser or electron beam. This process is suitable for repairing implants already in the patient’s body or producing large structures [4]. Directed energy deposition (DED) has been used in spinal applications to create patient-specific intervertebral fusion devices, allowing for constructing intricate geometries that meet unique anatomical specifications.
DED is a sophisticated additive manufacturing process that presents high material utilization, rapid deposition rates, and the capability for repairing or remanufacturing metal parts [4]. It supports multi-material printing and large-scale production, which are beneficial for industrial and aerospace use. Compared to powder bed fusion DED has some restrictions, such as reduced resolution, increased porosity, and constrained geometric complexity. The high thermal input may induce residual stress and distortions, which need post-processing [4]. DED usually employs lasers, electron beams, or plasma arcs to melt the metal powder or wire feedstock under a controlled condition, and is therefore, appropriate for structural and biomedical applications.
Stereolithography (SLA) builds parts layer by layer by curing a vat of liquid photopolymer resin with a UV laser. It has been shown that SLA may be used to create patient-specific spine models, which can help with preoperative planning and implant design. Complex knee joint prototypes have been created using SLA, enabling precise anatomical reproduction and prosthetic design testing.
SLA in 3D printing of biometals for prosthetics and implants provides high accuracy, good surface finish, and the capability to produce intricate geometries necessary for patient-specific models [21]. Its drawbacks are that it requires extra steps since SLA alone cannot directly print metals—resin-based casting or hybrid methods are needed. Photopolymer residues raise biocompatibility issues, and mechanical strength is less than in direct metal printing techniques [22]. Notwithstanding, its benefits include a higher resolution for complex lattice structures that optimize osseointegration [21]. Notable characteristics involve UV-curable resin patterns to cast in investments, compatibility with bioactive coatings, and utilization in manufacturing detailed surgical guides or metal implant scaffolds [22].
Sheets of material are stacked, bonded, and then trimmed to form a 3D structure. Large-scale anatomical models for surgical planning in hip replacement surgeries have been created using sheet lamination, which provides quick and affordable fabrication [23]. Sheet lamination has been utilized in knee prostheses to produce personalized cutting guides that improve surgical accuracy.
Sheet lamination for 3D printing of biometals into prostheses and implants involves bonding and piling thin sheets of metal on top of one another, then cutting them into shape with ultrasonic or laser techniques [1]. The benefits are cost efficiency, high rates of production, and the use of multiple metals in a single build, which is useful in functionally graded implants [1]. It also generates less heat than other metal 3D printing techniques, lowering residual stresses [24]. However, its shortcomings are that it has less resolution, has poorer interlayer bonding than powder-based metal printing, and has extended post-processing, such as machining and sintering, to attain final mechanical properties [24]. Special features involve the utilization of biocompatible metal sheets (e.g., titanium), using ultrasonic consolidation to bond the layers, and the ability to produce patient-specific prosthetics with layered material properties [24].
Every technology has its advantages and should be selected based on the type of material, requirements for an application, and considerations in cost. A typical schematic diagram showing the different 3D printing processes is depicted in Figure 1 and their comparison is presented in Table 1.
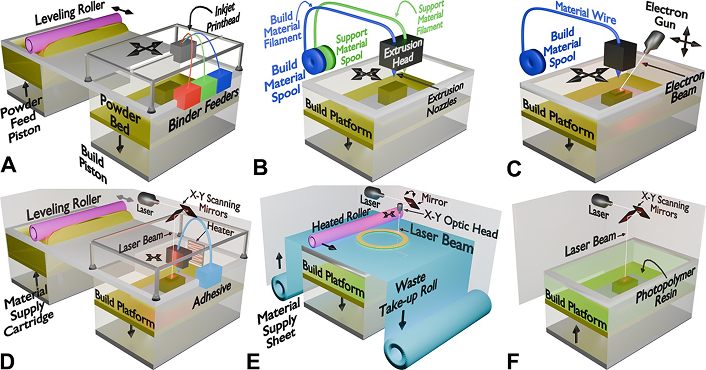
Schematic representation of different metal additive manufacturing (AM) techniques. (A) Binder jetting—A liquid binder is selectively deposited onto a powder bed to bond metal particles, followed by sintering or infiltration to form the final part. (B) Fused deposition modeling (FDM)—Thermoplastic or metal-filled filaments are extruded layer-by-layer through heated nozzles to build the part. (C) Directed energy deposition (DED)—A focused energy source (electron beam or laser) melts metal wire or powder as it is deposited onto the build platform, enabling near-net-shape fabrication. (D) Selective laser melting (SLM)—A laser selectively fuses powdered material layer by layer, forming a solid structure with precise control over porosity. (E) Sheet lamination—Thin sheets of metal or composite material are successively bonded and cut to shape using lasers or ultrasonic energy. (F) Stereolithography (SLA)—A laser selectively cures liquid photopolymer resin, layer by layer, to produce highly detailed and accurate 3D structures
Characteristics of various 3D printing techniques
| 3D Printing Technique | Materials Used | Precision* | Strength# | Surface Finish@ | Applications |
|---|---|---|---|---|---|
| Binder jetting [25] | Metals, ceramics, polymers | Moderate | Moderate (post-processed) | Rough (needs post-processing) | Custom bone scaffolds, complex implants |
| Fused deposition modeling (FDM) [26] | Thermoplastics (PLA, ABS, PEEK) | Low to moderate | Moderate | Rough (can be smoothed) | Custom prosthetic limbs, knee joint models |
| Directed energy deposition (DED) [4] | Metals (Ti, co-Cr alloys) | High | High | Moderate | Hip implant repairs, spinal fusion devices |
| Selective laser sintering (SLS) [27] | Polymers, metals, composites | High | High | Moderate | Spinal cages, acetabular cups for hip implants |
| Sheet lamination [28] | Paper, metal foils, polymers | Low to moderate | Moderate | Varies (depending on material) | Anatomical models, surgical cutting guides |
| Stereolithography (SLA) [29] | Photopolymers | Very high | Low to moderate | Excellent (smooth finish) | Detailed spinal models, knee prototypes |
* The ability to produce detailed, complex geometries; # depends on materials and post-processing techniques; @ indicates the smoothness of the final product without additional treatments
Titanium, specifically Ti-6Al-4V, is the gold standard in the field of biomedical implants by offering a higher strength-to-weight ratio, corrosion resistance, and excellent biocompatibility. It is light important for hip and knee replacement, spinal implants, and dental prosthetics. Its osseointegration property further helps stabilize and prolong the implant’s life (see Table 2) [30].
| Metal | Bio-compatibility | Corrosion Resistance | Strength-Weight Ratio (MPa/g/cm3) | Elastic Modulus (GPa) | Cost | Key Applications |
|---|---|---|---|---|---|---|
| Magnesium | Good | Low | ~130–150 | ~45 | Moderate | Temporary implants, biodegradable screws |
| Cobalt-chromium (Co-Cr) | Good | Excellent | ~70–90 | ~200–240 | Moderate | Joint replacements (hips, knees) |
| Stainless steel (316L) | Moderate | Good | ~25–30 | ~200 | Low | Bone plates, screws, temporary implants |
| Gold (Au) | Excellent | Excellent | ~5–10 | ~80 | Very high | Neural and cochlear implants |
| Titanium (Ti) | Excellent | Excellent | ~100–120 | ~110 | High | Orthopedic implants, dental implants |
| Platinum (Pt) | Excellent | Excellent | ~10–20 | ~170 | Very high | Electrodes, specialized implants |
Titanium and titanium alloys, particularly Ti-6Al-4V, are commonly used in medical implants because of their desirable mechanical properties and biocompatibility. Improvements in surface characteristics by processes such as nano-texturing have been demonstrated to profoundly effect on the osseointegration process—the direct structural and functional bond between living bone and the implant surface.
Nano-textured titanium alloy surfaces enhance osseointegration by:
Increased Osteogenic Activity: Nanofeatures trigger bone-forming cells, which results in better bone integration [30].
Enhanced Protein Adsorption: The higher surface area and energy of nano-textured surfaces allow for increased protein adsorption, essential for cell adhesion and proliferation [30].
Cobalt-chromium (Co-Cr) alloys also find applications in orthopedic implants. Although these have high strength and wear resistance, their osseointegration properties could differ from titanium alloys. Co-Cr alloys have been studied with titanium coatings to increase their surface properties. Titanium coatings done through direct metal fabrication (DMF) and titanium plasma spraying (TPS) have been compared for their effect on osseointegration.
The development of 3D printing technologies like SLM has changed the manufacturing of titanium implants with their ability to have complex geometries and patient-specific models. Nonetheless, the corrosion resistance of such implants is still a vital concern. Some recent developments aimed at improving corrosion resistance include:
Surface Modification Techniques: Chemical, physical, and biological surface modifications have enhanced corrosion resistance in 3D-printed titanium alloys. The modifications exhibit improved corrosion resistance and enhance antibacterial activity and osteogenesis [31].
Computational Alloy Design: Scientists use computational techniques to develop titanium alloys with enhanced corrosion and wear resistance. With the optimization of alloy composition, properties like phase stability, biocompatibility, and strength can be improved, lowering corrosion susceptibility [32].
These developments play a role in creating more robust and dependable titanium implants, eventually leading to better patient outcomes in orthopedic and dental procedures.
Stainless steel, especially 316L, is commonly used due to its cost-effectiveness, strength, and corrosion resistance. Temporary implants and surgical instruments are often made from it. Co-Cr alloys are famous for their high wear resistance and are often used in load-bearing applications such as knee and hip joints. These materials are very suitable for SLM and EBM technologies as they can be used to produce complex and robust designs (see Table 2) [3].
Magnesium alloys are an emerging class of biodegradable materials that can serve as temporary implants. These alloys facilitate natural healing through the biodegradation process of the implant through gradual dissolution. This aspect allows for reducing secondary surgery, thus minimizing patient discomfort and lowering costs associated with healthcare. Some other issues, including degradation rate control and mechanical stability during the healing process, remain under study (see Table 2) [1].
Magnesium (Mg) alloys are receiving interest in orthopedic devices because they are biodegradable and have mechanical properties like natural bone. Nevertheless, various challenges must be overcome for their degradation rate to be successfully controlled and for their mechanical performance to match the needs of clinical applications.
Challenges in controlling degradation rate:
Rapid Corrosion: Mg alloys tend to corrode rapidly in physiological environments, resulting in premature mechanical integrity loss prior to bone healing completion. Rapid degradation may lead to structural failure of the implant [33].
Hydrogen Gas Evolution: The corrosion of Mg alloys results in the production of hydrogen gas. Excess gas buildup can create pockets around the implant site and result in complications such as delayed healing or tissue damage [34].
pH Rise and Alkalization: Mg alloy degradation can result in a rise in local pH values, which can cause alkalization of the surrounding tissue. Such a shift in pH may harm cell viability and the healing process [33, 34].
These challenges may be tackled by incorporating elements like Al, Zn and Ca to enhance the corrosion resistance and maintain mechanical integrity. However, careful optimization of the concentration of the elements must be made so as not to disturb the biocompatibility aspect of these alloys. Surface modification techniques like coating with a biocompatible material and anodization help generate a protective layer that slows the corrosion rate and improves biocompatibility.
A comparison of Mg alloys with established materials like Ti in terms of their mechanical properties is tabulated in Table 3.
Mechanical properties of Mg Alloys and their comparison with cortical bones and Ti alloy
Controlled porosity is important for the proper functioning of 3D-printed implants because it allows the growth of bone and vascular tissues, thus promoting osseointegration and improving the stability of the implant in general. The microstructural features, such as grain size and phase composition, affect the mechanical properties and fatigue resistance of the implant [24]. Advanced simulation tools can be used to optimize these features to ensure that the implants produced closely match the properties of natural bone [37].
Optimization of the production process of 3D-printed titanium and stainless steel implants is critical to reducing porosity and residual stresses that can undermine mechanical integrity and function. Some of the steps that can be incorporated are:
Powder Quality and Preparation: Using of metal powders with spherical, uniform particles and a narrow particle size distribution favors uniform melting and solidification and minimizes porosity. Powder cleanliness and contamination prevention ensure material integrity throughout printing [38].
Process Parameter Control: Controlling Laser power and scan speed optimizes melting and fusion of powder layers, reducing defects. Additionally, layer thickness optimization can improve resolution and decrease porosity but increase build time [39].
Post-Processing Method: Suitable heat treatments can remove residual stresses and enhance mechanical properties. Treating the printed part by HIP under high temperature and pressure minimizes porosity and increases density [2].
Surface Treatment: Laser shock peening and ultrasonic nanocrystal surface modification can enhance surface integrity and minimize residual stresses [31].
Additive manufacturing techniques can introduce anisotropy, a condition in which mechanical properties differ concerning the direction of fabrication, potentially influencing the functionality of load-bearing devices. Additionally, anisotropic behavior can result in lower strength and fatigue resistance in some directions, posing a risk in critical use. The approaches that can be taken to minimize the impact of anisotropy on optimal mechanical performance are:
Orientation of the build direction along the central load-carrying axis can improve mechanical performance [40].
Customizing parameters such as laser power and scanning strategy can facilitate more isotropic microstructures, minimizing anisotropy [40].
Heat treatments can homogenize microstructures, counteracting anisotropic effects [41].
Integration of additive manufacturing with conventional techniques, such as machining key surfaces, can improve mechanical properties and minimize anisotropy [41].
The application of these strategies depends on a detailed knowledge of material behavior and process dynamics to enable the production of consistent, high-performance 3D-printed implants.
Grain size and phase composition are particularly important factors controlling the fatigue resistance and general mechanical performance of 3D-printed metals.
Grain Size: Finer, equiaxed grains generally improve mechanical properties, such as fatigue resistance, by hindering dislocation movement. In additive manufacturing, fast solidification creates fine microstructures, which may enhance strength and fatigue life. However, columnar grains oriented in the build direction can introduce anisotropy, which may compromise fatigue performance in some orientations [42].
Phase Composition: Phase distribution and stability in the microstructure profoundly influence on mechanical behavior. For example, cellular structure formation during laser powder bed fusion (L-PBF) in materials such as stainless steel 316L can impact fatigue behaviour [43].
Sophisticated simulation software has been created to forecast how process parameters—layer thickness and scan speed, for example—affect the mechanical properties and microstructure of 3D-printed implants. Some of them are discussed below.
Finite Element Analysis (FEA): FEA simulations model the thermal and mechanical responses throughout printing, including insight into residual stress, distortion, and the risk of defect creation. Optimized process parameters can be achieved with these simulations in order to acquire the desired mechanical performance [44].
Cellular Automaton Techniques: These simulations simulate microstructural development by modelling how metal grains develop due to thermal gradients as the material solidifies. When these techniques are combined with FEA, scientists can forecast microstructure development and its effect on mechanical properties [45].
Machine Learning Models: Data-driven approaches utilize experimental data to predict mechanical properties based on processing parameters. By training models on extensive datasets, these tools can forecast outcomes for various materials and printing conditions, aiding process optimization [46].
These simulation software packages allow manufacturers to customize process parameters efficiently to obtain optimal microstructures and mechanical properties in 3D-printed metal implants.
Topology optimization improves the design process by minimizing material usage while maintaining mechanical strength. Lattice structures have been highlighted as one of the most promising methods for weight reduction, flexibility enhancement, and tissue integration [8].
The process parameters, including laser power, scan speed, and layer thickness, must be strictly controlled to obtain the desired precision and mechanical properties in metal 3D printing [47]. Deviations may lead to defects such as porosity, residual stresses, and anisotropic properties, which may compromise the performance of the implant [37]. Heat treatment (sintering) and surface finishing are usually applied as post-processing techniques to mitigate these issues and improve the quality of the final product.
Such devices printed by 3D printing require standard quality assurance protocols in device manufacturing to ensure their reliability and safety. Long-term biocompatibility studies are also essential for garnering approval for any clinical use, apart from regulatory compliance.
Biocompatibility testing of 3D-printed metal implants involves chemical, mechanical, and biological tests to ascertain safety and effectiveness. Standard protocols from organizations such as the Food and Drug Administration (FDA) and the International Standard Organization (ISO) offer guidelines for these tests. Some of the regulatory hurdles are as follows:
Traditional manufacturing processes benefit from established standards ensuring product consistency. In contrast, the layer-by-layer nature of 3D printing introduces variables that can affect the mechanical properties and reliability of implants. Regulators require comprehensive validation to ensure each customized implant meets stringent safety and efficacy criteria [48].
Though customization improves patient outcomes, it makes the regulatory approval process more complex. Every custom implant can require separate evaluation, making it difficult for timely approvals [48].
Ensuring reproducibility of 3D printing operations and the production of implants to specified consistent requirements is essential. This involves verifying parameters like laser power, scan speed, and layer thickness [48].
Repeatability of 3D printing operations, such that the produced implants have uniformly predetermined specifications, is essential. This involves qualifying parameters like the power of a laser, a scan’s velocity, and the thickness of layers [48].
Post-processing treatments such as heat treatment and surface finishing are critical to obtaining implant properties of interest. Regulators evaluate these steps to ensure that they do not add defects or impair implant integrity [48].
Biocompatibility tests for 3D-printed metal implants are performed differently from conventional manufacturing processes because of differences in surface chemistry, mechanical properties, and possible contamination from additive manufacturing processes [49]. Conventional processes such as forging and machining yield well-characterized, homogenous surfaces, while 3D-printed implants tend to be characterized by anisotropic mechanical response due to unresolved residual powders and higher surface roughness because of their layer-by-layer generation [50]. Therefore, these additive manufacturing implants require additional chemical characterization (ISO 10993-18), fatigue testing (ASTM F3122), and evaluation of powder contamination. In addition, controlled porosity within the 3D printed implant, providing the osseointegration benefits, requires additional assessment according to ISO 23317 for testing the bone ingrowth and the bacterial adhesion risk [51]. Sterilization is also more complicated, as there is a potential for powders to become trapped in intricate porous structures, so ASTM F3328 has been developed to clean additive manufacturing components. To improve test protocols, standards for biocompatibility need to be established specifically for additive manufacturing, with enhanced in vivo and in vitro studies that are used further to comprehend porous surface interactions and localized corrosion hazards [51]. In addition, more advanced post-processing techniques like electropolishing, laser peening, and HIP are required to minimize contamination, enhance fatigue life, and provide long-term patient-specific implant safety [51].
Resolving these testing and regulatory issues is essential to maximizing the advantages of 3D-printed, patient-specific metal implants in the clinical environment.
Technological innovations in reconstructive surgery and 3D prostheses have transformed patient outcomes with novel technology and collaborative techniques. The journey started with making the first craniofacial model in 1995 using a milling technique, which paved the way for high-precision surgical planning. By 2014, creating the “Haptic Reverse Model” for preoperative planning significantly enhanced outcomes in soft tissue repair by enabling surgeons to accurately model operations [52]. One year later, 4D printing and scanning technology revolutionized medical modelling by producing dynamic, patient-specific haptic models, enhancing surgical preparation and patient care [53].
The creation of titanium alloy prostheses to reconstruct of chest walls demonstrated the amalgamation of compatible materials and complex manufacturing processes. The initial use of 3D printed custom-made titanium implants for bone chest wall reconstruction was reported by Turna et al. in 2014 [53]. Later (2020), upgraded imaging and computer modelling streamlined preoperative planning for complex hypertrophic excisions, enabling more accurate and personalized procedures. Computed Tomography (CT), Magnetic Resonance Imaging (MRI), and Ultrasound (US) are some of the conventional imaging modalities that give fixed two-dimensional (2D) images that render complex anatomy challenging to interpret [54]. Through 3D image post-processing and image segmentation, advanced volumetric medical imaging enables the generation of 3D volume renderings, thereby enhancing the visibility of pertinent anatomic details in 3D [55].
Identifying lipochondrocytes (LCs) in 2025 has opened new avenues in reconstructive surgery with new applications in cartilage reconstruction and flexible structural support for complex face reconstructions [56]. With advancements in the field, the incorporation of tissue-engineered muscle implants for mind-controlled prosthetics has the potential to restore not just form but function, with direct neural pathway integration for intuitive control.
The timeline, outlined in Figure 2, shows the revolutionary evolution of reconstructive prosthetics and surgery, fueled by the convergence of regenerative medicine, biomaterials, and 3D printing. Future development will likely be focused on patient-specific, completely integrated solutions that span the engineering-biology divide to provide novel surgical outcomes.
In March 2024, Nature (Scientific Reports) published a study detailing the production of 3D-printed prosthetic leg sockets. A positive mould of the residual limb is developed through a 3D scanner, which forms a highly accurate 3D model that can be customized per patient. It enhances the fit and comfort of prosthetic wearers [56]. Four compounds—carbon fiber, carbon-Kevlar fiber, fiberglass, and cement are employed to manufacture and strengthen fifteen polylactic acid+ (PLA+) prosthetic leg sockets. Axial compression and scanning electron microscopy (SEM) test the mechanical and microstructural properties of the sockets. The tests demonstrate that cement-reinforced sockets possess better properties, with yield strength increased up to 89.57% and Young’s modulus up to 76.15% compared to their counterparts [57].
Metal 3D printing, or additive manufacturing, has revolutionized the field of orthopedic implants, particularly in developing hip, knee, and spinal devices. Additive manufacturing allows the fabrication of patient-specific implants with complex geometries, enhancing functionality and biocompatibility. During hip replacement surgeries, 3D printing facilitates the manufacture of implants personalized to every person’s anatomy. The custom fit enhances the fit of the implant and integration into bone structure. The hip joint consists of the femur or thigh bone and the pelvis. The socket is a bowl-like depression surrounding the lower end of the pelvis (acetabulum), and the ball is the rounded margin of the femur. Among the important conditions causing hip pain are cancer, pinched wounds, arthritis, injuries, and other lifestyle-related issues [58]. Treatments involve non-surgical and surgical procedures, including hip replacement. Due to the processing method, various 3D printing methods have been suggested over the last four decades. Notably, the ASTM/ISO 52900:2021 standard recognized over 50 3D methods that belong to the following categories: binder jetting, FDM, DED, SLM, sheet lamination, and SLA [59, 60].
In one research conducted by Lee et al. [59] the practical fabrication of silicone components with a tensile strength of about 0.12 MPa and elongation at a break of about 60% was achieved using the binder jetting method. The mechanical properties were found to be adequate for clinical use in maxillofacial prosthetics [58]. In another investigation, Hagman et al. [60] determined the viability of fabricating removable partial dental prostheses in titanium through binder jetting. The titanium framework had extremely high surface roughness and achieved an unesthetic finish upon polishing. Additionally, the fit of the titanium framework was also not clinically acceptable, concluding that the binder jetting process for titanium requires optimization for such purposes. In one of the reports on FDM by Wang et al. [61], the potential to produce customized PEEK was explored. The PEEK implants produced by FDM showed low crystallinity (< 25%), suggesting the significance of the process for building chest wall defects. Another study presented results on the mechanical properties of FDM printed parts with different infill ratios. Specimens with an 80% infill ratio had higher tensile strength, and with a 100% infill ratio had greater flexural strength. The research demonstrated that by varying the infill ratio, the mechanical properties of the implants can be optimized for patient-specific use [62]. Ryu et al. [63] discussed the use of DED in fabricating porous surface coatings on joint implants to provide stability and longevity for prosthetic devices. The porous coatings exhibited enhanced osseointegration and more enhanced bone ingrowth compared to solid non-porous implants, thus facilitating the integration of the implants with the host bones. In a fascinating paper by Petruse et al. [64], the potential application of DED for the repair and improvement of medical equipment was reported. The research showed that the procedure could restore the mechanical properties of the parts with great effectiveness, opening the prospect of applying DED in the maintenance and customization of medical devices and implants. Alnazzawi et al. [65] have also presented the application of the SLM process over the conventional casting technique, producing customized Co-Cr metal posts for dental implants. The research has highlighted the prospect of employing SLM to develop custom metal posts with high accuracy compared to the conventional processes available.
Implantable biomaterials must be rigorously tested for mechanical performance and biocompatibility before they can be applied in the clinic. The most important performance parameters for implants are osseointegration, wear resistance, and corrosion resistance, which are significant to their lifespan and efficacy. Material composition, surface topography, and bioactivity influence osseointegration, which is the direct structural and functional connection between live bone and the surface of an implant. Titanium and its alloys are still the gold standard because of its superior mechanical properties and biocompatibility [66]. Surface modifications such as nano-texturing and bioactive coatings (e.g., hydroxyapatite) have enhanced bone-implant integration by promoting cellular adhesion and proliferation [67]. In addition, more recent studies highlight the need for 3D-printed porous scaffolds to trigger vascularization and bone development [68].
Resistance to wear is essential in load-bearing implants, especially in dental and orthopedic applications. Wear debris may initiate inflammatory responses, leading to implant failure and osteolysis. Some recent developments involve the employment of ceramic composites like zirconia-toughened alumina, with high hardness and low wear rates [69]. In addition, surface coatings like DLC and TiN have significantly reduced wear particle generation and improved durability against cyclic loading conditions [70].
Corrosion resistance is essential to prevent the release of metal ions, which are responsible for local and systemic toxicity. Cobalt-chromium and titanium alloys are extensively used because of their passive oxide layer, which provides intrinsic corrosion resistance. Nano-structured oxide coating technology has improved these characteristics, promoting lifetime and biocompatibility [71]. Electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization are popular methods of testing corrosion performance in simulated physiological conditions [72, 73].
Surface modification techniques are critical in enhancing the biocompatibility and mechanical properties of biomaterials applied in medical implants. The techniques enhance interactions with biological tissues by modifying surface characteristics without altering bulk features, leading to improved integration with their host, thus enhancing their utility. Advances in surface modification technologies have significantly enhanced the biocompatibility and mechanical performance of medical implants. A comprehensive overview of surface modification processes aimed at promoting the osseointegration and antibacterial behavior of titanium-based implants was provided by Yuan et al. [73]. Acid etching, sol-gel processing, chemical vapor deposition, electrochemical methods, layer-by-layer self-assembly, and chemical grafting were some of the processes included in the review. The advantages, limitations, and potential applications of each method were discussed, presenting information regarding the latest findings in enhancing implant surface properties. The influence of nitriding and boriding surface treatments on stainless steel implants from SS410 and SS316L was investigated by Sivakumaran et al. [74]. Improved wear resistance and surface hardness were the outcomes of the treatments. Interestingly, boriding increased hardness to a maximum, reduced wear losses, and significantly enhanced surface roughness.
Furthermore, both treatments exhibited antibacterial activity against Bacillus subtilis, which implies that they can increase implant life and performance. To enhance the mechanical, tribological, corrosion, wetting, and biocompatibility characteristics of Ti-6Al-4V alloys, Kumar et al. [75] explored a range of innovative coating materials and techniques. Coatings like metal nitrides, diamond-like carbon, metal oxides, high-entropy alloys, and polymer-metal oxide composites were studied. The authors present detailed descriptions of the working mechanisms of these coatings and how they promote implant function. A comprehensive review of surface modification methods for 3D-printed titanium implants was conducted by Long et al. [76]. The research considered conventional and innovative methods, including chemical modifications, bioconvergence technologies, physical-mechanical processes, and functional composite methods. To attain multifunctional and tailored implant designs, the authors emphasized the importance of precise control over implant surface morphology to enhance osteogenic properties and antimicrobial performance. The effects of surface modification on long-term biocompatibility and performance are as follows:
Micro- and nano-scale surface topographies produced through surface treatments favor osteoblast adhesion, proliferation, and differentiation for better bone integration. For example, micro-arc oxidation has been used to manufacture coatings on intricate-structured titanium implants and is reported to induce better biocompatibility and osseointegration [77].
Some surface treatments give titanium implants antibacterial properties, which decrease the chances of infection. Processes like femtosecond laser-induced surface texturing have proven to prevent bacterial adhesion and biofilm development, thus increasing the longevity and functionality of the implant [76].
Some of the other significant surface modification techniques are listed below:
Hydroxyapatite Coatings: Hydroxyapatite, occurring naturally in bone tissue, is deposited on the surface of titanium to simulate the mineral phase of the bone. The coatings have been demonstrated to enhance osseointegration by presenting a bioactive surface that stimulates the attachment and proliferation of bone cells [77].
Micro-Arc Oxidation: Micro-Arc Oxidation is anodic oxidation of the titanium surface in an electrolyte, which forms a porous oxide layer. The method improves biocompatibility and can be modified to enhance osseointegration and antibacterial functions by adding bioactive components to the oxide layer [78].
Laser Surface Texturing: This process uses laser beams to develop specific patterns on the implant surface. Laser-induced hybrid groove structures on Ti-6Al-4V alloys enhanced osseointegration by inducing osteoblast activity and protein adsorption [79].
Bioactive Glass Coatings: In this process, the implant surface is coated with bioactive glass materials. Such coatings have been shown to accelerate bone ingrowth in early healing periods, improving osseointegration [80].
Antibacterial-Osteogenic Composite Coatings: Coating development incorporating antibacterial components and osteogenic factors has been demonstrated to inhibit bacterial adhesion and induce the activity of bone cells, thus increasing infection resistance as well as osseointegration [81].
These surface modification techniques applied over 3D printed Ti can enhance the integration with bone tissue and reduce infection risks. The process leads to the long-term performance of the implants and the satisfaction of patients.
Prosthesis and implant customization have been significantly enhanced due to metal 3D printing, which now offers customized treatments for every patient. Nevertheless, some challenges still stand in the path of its increased use in the medical field. Metal implants can undergo deformation or failure due to residual stresses imparted by the rapid heating and cooling phases that characterize additive manufacturing. To mitigate such pressures, strategies such as post-processing thermal treatments and optimized scan patterns are currently being researched [82]. Anisotropic mechanical properties, where material exhibits different strengths along different orientations, are often the product of layer-by-layer fabrication used in 3D printing. The mechanical reliability of implants with physiological stresses might be compromised through this anisotropy. The techniques most researched to correct this problem are altering print settings and post-processing methods. Typical defects such as porosity and incomplete fusion can harm the mechanical stability and biocompatibility of implants. In order to detect and minimize these defects during manufacturing, advanced in-process monitoring and control methods are being evolved.
To ensure safety and efficacy, strict regulatory oversight is needed when implementing 3D-printed metal implants into practice. Standardization is challenging due to the significant differences in 3D printing technologies. It is important to establish standardized test protocols for biocompatibility and mechanical properties [83]. Custom implant approval processes must be individualized for customized implants manufactured for each patient and can be costly and time-consuming. To allow for instant patient treatment, these processes should be streamlined. Moral issues surrounding equal access and potential misuse arise due to the ability to create implants that are tailor-made for the patient. Among the significant challenges is ensuring all patients, including those from differing socioeconomic statuses, enjoy 3D printing advances.
With various creative opportunities being explored, metal 3D printing for biological applications looks promising. Osseointegration can be enhanced, and the chances of infection can be reduced using bioactive coatings like drug-releasing surfaces or antimicrobial coatings when applied over 3D-printed implants. Such coatings prepared by technologies like plasma spraying are increasingly used [76]. For enhanced patient results, 3D printing and sensor technology are merged to enable intelligent implants to monitor healing trends and offer insights to healthcare experts. Advances in 3D printing enable one to design implants with architectures for the highest level of mechanical toughness and biological functionality by conjoining of biocompatible metal and biodegradable polymers [62].
Hybrid manufacturing, which combines 3D printing with conventional methods such as casting or forging, significantly improves the fabrication of biometal implants. These improvements can be listed as:
Enhanced Mechanical Properties: Conventional processes like forging create parts with better mechanical strength because they can hone grain structures and remove internal flaws. Combining these processes with 3D printing allows manufacturers to produce implants that enjoy the flexibility of additive manufacturing design and the strength of traditional processes [84].
Improved Surface Finish and Dimensional Tolerance: Post-processing 3D-printed components via machining can greatly enhance surface finish and meet tight dimensional tolerances. The hybrid method mitigates the surface roughness typically seen with additive manufacturing, creating implants with finer finishes that are important for biocompatibility and functionality [85].
Advances in material science have given rise to new materials explicitly designed for 3D printing to be stronger, more durable, and more biocompatible than existing biomaterials.
Biodegradable Polymers: Polymers like polycaprolactone (PCL) and its copolymers are being designed with tunable mechanical properties and degradation rates compatible with tissue engineering applications. The polymers are printable via 3D printing to create scaffolds that promote tissue regeneration and degrade harmlessly within the body over time [86].
Ceramic Materials: Ceramics such as hydroxyapatite, alumina (Al2O3), and zirconia (ZrO2) are well known for their high biocompatibility and wear resistance. Advances in 3D printing technology have made it possible to create complex ceramic structures that enhance bone integration and longevity in orthopedic implants [4].
Hybrid manufacturing technologies and the creation of advanced materials are leading the way for the next generation of biometal implants with better performance and patient outcomes.
Metal 3D printing has revolutionized the manufacture of biomedical implants and prostheses with its incomparable advantages in terms of material efficiency, customization explicitly tailored to patients, and design sophistication. Some of the advantages contributed by technologies such as DED, binder jetting, EBM, and SLM are high mechanical properties, precision, and cost. Implant performance, lifespan, and biocompatibility largely depend on the choice of biometals, such as titanium, stainless steel, cobalt-chromium alloys, and recent developments in biodegradable magnesium alloys.
Despite these advances, problems such as residual stress, anisotropy of mechanical properties, and surface defects still hinder extensive clinical applications. For enhanced performance and reliability of 3D-printed implants, scientists are exploring advanced post-processing techniques, topology optimization, and online monitoring systems. Also, to ensure safety and efficacy for medical uses, regulatory frameworks must be revised to consider the unique characteristics of additive manufacturing.
The integration of 3D-printed implants into human tissue will be further enhanced by advances in biomaterials, bioactive coatings, and hybrid processes in future studies. Metal 3D printing can revolutionize implant and prosthetic production with developing technology, paving the way for a new era of highly effective and personalized healthcare treatments.
DED: directed energy deposition
EBM: electron beam melting
FDM: fused deposition modeling
FEA: Finite Element Analysis
HIP: Hot Isostatic Pressing
PEEK: Polyether Ether Ketone
SLA: stereolithography
SLM: selective laser melting
AD and PR: Conceptualization, Methodology, Validation, Visualization, Writing—original draft, Writing—review & editing.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Fariborz Tavangarian ... Anilchandra Attaluri
Minhaz Husain ... J. P. Davim
Bharat Kalia ... Gurwinder Singh
Antonio Ziranu ... Greta Tanzi Germani
Joshua D. Smith ... Matthew E. Spector
