Affiliation:
1Rutgers-Robert Wood Johnson Medical School, Piscataway, NJ 08855, USA
2Current address: Department of Surgery, Morristown Medical Center, Morristown, NJ 07960, USA
Affiliation:
3Rutgers Biomedical Health Sciences School of Graduate Studies, Newark, NJ 07103, USA
4Current address: Rutgers-New Jersey Medical School, Newark, NJ 07103, USA
ORCID: https://orcid.org/0009-0009-5581-9498
Affiliation:
3Rutgers Biomedical Health Sciences School of Graduate Studies, Newark, NJ 07103, USA
4Current address: Rutgers-New Jersey Medical School, Newark, NJ 07103, USA
ORCID: https://orcid.org/0009-0001-7660-9758
Affiliation:
5Department of Orthopaedic Surgery, Jersey City Medical Center, Jersey City, NJ 07302, USA
ORCID: https://orcid.org/0000-0002-0397-2951
Affiliation:
5Department of Orthopaedic Surgery, Jersey City Medical Center, Jersey City, NJ 07302, USA
ORCID: https://orcid.org/0000-0003-0086-0740
Affiliation:
5Department of Orthopaedic Surgery, Jersey City Medical Center, Jersey City, NJ 07302, USA
Affiliation:
5Department of Orthopaedic Surgery, Jersey City Medical Center, Jersey City, NJ 07302, USA
ORCID: https://orcid.org/0009-0009-0000-6342
Affiliation:
6Department of Orthopaedics, Rutgers-New Jersey Medical School, Newark, NJ 07103, USA
7Current address: Shift Health, Toronto, ON M5R 3N5, Canada
Affiliation:
6Department of Orthopaedics, Rutgers-New Jersey Medical School, Newark, NJ 07103, USA
ORCID: https://orcid.org/0000-0001-6463-414X
Affiliation:
3Rutgers Biomedical Health Sciences School of Graduate Studies, Newark, NJ 07103, USA
6Department of Orthopaedics, Rutgers-New Jersey Medical School, Newark, NJ 07103, USA
ORCID: https://orcid.org/0000-0002-6639-428X
Affiliation:
3Rutgers Biomedical Health Sciences School of Graduate Studies, Newark, NJ 07103, USA
6Department of Orthopaedics, Rutgers-New Jersey Medical School, Newark, NJ 07103, USA
Affiliation:
3Rutgers Biomedical Health Sciences School of Graduate Studies, Newark, NJ 07103, USA
6Department of Orthopaedics, Rutgers-New Jersey Medical School, Newark, NJ 07103, USA
Email: oconnojp@njms.rutgers.edu
ORCID: https://orcid.org/0000-0003-0282-1104
Explor BioMat-X. 2025;2:101341 DOI: https://doi.org/10.37349/ebmx.2025.101341
Received: April 15, 2025 Accepted: June 19, 2025 Published: July 07, 2025
Academic Editor: Lucian Baia, ”Babeș-Bolyai” University, Romania
Aim: Zinc is essential for normal bone growth and can promote bone regeneration. Processed human bone allograft treated with zinc shows improved bone formation activity. Various factors were tested for effects on zinc binding to bone allograft with the long-term goal of developing methods to enhance the bone formation activity and safety of bone allograft in orthopaedic applications.
Methods: The amount of zinc bound to allograft was measured using Inductively Coupled Plasma-Mass Spectrometry (ICP-MS). Fluorescent visualization of zinc bound to allograft was accomplished using Zinpyr-1. The potential anti-microbial property of zinc-treated allograft was measured by exposing allograft to Staphylococcus aureus. After washing, the exposed allograft was cultured in bacterial media to measure residual Staphylococcus aureus. Data were analyzed using standard parametric methods.
Results: Rapid binding of zinc to bone allograft (1–15 min) was relatively insensitive to zinc concentration, incubation time, pH, or divalent cation competition. In contrast, zinc salt counter ions had significant effects, with zinc acetate producing more rapid zinc binding than zinc chloride or zinc picolinate. The ability of Staphylococcus aureus to contaminate bone allograft was also significantly reduced by prior zinc treatment.
Conclusions: The study results provide guidelines for modifying the processing of bone allograft to enhance bone formation activity while also improving the resistance of the allograft to bacterial contamination.
Large bone defects caused by trauma or therapeutic resection are difficult to treat. Often, the defect void is filled with bone graft to provide structural integrity, osteo-conduction, osteo-induction, or a combination thereof. Autograft bone harvested from the patient yields the best clinical outcomes. However, the amount of autograft that can be harvested is limited, and donor site morbidity is a significant clinical concern. Alternative approaches may include using the Masquelet method to generate additional tissue for grafting or employing limb-lengthening procedures to avoid grafting altogether [1–4]. Allograft and synthetic bone graft materials are commonly used to treat large bone defects [5]. Allograft is generally abundant and available in different physical forms that can be tailored to the required need. However, the ability of allograft or synthetic bone graft to promote osteogenesis within a bone defect is significantly less than that of autograft [6, 7].
Zinc is an essential nutrient that concentrates in bone and appears to have one or more physiological roles in the skeleton [8]. Previous studies found that dietary Zn deficiency leads to reduced levels of Zn in bone and can impair adolescent growth in humans and animal models [9–13]. Clinical studies have found that elevated serum Zn levels correlate with increased bone mineral density and reduced fracture risk [14]. Conversely, patients with osteoporosis were found to have reduced serum Zn levels and reduced bone tissue levels of Zn [15, 16]. Animal models and in vitro studies have also found that Zn can inhibit osteoclast activity and stimulate osteoblast activity [17–20].
Zinc can also promote bone regeneration when applied to sites of bone healing. Using a rat femur fracture model, local application of Zn to the fracture site significantly improved healing as shown by enhanced callus cellularity, increased callus levels of insulin-like growth factor-1 and vascular endothelial growth factor, increased percentages of callus cartilage and bone, and increased callus mechanical properties, demonstrating the potential therapeutic use of Zn for bone regeneration [21–23]. Local application of Zn with autograft improved posterolateral spinal fusion in a rat model [24]. Several studies have also noted that incorporating Zn into synthetic materials can enhance the osteogenic activity of those materials [25–27]. A recent experiment also found that adsorbing Zn to morselized, processed human allograft significantly improved osteogenesis in critically-sized rat femoral defects as compared to non-adsorbed control allograft [28].
Zinc also has anti-microbial properties. Using zinc acetate, Atmaca et al. [29] found that soluble Zn inhibited Staphylococcus aureus (S. aureus) and Staphylococcus epidermidis (S. epidermidis) growth at concentrations above 2.2 mM but had no effect on the growth of Pseudomonas aeruginosa (P. aeruginosa). Gugala et al. [30] also found that approximately 2 mM Zn could inhibit the growth or biofilm formation of S. aureus, but was less effective against P. aeruginosa or Escherichia coli (E. coli). Inclusion of Zn into bioactive glass at 1.2% Zn by weight or hydroxyapatite at 1.6% Zn by weight inhibited the growth of Streptococcus mutans (S. mutans) and S. aureus, respectively [31, 32]. Thus, treating bone allograft with Zn may also provide a beneficial anti-microbial effect during and after allograft preparation.
The improved osteogenic capacity of the Zn-treated allograft suggests that binding of Zn to the allograft can be adjusted to improve clinical performance. To that end, this study measured the effects of Zn concentration, incubation time, potentially competitive divalent cations, and different Zn salts on Zn-binding to human allograft. By examining adsorption parameters that affect Zn binding to allograft, we aim to better understand how allograft Zn treatment can be optimized for future in vivo testing. The potential use of Zn-binding to allograft to reduce bacterial burden was also investigated.
Processed human cortical cancellous bone allograft was obtained from a commercial vendor (SpineFrontier, Beverly, MA, USA). Tissue banks must obtain consent from the donor or the donor’s family before collecting any tissue. The allograft was pulverized and sieved to obtain 0.5 to 1 mm size granules, which were used in all subsequent experiments. Micro-spin filters [0.2 µm pore size polyvinylidene fluoride (PVDF) membrane, 850 µL capacity, polypropylene housing] in 2 mL microcentrifuge tubes were purchased from Analytical Sales and Services, Inc. (Flanders, NJ, USA). PVDF midi-spin filters (0.2 µm pore size membrane, 4 mL capacity, polypropylene housing) in 7 mL tubes were purchased from Ciro Manufacturing Corp. (catalog number BPV02, Deerfield Beach, FL, USA). Anhydrous zinc chloride and zinc acetate dihydrate were purchased from Sigma-Aldrich (St. Louis, MO, USA). Zinc picolinate was purchased from Spectrum Chemical Mfg. Corp. (New Brunswick, NJ, USA). The different Zn salts were dissolved in Tris-buffered saline (TBS) that consisted of 50 mM Tris-Cl and 150 mM NaCl, pH 7.4.
Staphylococcus aureus, subspecies aureus, strain NCTC 8532 (S. aureus) was purchased from the American Type Culture Collection (Manassas, VA, USA). S. aureus was cultured at 37°C in Difco Nutrient Broth media or agar plates (Fisher Scientific, Pittsburgh, PA, USA). Colonies of S. aureus from agar plates were used to inoculate 3 mL of Difco Nutrient Broth, that were cultured overnight at 37°C with shaking. The overnight cultures were first diluted to an optical density (OD600) of 0.1 [equal to 107 colony forming unit (CFU) per mL] in fresh Difco Nutrient Broth and then to 103 CFU/mL in TBS in preparation for treating bone allograft. Only fresh, overnight cultures were used for experiments.
To each pre-tared micro-spin centrifuge tube, approximately 0.1 g of dry, morselized bone allograft was added to the micro-spin filter cup. The test Zn solution (0.5 mL) or diluent control (TBS, 0.5 mL) was added to each allograft aliquot in the micro-spin filter and incubated at room temperature with shaking for the desired test time. The Zn solution or control TBS was removed by centrifugation at 2,000 revolutions per minute (RPM) for 2 min at room temperature. The allograft was then washed 5 times with 0.5 mL of TBS. The allograft sample was then dried in vacuo. The micro-spin centrifuge tube with filter and treated allograft was weighed to determine the exact amount of allograft. Nitric acid (0.5 mL) was added to each micro-spin filter cup and then the micro-spin tube re-weighed to verify the exact volume of added nitric acid. Samples were incubated in a 65°C water bath until the allograft was dissolved (approximately 3 hours). Zn was measured by Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) after diluting an aliquot of each nitric acid extract with water (1:50 to 1:200). ICP-MS data were corrected for dilutions and normalized as mg of Zn per g of dry bone allograft. ICP-MS measurements were conducted by Dr. Larisa Krishtopa (Otto H. York Center for Environmental Engineering and Science, New Jersey Institute of Technology) using an Agilent 7900 ICP-MS instrument.
To assess the utility of different Zn salts on Zn binding to allograft, zinc chloride, zinc acetate dihydrate, and zinc picolinate were dissolved in TBS to approximately 15 mM final concentrations (see Table 1). Binding times for the different Zn salt solutions with bone allograft were 7, 20, and 60 min at room temperature. Binding procedures, TBS washes, nitric acid extraction, and Zn quantitation were conducted as described above.
Characteristics of Zn salts
| Compound | Molecular weight (g/mol) | Experimental concentration | Water solubility | PubChem CID | ||
|---|---|---|---|---|---|---|
| Percent (w/v) | mM | Relative solubility | g per 100 g water at 25°C | |||
| Zn chloride | 136.3 | 0.2 | 14.7 | High | 432 | 5727 |
| Zn acetate dihydrate | 219.5 | 0.32 | 14.6 | Moderate | 40 | 11192 |
| Zn picolinate | 309.6 | 0.46 | 14.9 | Low | — | 9904746 |
To assess potential competitive effects of divalent cations on Zn binding to allograft, magnesium chloride (MgCl2-6H2O) was dissolved to 0%, 0.1%, 0.5%, 1%, and 2% (w/v) in TBS containing 0.5% w/v ZnCl2. Binding time for the different Zn-Mg solutions with bone allograft was 7 min at room temperature. Binding procedures, TBS washes, nitric acid extraction, and Zn quantitation were conducted as described above. Mg quantitation from the same extracts was also conducted using the Agilent 7900 ICP-MS.
To assess the effects of pH on Zn binding to allograft, morselized allograft was bound with Zn for 30 min using 0.5% ZnCl2 dissolved in TBS at pH 6.0, 6.3, 6.6, 7.0, 7.4, and 8.0. Following binding, the allograft samples were rapidly washed 6 times with the same pH-adjusted TBS using micro-spin tubes. Bound Zn was measured by ICP-MS as described above.
Aliquots of morselized bone allograft (0.15 g) were placed in 4 mL midi-spin filters. Allograft aliquots were treated with 3 mL of 0.5% ZnCl2 in TBS or 3 mL of TBS for 1, 7, 15, or 60 min and then washed 6× with 3 mL of TBS. ZnCl2 and TBS were separated from the allograft by centrifugation at 500 RPM for 3 mins. Treated allograft aliquots were transferred into 15 mL conical tubes and then sterilized with gamma-irradiation (410 cGy/min for 15 min).
Treated allograft aliquots were inoculated with 3 mL of S. aureus diluted to 103 CFU/mL in TBS (pH 7.4) for 30 min at 37°C with shaking. The inoculated allograft aliquots were washed 6 times with TBS as described above. After washing, 10 mL of Difco Nutrient Broth was added to each aliquot and cultured at 37°C with shaking for 14 hours. Optical density (OD600) was measured hourly beginning 9 hours after Nutrient Broth addition. The OD600 values for each aliquot were plotted versus culture time and the Area Under the Curve (AUC) from 9 to 14 hours was determined using SigmaPlot (version 15, Systat Software, Inc. San Jose, CA). A minimum of 6 TBS-treated and 6 Zn-treated allograft aliquots were assayed for S. aureus growth for each Zn binding time (1, 7, 15 and 60 min). In general, 6 control-treated allograft samples and 6 Zn-treated allograft samples were inoculated with S. aureus at the same time to assess the effects of allograft Zn-treatment on S. aureus growth.
A morselized allograft that was approximately 1 mm in diameter was used. The Allograft was treated with 0.5% ZnCl2 in TBS for 1 hour. After binding, the allograft was rapidly washed 3 times with TBS, followed by an additional 5 washes with TBS for 5 min each. The allograft was then stained with 6.5 µM Zinpyr-1 (MedChem Express, Monmouth Junction, NJ). The allograft was stained for 30 min at room temperature with the Zinpyr-1 with gentle agitation and protected from light. After staining, the allograft morsels were washed with TBS 3 times for 15 min each. The morsels were then glued onto a microscope slide using a methylmethacrylate cement. The morsels were briefly polished to create a flat surface and expose the interior of the morsels. Zinpyr-1 staining was then detected by epifluorescence using an Olympus BX50 microscope.
Data were analyzed and compared using GraphPad Prism version 10.1 software (Graphpad Software, LLC, Boston, MA). Unless otherwise indicated, data were compared using 1-way or 2-way ANOVA and Tukey’s corrected post-hoc tests. Data are reported as mean values with standard deviations.
In a prior study, we found that treating morselized human bone allograft with 0.1% w/v ZnCl2 for 30 min improved the osteogenic capacity of the allograft in a rat femur defect model [28]. Within that study, experiments showed that Zn binding to allograft occurred in a time- and concentration-dependent manner [28]. However, time-dependent binding was only measured using a relatively high concentration of Zn (1% w/v ZnCl2), while concentration-dependent binding was only measured after an extended (24-hour) binding time.
To better characterize Zn-allograft binding, we measured Zn binding to morselized human bone allograft at lower ZnCl2 concentrations (0.01%, 0.1%, and 1% w/v) and at shorter binding times (0.5, 3, and 24 hours; Figure 1A). Again, we found that Zn binding was concentration- (p < 0.001) and time-dependent (p < 0.001) between 0.01% and 1% w/v ZnCl2 and 0.5 and 24 hours of binding time.
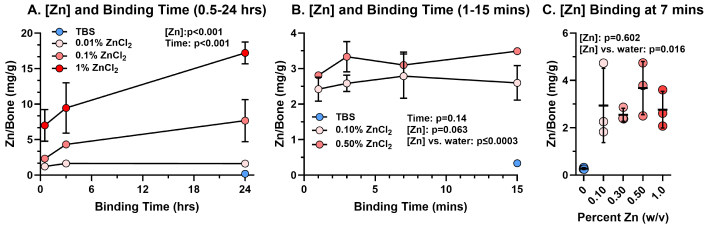
Zn binding to bone allograft: [Zn] and time effects. Morselized human bone allograft was treated with ZnCl2 or TBS. After washing, Zn was extracted from the allograft with nitric acid and measured by ICP-MS. (A) Allograft was treated with ZnCl2 at 0.01%, 0.1%, and 1% (w/v) for 0.5, 3, or 24 hours or with TBS for 24 hours. (B) Allograft was treated with 0.1% or 0.5% w/v ZnCl2 for 1, 3, 7, or 15 mins or with TBS for 15 mins. (C) Allograft was treated for 7 mins with TBS or 0.1, 0.3, 0.5, and 1% (w/v) ZnCl2. (A, B) Values shown are the means (± SD) from 3 independent replicates (2 independent replicates for TBS and 6 independent replicates for 1% ZnCl2 at 24 hours, panel A). (C) Bars represent the mean and SD of the 3 independent replicates for each Zn concentration. The amount of Zn bound per gram of allograft was compared to binding time (A, B) and to Zn concentration (A, B, C). p-values from the 2-way ANOVA analyses for effects of Zn concentration [Zn] and time on Zn bound to allograft are shown in each graph (A, B). p-values from a 1-way ANOVA for effects of Zn concentration [Zn] on Zn bound to allograft are shown (C)
Closer examination of the binding data in Figure 1A indicated that Zn binding occurred rapidly within the first 30 minutes, with respective averages of 1.2, 2.3, and 7.0 mg of Zn bound per g of allograft for the 0.01%, 0.1%, and 1% ZnCl2 concentrations. Consequently, the potential effects of Zn concentration over shorter binding times were evaluated (Figure 1B). However, no effect of binding time was detected between 1, 3, 7, and 15 min of Zn binding to allograft bone when using ZnCl2 concentrations (w/v) of 0.1% and 0.5% (p = 0.14; Figure 1B), nor was any effect detected when Zn concentrations (w/v) of 0.1%, 0.3%, 0.5%, and 1% ZnCl2 were bound to allograft bone for 7 min (p = 0.60; Figure 1C).
As a divalent cation, Zn can form a variety of salts with differing water solubility (Table 1). The ability of different Zn salts to affect Zn adsorption to allograft was measured. Approximately 15 mM solutions of Zn, as Zn chloride (0.2% w/v), Zn acetate (0.32% w/v), and Zn picolinate (0.46% w/v) were used to treat morselized human bone allograft for 7, 20, or 60 min. As shown in Figure 2, the amount of Zn bound to the allograft increased with binding time for all salts (p < 0.001). The Zn salt used also affected the amount of Zn bound to the allograft (p < 0.001). Interestingly, the amount of Zn bound to the allograft was significantly greater after 7 min incubation in Zn acetate (0.82 mg of Zn per g of allograft) as compared to ZnCl2 (0.44 mg of Zn per g of allograft). In contrast, the amount of Zn bound to allograft bone from Zn picolinate (0.25 mg of Zn per g of allograft) was significantly lower than that from Zn chloride after 7 min and from Zn acetate after 7, 20, and 60 min of binding.
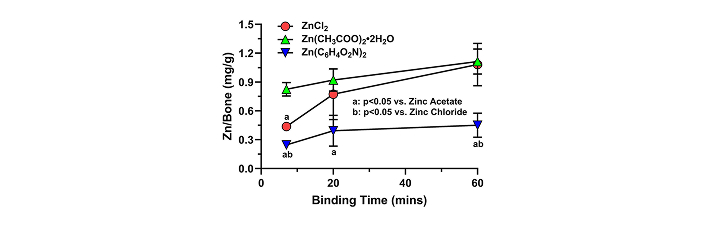
The effects of different Zn salts on Zn binding to bone allograft. Binding of Zn to allograft is dependent on the Zn salt used. Aliquots of morselized human allograft were incubated in approximately 15 mM solutions of zinc chloride, zinc acetate, or zinc picolinate for 7, 20, or 60 min at room temperature (n = 3 per salt and per time). Bound Zn was extracted with nitric acid and measured using ICP-MS. Shown are mean values (± SD). Overall, more Zn was bound to the allograft using zinc acetate (green triangles) after 7 min than from either zinc chloride (red circles) or zinc picolinate (blue inverted triangle). Conversely, less Zn was bound to allograft using zinc picolinate than from either zinc acetate or zinc chloride and at all time points tested. Statistical significance is indicated in the graph
The potential effect of other cations competing for Zn binding sites on the bone allograft was investigated using increasing concentrations of Mg (0–2% w/v of MgCl2-6H2O) and a constant concentration of 0.5% w/v ZnCl2 (Figure 3). Only a single binding time of 7 min was investigated. The baseline level of Mg in the bone allograft (0.71 ± 0.15 mg/g) was approximately 4-fold higher than the average baseline level of Zn detected in bone allograft incubated in TBS (0.16 ± 0.12 mg/g, Figure 1). No difference in binding of Mg to the allograft was detected after 7 min of incubation for all Mg concentrations tested (ANOVA p = 0.064; linear regression p = 0.142, Figure 3A). However, a significant difference was detected when comparing allograft samples incubated with 0% Mg (0.71 ± 0.15 mg/g) to all allograft samples treated with ≥ 0.1% Mg (0.96 ± 0.10 mg/g; t-test, p = 0.003), indicating that approximately 0.25 mg of Mg bound per g of bone allograft.
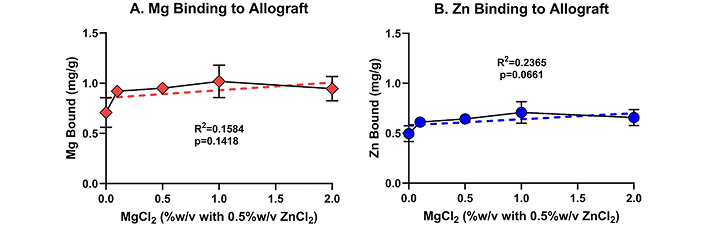
Divalent cation effects on Zn binding to bone allograft. Rapid binding of Zn to bone allograft is not affected by Mg concentration. Aliquots of bone allograft were treated for 7 min at room temperature with 0.5% (w/v) ZnCl2 in TBS and containing 0%, 0.1%, 0.5%, 1%, or 2% (w/v) MgCl2-6H20. Mg (panel A) and Zn (panel B) that remained bound to the allograft were extracted using nitric acid and measured by ICP-MS. Shown are mean values (± SD) from 3 independent replicates for each Mg concentration. Linear regression found no significant effect of increasing Mg levels on Zn binding after 7 min of binding time (B, p = 0.066)
The addition of Mg to the Zn solution did not inhibit Zn binding to the bone allograft during the 7 min of binding (Figure 3B). Comparing the amount of Zn bound from 0.5% w/v ZnCl2 with no added MgCl2 (0.495 ± 0.079 mg/g) to the amount of Zn bound from the 0.5% ZnCl2 with ≥ 0.1% w/v MgCl2 (0.655 ± 0.068 mg/g) found that in the presence of MgCl2, 32% more Zn bound to the allograft during the 7 min binding reactions (t-test, p = 0.004).
The potential effect of buffer pH on Zn binding to bone allograft was measured. The pH of TBS was adjusted to yield solutions in the pH range of 6 to 8. ZnCl2 was dissolved in the pH adjusted TBS to 0.5% (w/v). No effect on pH after dissolving the ZnCl2 was detected. Aliquots of bone allograft were treated with pH adjusted TBS-ZnCl2 solutions for 30 min at room temperature with shaking. The amount of Zn bound to the allograft was measured (Figure 4). The amount of Zn bound remained constant over the pH range tested (p = 0.42).
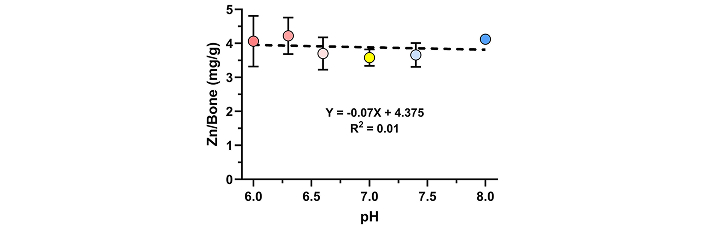
pH-independent Zn binding to bone allograft. Aliquots of morselized human allograft were treated with 0.5% (w/v) ZnCl2 dissolved in TBS buffered to pH 6.0, 6.3, 6.6, 7.0, 7.4, or 8.0 for 30 min and then washed extensively with TBS buffered to the same pH. Zn that remained bound to the allograft was extracted with nitric acid and measured by ICP-MS. Shown are mean (± SD) values from 3 independent replicates for each pH tested. A comparison of the amount of Zn bound to allograft found no statistical difference between the groups (p = 0.42)
The rapid binding of Zn to the bone allograft indicated that binding was occurring primarily on the surface of the bone allograft morsels. To confirm that Zn was bound to the surface, allograft was bound with 0.5% ZnCl2 for 1 hour. The bound Zn was then detected using Zinpyr-1, which fluoresces green when bound to Zn [33]. As shown in Figure 5, Zinpyr-1 fluorescence was primarily detected on the surface of the allograft morsels, indicating that Zn binding occurred on the surface of the allograft morsels.
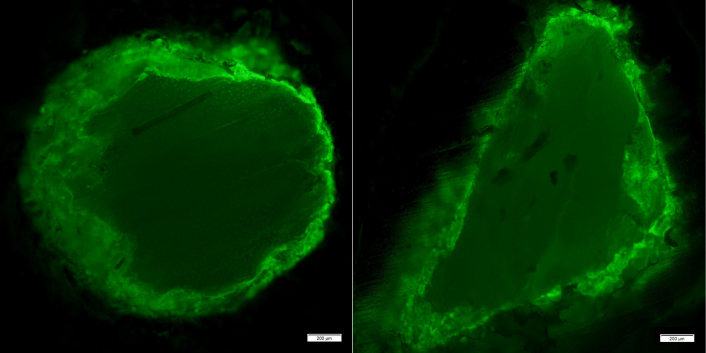
Surface binding of Zn to bone allograft. Morselized human allograft was treated with 0.5% (w/v) ZnCl2 dissolved in TBS buffered to pH 7.4 for 60 min and then washed extensively with TBS before detecting bound Zn with the fluorescent stain, Zinpyr-1. The stained allograft morsels were polished to reveal the interior of the bone morsels before epi-fluorescent detection of the Zinpyr-1. Shown are two, approximately 1 mm diameter allograft morsels; scale bars are 200 µm
Binding Zn to allograft bone is likely to alter the surface chemistry of the allograft. The bound Zn could potentially reduce bacterial adherence or present a sufficiently high local Zn concentration to inhibit bacterial growth. To test this, morselized human bone allograft was incubated in 0.5% ZnCl2 or TBS for 1, 7, 15, or 60 min, washed multiple times with TBS pH 7.4, dried, and then sterilized by gamma-irradiation. The allograft aliquots were inoculated with S. aureus and, after washing, cultured at 37°C with shaking in Nutrient Broth. The optical density (OD600) of each allograft-S. aureus culture was measured from 9 to 14 hours to determine the growth of S. aureus and, therefore, the relative amount of viable S. aureus retained by the allograft. Growth curves for S. aureus were plotted for each allograft culture and the AUC was calculated for each growth curve (Figure 6). Growth curves appeared similar between control allograft and allograft treated for 1 min with ZnCl2, but apparent reductions in S. aureus growth were evident in cultures of allograft treated with ZnCl2 for 7 min or more (Figure 6A–D). AUCs were significantly different between control and Zn-treated allografts for all ZnCl2 treatment times tested (Figure 6A–D). A comparison of all the control allograft AUCs and all the Zn-treated allograft AUCs showed a significant reduction in S. aureus growth in the Zn-treated allograft cultures (Figure 6E).
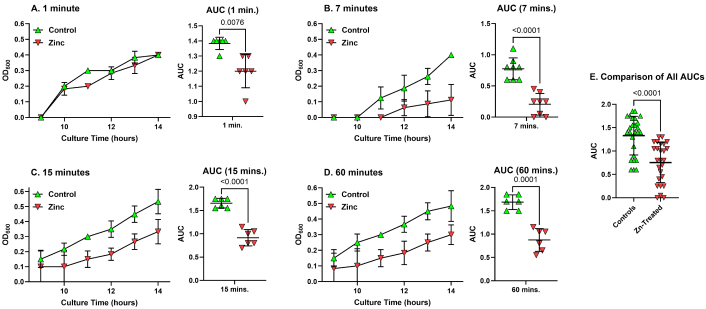
Staphylococcus aureus growth curves in bone allograft cultures. Bone allograft samples were incubated in 0.5% (w/v) ZnCl2 or TBS for 1, 7, 15, or 60 minutes, washed, and then inoculated with Staphylococcus aureus. After 30 min, the allograft samples were extensively washed with TBS before bacterial growth media was added. Bacterial growth was measured by optical density (OD600) from 9 to 14 hours after the addition of bacterial media. Panels A–D show the mean (± SD) OD600 values from 9 to 14 hours after Nutrient Broth addition for the 1-, 7-, 15-, and 60-min treatment times (respectively, panels A–D, left graph). AUCs were calculated from 9–14 hours for each allograft aliquot. Within each treatment time, the AUCs were compared (panels A–D, right graph). All treatment times with ZnCl2 showed a reduction in Staphylococcus aureus growth based on the AUCs (p values are indicated in each graph). Panel E shows the AUCs from all control- and Zn-treated bone allograft cultures. An unpaired t-test was used to compare the AUC values for the control-treated and Zn-treated allograft cultures, which detected a significant reduction in Staphylococcus aureus growth (p < 0.0001). Green triangles represent the control, TBS-treated allograft cultures. Red, inverted triangles represent the Zn-treated allograft cultures
The experimental results indicate that the amount of Zn binding to the allograft is not dependent on ZnCl2 concentration (from 0.1% to 1% w/v) when treatment time is relatively short (< 15 min, Figure 1). However, Zn binding to allograft is dependent on ZnCl2 concentration when treatment time is longer (1 hour or more) and the ZnCl2 solution is > 0.1% ZnCl2 (Figure 1) [28]. This suggests that Zn binds to allograft bone through more than one modality. As bone is composed of both organic and mineral components, the fast, concentration-independent binding and slow, concentration- and time-dependent binding of Zn may reflect differential Zn binding affinities between the organic and mineral components of bone or between surface binding of Zn to bone hydroxyapatite versus Zn substitution of calcium within the hydroxyapatite crystal [34]. In support of the former, a study from Fuierer et al. found that Zn bound to the surface of synthetic, non-sintered hydroxyapatite, most likely as a zinc phosphate surface precipitate, while Haumont detected Zn in the calcifying cartilage of rat growth plates and the osteoid (non-mineralized bone protein matrix) of canine Haversian canals [35, 36]. We found that even after 1 hour of incubation in 0.5% ZnCl2, binding of Zn to the bone allograft occurred primarily on the surface (Figure 5). The presence of Tissue Non-specific Alkaline Phosphatase (TNSALP) at sites of calcifying cartilage and mineralizing osteoid would contribute to Zn localization at these sites since Zn is a co-factor for TNSALP [37, 38]. However, phosphate produced from TNSALP enzymatic activity at sites of calcifying cartilage and osteoid may amplify local Zn levels by precipitating Zn as zinc phosphate. Interestingly, Fuierer et al. [36] also found that Zn binding to the surface of hydroxyapatite inhibited further hydroxyapatite crystal growth. Thus, controlling soluble levels of Zn at sites of active bone formation may be a means to regulate bone mineral deposition.
Differences in Zn binding to allograft from the different zinc salts were somewhat unexpected (Table 1 and Figure 2). The expectation was that the aqueous solubility of each zinc salt would correlate with the concentration of Zn2+ ions available to bind to the bone allograft. As expected, the low aqueous solubility of zinc picolinate leads to reduced binding of Zn to the allograft bone as compared to the highly aqueous soluble zinc chloride. In contrast, the moderately soluble zinc acetate produced more Zn binding to the bone allograft than the highly soluble zinc chloride. The difference between the rapid binding of Zn to bone allograft from zinc acetate versus zinc chloride is not known. However, the ability of Zn2+ to interact with water appears to be affected by how well the anion can buffer in order to prevent Zn precipitation [39]. Since the buffering capacity of acetate is much greater than chloride, perhaps this could account for the faster binding of Zn to bone allograft from a zinc acetate solution than a zinc chloride solution.
The mechanism by which Zn binds to the allograft is not known. Spadaro et al. [40] found that Zn bound to both the organic (collagen) and mineral phases of bone, indicating multiple mechanisms for sequestering Zn in bone tissue. Several trace metals are found in normal bone tissue, and among those trace metals, Mg and Zn are two of the more abundant elements [41, 42]. Mg and Zn dissolve as divalent cations. Thus, if Zn binding to the bone allograft was strictly through an ionic interaction, excess levels of Mg2+ should compete for Zn2+ binding. However, we detected no competitive effect of Mg on Zn binding even at an approximate 2.5-fold excess of Mg (0.5% w/v ZnCl2 and 2.0% w/v MgCl2-6H2O, Figure 3). In addition, binding of Zn was independent of pH over the pH 6 to pH 8 range tested (Figure 4). This indicates that Zn binding to the allograft has more specificity than a simple ionic interaction.
In addition to improving the osteogenic activity of processed bone allograft [28], Zn binding to allograft also appears to reduce the ability of bacteria to attach to allograft or to remain viable when attached to the Zn-bound allograft. As shown in Figure 6, Zn-bound allograft was resistant to binding viable S. aureus, one of the most common pathogens associated with osteomyelitis [43]. While even 1 min of treating the allograft with 0.5% w/v ZnCl2 reduced viable S. aureus binding, longer ZnCl2 incubation times appeared to be more effective.
Use of Zn in orthopaedic applications is an area of increasing interest because of the positive effects of Zn on osteogenesis and because Zn can be used to make biodegradable metal fixation devices [8, 44]. Use of Zn as an allograft adjuvant to enhance bone regeneration offers significant advantages over other approaches. Unlike growth factors or other protein adjuvants, Zn should not be affected by standard sterilization procedures and would allow for convenient, room temperature storage of the Zn bound allograft. As noted, Zn is an essential trace mineral that is necessary for multiple molecular processes. An estimated 10% of the human proteome binds Zn [45]. Sufficient amounts of Zn are generally consumed through a normal diet, but oral supplements are commonly used. The recommended daily allowance for Zn consumption in the United States is 8 mg/day for women and 11 mg/day for men, with a recommended maximum of 40 mg/day. The LD50 for oral Zn toxicity is 3 g/kg body weight [46, 47]. Zn is used therapeutically for topical and enteric applications. Zn is considered a generally recognized as safe and effective substance by the US Food and Drug Administration. In our prior work, we found that Zn bound to allograft at 0.4% w/w was effective in promoting bone formation, which would equate to 40 mg of Zn per 10 g of bone allograft [28]. At the 0.4% w/w dose level of Zn to allograft and given the apparent slow-release of Zn from allograft, use of Zn-bound allograft to treat skeletal injuries would be unlikely to produce any safety concerns.
The results of this study will be useful for determining how best to restore trace metals or other substances to bone allograft that are lost during processing. In addition, the results of this study indicate that trace substances do not necessarily bind to bone through simple ionic interactions with the hydroxyapatite mineral phase of bone. Whether the binding mechanism utilized to restore Zn levels to processed allograft is similar to how Zn normally binds to bone in vivo will require additional investigation. Still, the results of this study can be used to help optimize methods that will enhance the osteogenic activity and bacterial resistance of bone allograft to provide better clinical outcomes.
AUC: Area Under the Curve
CFU: colony forming unit
ICP-MS: Inductively Coupled Plasma-Mass Spectrometry
PVDF: polyvinylidene fluoride
RPM: revolutions per minute
TBS: Tris-buffered saline
TNSALP: Tissue Non-specific Alkaline Phosphatase
Authors may acknowledge those individuals who provided help during the research and preparation of the manuscript.
TH: Investigation, Formal analysis, Writing—review & editing. JFT: Investigation, Formal analysis, Writing—review & editing. DAN: Investigation, Formal analysis, Writing—review & editing. LGM: Investigation, Formal analysis, Writing—review & editing. JF: Investigation, Formal analysis, Methodology, Writing—review & editing. JM: Investigation, Formal analysis, Writing—review & editing. KA: Investigation, Formal analysis, Writing—review & editing. DK: Investigation, Fornal analysis, Writing—review & editing. MDC: Investigation, Formal analysis, Methodology, Supervision, Writing—review & editing. SL: Resources, Funding acquisition, Writing—review & editing. JB: Resources, Funding acquisition, Writing—review & editing. JPOC: Conceptualization, Funding acquisition, Supervision, Project administration, Formal analysis, Writing—original draft, Writing—review & editing. All authors read and approved the submitted version.
SL and JB are founders of CreOsso, LLC, a company focused on bone healing adjuncts. SL, JB, and JPOC are listed as inventors of patent numbers US9265794 and US9999636 granted to Rutgers University. The remaining authors declare no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
This research was supported by funds from CreOsso, LLC. Members of the CreOsso, LLC board reviewed the research proposal prior to funding. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2604
Download: 47
Times Cited: 0
