



Aim:
This study aimed to identify and analyze the top 100 most cited digital health and mobile health (m-health) publications. It could aid researchers in the identification of promising new research avenues, additionally supporting the establishment of international scientific collaboration between interdisciplinary research groups with demonstrated achievements in the area of interest.
Methods:
On 30th August, 2023, the Web of Science Core Collection (WOSCC) electronic database was queried to identify the top 100 most cited digital health papers with a comprehensive search string. From the initial search, 106 papers were identified. After screening for relevance, six papers were excluded, resulting in the final list of the top 100 papers. The basic bibliographic data was directly extracted from WOSCC using its “Analyze” and “Create Citation Report” functions. The complete records of the top 100 papers were downloaded and imported into a bibliometric software called VOSviewer (version 1.6.19) to generate an author keyword map and author collaboration map.
Results:
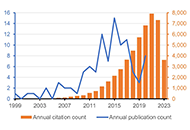
The top 100 papers on digital health received a total of 49,653 citations. Over half of them (n = 55) were published during 2013–2017. Among these 100 papers, 59 were original articles, 36 were reviews, 4 were editorial materials, and 1 was a proceeding paper. All papers were written in English. The University of London and the University of California system were the most represented affiliations. The USA and the UK were the most represented countries. The Journal of Medical Internet Research was the most represented journal. Several diseases and health conditions were identified as a focus of these works, including anxiety, depression, diabetes mellitus, cardiovascular diseases, and coronavirus disease 2019 (COVID-19).
Conclusions:
The findings underscore key areas of focus in the field and prominent contributors, providing a roadmap for future research in digital and m-health.
Aim:
This study aimed to identify and analyze the top 100 most cited digital health and mobile health (m-health) publications. It could aid researchers in the identification of promising new research avenues, additionally supporting the establishment of international scientific collaboration between interdisciplinary research groups with demonstrated achievements in the area of interest.
Methods:
On 30th August, 2023, the Web of Science Core Collection (WOSCC) electronic database was queried to identify the top 100 most cited digital health papers with a comprehensive search string. From the initial search, 106 papers were identified. After screening for relevance, six papers were excluded, resulting in the final list of the top 100 papers. The basic bibliographic data was directly extracted from WOSCC using its “Analyze” and “Create Citation Report” functions. The complete records of the top 100 papers were downloaded and imported into a bibliometric software called VOSviewer (version 1.6.19) to generate an author keyword map and author collaboration map.
Results:
The top 100 papers on digital health received a total of 49,653 citations. Over half of them (n = 55) were published during 2013–2017. Among these 100 papers, 59 were original articles, 36 were reviews, 4 were editorial materials, and 1 was a proceeding paper. All papers were written in English. The University of London and the University of California system were the most represented affiliations. The USA and the UK were the most represented countries. The Journal of Medical Internet Research was the most represented journal. Several diseases and health conditions were identified as a focus of these works, including anxiety, depression, diabetes mellitus, cardiovascular diseases, and coronavirus disease 2019 (COVID-19).
Conclusions:
The findings underscore key areas of focus in the field and prominent contributors, providing a roadmap for future research in digital and m-health.
DOI: https://doi.org/10.37349/edht.2024.00013

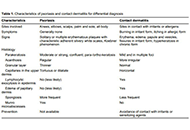
Allergic and immunologic skin diseases are becoming increasingly common and this requires clinicians to be able to recognize and diagnose them. A joint meeting (GET TOGETHER 2022) of the Italian Society of Allergy, Asthma and Clinical Immunology (SIAAIC) and the Italian Society of Allergological, Occupational and Environmental Dermatology (SIDAPA) aimed to review the current knowledge on the differential diagnosis of contact dermatitis, atopic dermatitis, hereditary angioedema, urticaria, and cutaneous mastocytosis. The most important aspects to take into consideration when faced with a new cutaneous manifestation are the clinical features of the lesions, their distribution, age of onset, and comorbidities/aggravating factors. The document does not aim to provide an exhaustive and comprehensive description of all allergic and immunologic skin diseases. Instead, it should be a reference tool for the clinician who is faced with the onset of a new skin manifestation and its differential diagnosis.
Allergic and immunologic skin diseases are becoming increasingly common and this requires clinicians to be able to recognize and diagnose them. A joint meeting (GET TOGETHER 2022) of the Italian Society of Allergy, Asthma and Clinical Immunology (SIAAIC) and the Italian Society of Allergological, Occupational and Environmental Dermatology (SIDAPA) aimed to review the current knowledge on the differential diagnosis of contact dermatitis, atopic dermatitis, hereditary angioedema, urticaria, and cutaneous mastocytosis. The most important aspects to take into consideration when faced with a new cutaneous manifestation are the clinical features of the lesions, their distribution, age of onset, and comorbidities/aggravating factors. The document does not aim to provide an exhaustive and comprehensive description of all allergic and immunologic skin diseases. Instead, it should be a reference tool for the clinician who is faced with the onset of a new skin manifestation and its differential diagnosis.
DOI: https://doi.org/10.37349/eaa.2024.00055

Aim:
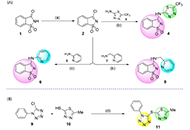
This study discloses the synthesis and the antimicrobial and anticancer activities of four molecules of structural basis saccharin-thiadiazolyl (4), saccharin-pyridyl (6, 8), and tetrazole-thiadiazolyl (11).
Methods:
Antimicrobial properties of the molecules were evaluated by the well-diffusion method, against Gram-positive bacteria [Staphylococcus aureus American Type Culture Collection (ATCC) 25923, Staphylococcus epidermidis ATCC 12228, Mycobacterium smegmatis ATCC 607], Gram-negative bacteria (Pseudomonas aeruginosa ATCC 27853) and yeast (Saccharomyces cerevisiae ATCC 2601 and Candida albicans ATCC 10231) strains. The anticancer activity of the compounds was assessed through i) proliferation assays for HCT116, MCF-7, and A375 human cell lines [cells were treated with serial dilutions of compounds and the effect on cell propagation was evaluated by sulforhodamine B (SRB) assay]; ii) antiproliferative and cytotoxic assays for glioma-type cell lines A172 (glioblastoma), U87 (brain-likely glioblastoma), and H4 (neuroglioma; cells were treated with diverse concentrations and the cell viability was assessed using a modified Alamar blue® assay).
Results:
Compound 11 exhibited significant inhibitory activity against S. aureus and S. epidermidis, with the further molecules demonstrating some inhibitory potential against all the tested Gram-positive, Gram-negative, and yeast strains. Similarly, derivative 11 showed an interesting antiproliferative activity against human colon adenocarcinoma (HCT116), human breast adenocarcinoma (MCF-7), and melanoma (A375) cells, with 50% growth inhibition (GI50) values varying from 3.55 µmol/L to 11.5 µmol/L, in the same order of magnitude of those shown by etoposide. Treatment of brain-like glioblastoma cells (U87) with 11, at the concentration of 100 µg/mL, induced a decrease in cell viability by 50% after 48 h and 72 h. Besides, results attained for A172 cells have shown that compound 11 only induces a significant decrease in cell viability upon treatment at 100 µg/mL for 72 h. A divergent observation was recorded for H4 cells, where the treatment with derivative 11 had promoted a significant decrease in cell viability (< 40–60%), even at concentrations as low as 0.39 µg/mL, after 24 h.
Conclusions:
This investigation reveals the potential of distinct azole-based conjugates, in particular the tetrazole-thiadiazolyl (11) derivative, as scaffolds worth further investigations, in the frame of antimicrobial and antineoplastic chemotherapy.
Aim:
This study discloses the synthesis and the antimicrobial and anticancer activities of four molecules of structural basis saccharin-thiadiazolyl (4), saccharin-pyridyl (6, 8), and tetrazole-thiadiazolyl (11).
Methods:
Antimicrobial properties of the molecules were evaluated by the well-diffusion method, against Gram-positive bacteria [Staphylococcus aureus American Type Culture Collection (ATCC) 25923, Staphylococcus epidermidis ATCC 12228, Mycobacterium smegmatis ATCC 607], Gram-negative bacteria (Pseudomonas aeruginosa ATCC 27853) and yeast (Saccharomyces cerevisiae ATCC 2601 and Candida albicans ATCC 10231) strains. The anticancer activity of the compounds was assessed through i) proliferation assays for HCT116, MCF-7, and A375 human cell lines [cells were treated with serial dilutions of compounds and the effect on cell propagation was evaluated by sulforhodamine B (SRB) assay]; ii) antiproliferative and cytotoxic assays for glioma-type cell lines A172 (glioblastoma), U87 (brain-likely glioblastoma), and H4 (neuroglioma; cells were treated with diverse concentrations and the cell viability was assessed using a modified Alamar blue® assay).
Results:
Compound 11 exhibited significant inhibitory activity against S. aureus and S. epidermidis, with the further molecules demonstrating some inhibitory potential against all the tested Gram-positive, Gram-negative, and yeast strains. Similarly, derivative 11 showed an interesting antiproliferative activity against human colon adenocarcinoma (HCT116), human breast adenocarcinoma (MCF-7), and melanoma (A375) cells, with 50% growth inhibition (GI50) values varying from 3.55 µmol/L to 11.5 µmol/L, in the same order of magnitude of those shown by etoposide. Treatment of brain-like glioblastoma cells (U87) with 11, at the concentration of 100 µg/mL, induced a decrease in cell viability by 50% after 48 h and 72 h. Besides, results attained for A172 cells have shown that compound 11 only induces a significant decrease in cell viability upon treatment at 100 µg/mL for 72 h. A divergent observation was recorded for H4 cells, where the treatment with derivative 11 had promoted a significant decrease in cell viability (< 40–60%), even at concentrations as low as 0.39 µg/mL, after 24 h.
Conclusions:
This investigation reveals the potential of distinct azole-based conjugates, in particular the tetrazole-thiadiazolyl (11) derivative, as scaffolds worth further investigations, in the frame of antimicrobial and antineoplastic chemotherapy.
DOI: https://doi.org/10.37349/eds.2023.00028

Aim:
Endothelial dysfunction has been associated with both cerebrovascular pathology and Alzheimer’s disease (AD). However, the connection between circulating endothelial cells and the risk of AD remains uncertain. The objective was to leverage data from the Framingham Heart Study to investigate various circulating endothelial subtypes and their potential correlations with the risk of AD.
Methods:
The study conducted data analyses using Cox proportional hazard regression and linear regression methods. Additionally, genome-wide association study (GWAS) was carried out to further explore the data.
Results:
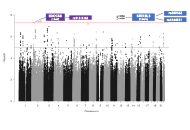
Among the eleven distinct circulating endothelial subtypes, only circulating endothelial progenitor cells (EPCs) expressing CD34+CD133+ were found to be negatively and dose-dependently associated with reduced AD risk. This association persisted even after adjusting for age, sex, years of education, apolipoprotein E (APOE) ε4 status, and various vascular diseases. Particularly noteworthy was the significant association observed in individuals with hypertension and cerebral microbleeds. Consistently, positive associations were identified between CD34+CD133+ EPCs and specific brain regions, such as higher proportions of circulating CD34+CD133+ cells correlating with increased volumes of white matter and the hippocampus. Additionally, a GWAS study unveiled that CD34+CD133+ cells influenced AD risk specifically in individuals with homozygous genotypes for variants in two stem cell-related genes: kirre like nephrin family adhesion molecule 3 (KIRREL3, rs580382 CC and rs4144611 TT) and exocyst complex component 6B (EXOC6B, rs61619102 CC).
Conclusions:
The findings suggest that circulating CD34+CD133+ EPCs possess a protective effect and may offer a new therapeutic avenue for AD, especially in individuals with vascular pathology and those carrying specific genotypes of KIRREL3 and EXOC6B genes.
Aim:
Endothelial dysfunction has been associated with both cerebrovascular pathology and Alzheimer’s disease (AD). However, the connection between circulating endothelial cells and the risk of AD remains uncertain. The objective was to leverage data from the Framingham Heart Study to investigate various circulating endothelial subtypes and their potential correlations with the risk of AD.
Methods:
The study conducted data analyses using Cox proportional hazard regression and linear regression methods. Additionally, genome-wide association study (GWAS) was carried out to further explore the data.
Results:
Among the eleven distinct circulating endothelial subtypes, only circulating endothelial progenitor cells (EPCs) expressing CD34+CD133+ were found to be negatively and dose-dependently associated with reduced AD risk. This association persisted even after adjusting for age, sex, years of education, apolipoprotein E (APOE) ε4 status, and various vascular diseases. Particularly noteworthy was the significant association observed in individuals with hypertension and cerebral microbleeds. Consistently, positive associations were identified between CD34+CD133+ EPCs and specific brain regions, such as higher proportions of circulating CD34+CD133+ cells correlating with increased volumes of white matter and the hippocampus. Additionally, a GWAS study unveiled that CD34+CD133+ cells influenced AD risk specifically in individuals with homozygous genotypes for variants in two stem cell-related genes: kirre like nephrin family adhesion molecule 3 (KIRREL3, rs580382 CC and rs4144611 TT) and exocyst complex component 6B (EXOC6B, rs61619102 CC).
Conclusions:
The findings suggest that circulating CD34+CD133+ EPCs possess a protective effect and may offer a new therapeutic avenue for AD, especially in individuals with vascular pathology and those carrying specific genotypes of KIRREL3 and EXOC6B genes.
DOI: https://doi.org/10.37349/emed.2024.00216
This article belongs to the special issue Neurophysiological Mechanisms of Aging and Dementia

Aim:
To evaluate angiotensin II (Ang II) and Ang-(1-7) levels and the cytokine profile in patients hospitalized with mild coronavirus disease 2019 (COVID-19) and contrast them with patients with identical clinical conditions but treated with high doses of vitamin D (vitD).
Methods:
From the 218 patients recruited (ClinicalTrials.gov NCT04411446), 16 participated in this sub-study and were randomized to a single oral dose of 500,000 IU vitD (n = 10) or placebo (n = 6). Plasmatic Ang II and Ang-(1-7) levels were determined by radioimmunoassay and interleukins (ILs) 1, 6, 8, and 10 and tumor necrosis factor alpha (TNF-α) by enzyme-linked immunosorbent assay before and after treatment. Parallel, serum 25-hydroxyvitamin D3 (25-OH vitD) concentrations as vitD status was measured by a chemiluminescence immunoassay.
Results:
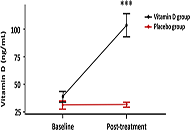
A trend towards an increase in Ang-(1-7) and a decrease in Ang II levels were observed in placebo- and vitD-treated COVID-19 patients compared to baseline values. There was no difference in Ang II and Ang-(1-7) levels between placebo- and vitD-treated COVID-19 patients. Similar results were obtained with ILs profile. COVID-19 patients showed an increase in the protective component of the RAS which was not improved by vitD treatment.
Conclusions:
VitD did not improve RAS disbalance in COVID-19. Notwithstanding, the authors visualize that acute treatment with high doses of vitD may show a trend to a decline in inflammatory ILs and an increase in protective markers. Finally, the authors would like to highlight the limitations of this preliminary study, namely the small number of patients and the use of a large single bolus dose of vitD rather than lower daily doses for extended periods with prolonged follow-up times. All these factors need special consideration in the designs of new vitD supplementation trials. All these factors need special consideration in the designs of new vitD supplementation trials (ClinicalTrials.gov identifier: NCT04411446).
Aim:
To evaluate angiotensin II (Ang II) and Ang-(1-7) levels and the cytokine profile in patients hospitalized with mild coronavirus disease 2019 (COVID-19) and contrast them with patients with identical clinical conditions but treated with high doses of vitamin D (vitD).
Methods:
From the 218 patients recruited (ClinicalTrials.gov NCT04411446), 16 participated in this sub-study and were randomized to a single oral dose of 500,000 IU vitD (n = 10) or placebo (n = 6). Plasmatic Ang II and Ang-(1-7) levels were determined by radioimmunoassay and interleukins (ILs) 1, 6, 8, and 10 and tumor necrosis factor alpha (TNF-α) by enzyme-linked immunosorbent assay before and after treatment. Parallel, serum 25-hydroxyvitamin D3 (25-OH vitD) concentrations as vitD status was measured by a chemiluminescence immunoassay.
Results:
A trend towards an increase in Ang-(1-7) and a decrease in Ang II levels were observed in placebo- and vitD-treated COVID-19 patients compared to baseline values. There was no difference in Ang II and Ang-(1-7) levels between placebo- and vitD-treated COVID-19 patients. Similar results were obtained with ILs profile. COVID-19 patients showed an increase in the protective component of the RAS which was not improved by vitD treatment.
Conclusions:
VitD did not improve RAS disbalance in COVID-19. Notwithstanding, the authors visualize that acute treatment with high doses of vitD may show a trend to a decline in inflammatory ILs and an increase in protective markers. Finally, the authors would like to highlight the limitations of this preliminary study, namely the small number of patients and the use of a large single bolus dose of vitD rather than lower daily doses for extended periods with prolonged follow-up times. All these factors need special consideration in the designs of new vitD supplementation trials. All these factors need special consideration in the designs of new vitD supplementation trials (ClinicalTrials.gov identifier: NCT04411446).
DOI: https://doi.org/10.37349/emed.2023.00137
This article belongs to the special issue Lung Fibrosis—Models and Mechanisms

In spite of the immense advancement in the diagnostic and treatment modalities, cancer continues to be one of the leading causes of mortality across the globe, responsible for the death of around 10 million patients every year. The foremost challenges faced in the treatment of this disease are chemoresistance, adverse effects of the drugs, and the high cost of treatment. Though scientific studies over the past few decades have foreseen and are focusing on the cancer-preventive and therapeutic potential of natural products and their underlying mechanism of action, many more of these agents are not still explored. Piperlongumine (PL), or piplartine, is one such alkaloid isolated from Piper longum Linn., which is shown to be safe and has significant potential in the prevention and therapy of cancer. Numerous shreds of evidence have established the ability of this alkaloid and its analogs and nanoformulations in modulating various complex molecular pathways such as phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin, nuclear factor-kappa B, Janus kinases/signal transducer and activator of transcription 3, etc. and inhibit different hallmarks of cancer such as cell survival, proliferation, invasion, angiogenesis, epithelial-mesenchymal-transition, metastases, etc. In addition, PL was also shown to inhibit radioresistance and chemoresistance and sensitize the cancer cells to the standard chemotherapeutic agents. Therefore, this compound has high potential as a drug candidate for the prevention and treatment of different cancers. The current review briefly reiterates the anti-cancer properties of PL against different types of cancer, which permits further investigation by conducting clinical studies.
In spite of the immense advancement in the diagnostic and treatment modalities, cancer continues to be one of the leading causes of mortality across the globe, responsible for the death of around 10 million patients every year. The foremost challenges faced in the treatment of this disease are chemoresistance, adverse effects of the drugs, and the high cost of treatment. Though scientific studies over the past few decades have foreseen and are focusing on the cancer-preventive and therapeutic potential of natural products and their underlying mechanism of action, many more of these agents are not still explored. Piperlongumine (PL), or piplartine, is one such alkaloid isolated from Piper longum Linn., which is shown to be safe and has significant potential in the prevention and therapy of cancer. Numerous shreds of evidence have established the ability of this alkaloid and its analogs and nanoformulations in modulating various complex molecular pathways such as phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin, nuclear factor-kappa B, Janus kinases/signal transducer and activator of transcription 3, etc. and inhibit different hallmarks of cancer such as cell survival, proliferation, invasion, angiogenesis, epithelial-mesenchymal-transition, metastases, etc. In addition, PL was also shown to inhibit radioresistance and chemoresistance and sensitize the cancer cells to the standard chemotherapeutic agents. Therefore, this compound has high potential as a drug candidate for the prevention and treatment of different cancers. The current review briefly reiterates the anti-cancer properties of PL against different types of cancer, which permits further investigation by conducting clinical studies.
DOI: https://doi.org/10.37349/etat.2021.00049

Aim:
Currently, malignant diseases represent a health issue worldwide. Among these, lung cancer is of growing importance, due to its high incidence and mortality. Chemotherapy, one of the most frequently used treatments, has shown its ability to induce accelerated immunosenescence in classic and as well non-classic lymphocyte compartments, being less described in the latter. The immune restoration strategies have demonstrated their ability to reverse immunosenescence and exhaustion markers in conventional lymphocyte subpopulations after chemotherapy. However, the possible immunorestorative effect on non-classical lymphocytes has not been widely reported. The aim of this study was to evaluate the effect of chemotherapy and the administration of a thymic polypeptide factor on non-classical lymphocyte populations in patients with advanced lung cancer.
Methods:
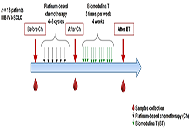
Eighteen patients with advanced lung cancer, were evaluated at baseline before and after platinum-based chemotherapy (4–6 cycles). All patients could complete treatment with a thymic polypeptide factor [Biomodulina T (BT)] at the end of chemotherapy. Blood from patients was collected by venipuncture in heparinized tubes before and after chemotherapy and at the end of BT treatment to analyze the frequencies of non-classical immune subpopulations by flow cytometry.
Results:
Natural killer (NK), natural killer T cells (NKT), and double-positive T lymphocyte (DPT) proportions reached normal values in patients diagnosed with advanced lung cancer before receiving cytotoxic treatment. Chemotherapy did not induce modifications in the total percent of NK, NKT, and DPT populations in these patients. However, the administration of BT decreased DPTs and NK cells expressing the cluster of differentiation (CD)57 molecule, which is considered a marker of immunosenescence.
Conclusions:
These results suggest a lower influence of platinum-based chemotherapy on non-classical lymphocytes and the potential to generate a reconstitution of lymphocyte subpopulations in patients with advanced lung cancer by using the thymic factor BT, which reveals a new possibility for improving the response to cancer immunotherapies [Cuban Public Registry of Clinical Trial (RPCEC, https://rpcec.sld.cu/en/trials/RPCEC00000358-En) identifier: RPCEC00000358].
Aim:
Currently, malignant diseases represent a health issue worldwide. Among these, lung cancer is of growing importance, due to its high incidence and mortality. Chemotherapy, one of the most frequently used treatments, has shown its ability to induce accelerated immunosenescence in classic and as well non-classic lymphocyte compartments, being less described in the latter. The immune restoration strategies have demonstrated their ability to reverse immunosenescence and exhaustion markers in conventional lymphocyte subpopulations after chemotherapy. However, the possible immunorestorative effect on non-classical lymphocytes has not been widely reported. The aim of this study was to evaluate the effect of chemotherapy and the administration of a thymic polypeptide factor on non-classical lymphocyte populations in patients with advanced lung cancer.
Methods:
Eighteen patients with advanced lung cancer, were evaluated at baseline before and after platinum-based chemotherapy (4–6 cycles). All patients could complete treatment with a thymic polypeptide factor [Biomodulina T (BT)] at the end of chemotherapy. Blood from patients was collected by venipuncture in heparinized tubes before and after chemotherapy and at the end of BT treatment to analyze the frequencies of non-classical immune subpopulations by flow cytometry.
Results:
Natural killer (NK), natural killer T cells (NKT), and double-positive T lymphocyte (DPT) proportions reached normal values in patients diagnosed with advanced lung cancer before receiving cytotoxic treatment. Chemotherapy did not induce modifications in the total percent of NK, NKT, and DPT populations in these patients. However, the administration of BT decreased DPTs and NK cells expressing the cluster of differentiation (CD)57 molecule, which is considered a marker of immunosenescence.
Conclusions:
These results suggest a lower influence of platinum-based chemotherapy on non-classical lymphocytes and the potential to generate a reconstitution of lymphocyte subpopulations in patients with advanced lung cancer by using the thymic factor BT, which reveals a new possibility for improving the response to cancer immunotherapies [Cuban Public Registry of Clinical Trial (RPCEC, https://rpcec.sld.cu/en/trials/RPCEC00000358-En) identifier: RPCEC00000358].
DOI: https://doi.org/10.37349/ei.2024.00150

Coronavirus disease 2019 (COVID-19) was declared a global pandemic by the World Health Organization (WHO) in March 2020. Despite the availability of therapies and the adoption of security measures, the most effective method to fight COVID-19 remains the induction of immunity through vaccines. Scientific communities have developed several types of COVID-19 vaccines since the beginning of the pandemic, including those with innovative messenger RNA (mRNA) technology. Patients with a history of allergic reactions may have an increased risk of hypersensitivity reactions to COVID-19 vaccines. Therefore, it is important that these patients are evaluated by an allergist to help monitor immediate-type adverse reactions and identify what vaccine component may elicit an allergic reaction. Various strategies have been suggested to prevent hypersensitivity reactions, including performing skin tests or in vitro tests before vaccination in high-risk patients, administering a different vaccine for the second dose in subjects reporting adverse reactions to the first dose, fractional dosing, or pretreating with anti-immunoglobulin E (IgE) monoclonal antibody. The scope of this review is to evaluate, through current evidence available in the literature, the accuracy of skin testing to the excipients of COVID-19 vaccines, especially polyethylene glycol (PEG) and polysorbate, in predicting allergic reactions to vaccination, despite the existing discordance of data and approaches to the question from the various clinical experiences, as to permit the safe administration of COVID-19 vaccines to populations around the globe.
Coronavirus disease 2019 (COVID-19) was declared a global pandemic by the World Health Organization (WHO) in March 2020. Despite the availability of therapies and the adoption of security measures, the most effective method to fight COVID-19 remains the induction of immunity through vaccines. Scientific communities have developed several types of COVID-19 vaccines since the beginning of the pandemic, including those with innovative messenger RNA (mRNA) technology. Patients with a history of allergic reactions may have an increased risk of hypersensitivity reactions to COVID-19 vaccines. Therefore, it is important that these patients are evaluated by an allergist to help monitor immediate-type adverse reactions and identify what vaccine component may elicit an allergic reaction. Various strategies have been suggested to prevent hypersensitivity reactions, including performing skin tests or in vitro tests before vaccination in high-risk patients, administering a different vaccine for the second dose in subjects reporting adverse reactions to the first dose, fractional dosing, or pretreating with anti-immunoglobulin E (IgE) monoclonal antibody. The scope of this review is to evaluate, through current evidence available in the literature, the accuracy of skin testing to the excipients of COVID-19 vaccines, especially polyethylene glycol (PEG) and polysorbate, in predicting allergic reactions to vaccination, despite the existing discordance of data and approaches to the question from the various clinical experiences, as to permit the safe administration of COVID-19 vaccines to populations around the globe.
DOI: https://doi.org/10.37349/eaa.2024.00028

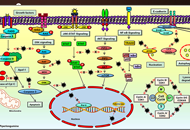
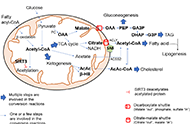
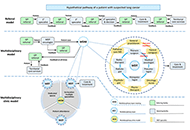
Mitochondria are present in all mammalian cells except matured red blood cells. Mitochondria consist of several metabolic pathways for glucose, fatty acids, amino acids, and bioenergetic pathways for ATP synthesis, membrane potential, and reactive oxygen production. In the liver, hepatic mitochondria play a key role in hepatic steatosis because mitochondrial metabolism produces acetyl-CoA which is the building block for synthesis of lipids and cholesterol. Mitochondria inner membrane is impermeable of metabolites, reducing equivalents, and small molecules such as phosphate, and sulfate. Thus, mitochondrial shuttles and carriers function as the routes of influx and efflux of these metabolites and molecules across the inner membrane. The signal regulation of these shuttles and mitochondrial enzymes could play a key role in coordinating the mitochondrial metabolism to adapt the cytosolic part of metabolic pathways in liver metabolic stress. Intriguingly, the interaction of mitochondria protein SH3 domain-binding protein 5 (SAB/SH3BP5) and c-Jun N-terminal kinase (JNK) was found as a pivotal role in sustained activation of JNK and phosphorylated-JNK (P-JNK) mediated activation of lipogenic pathway in nutritional excess. Knockout or knockdown of SAB prevented or reversed the hepatic steatosis, inflammation, and fibrosis, and improved metabolic intolerance and energy expenditure. Moreover, blocking the SAB peptide prevents palmitic acid-induced P-JNK interaction with SAB and inhibition of mitochondrial bioenergetics, implying the P-JNK effect on mitochondrial metabolism. This review focuses on the flow of mitochondrial metabolites in metabolic stress conditions and the contribution of mitochondria and mitochondrial stress signals in hepatic steatosis.
Mitochondria are present in all mammalian cells except matured red blood cells. Mitochondria consist of several metabolic pathways for glucose, fatty acids, amino acids, and bioenergetic pathways for ATP synthesis, membrane potential, and reactive oxygen production. In the liver, hepatic mitochondria play a key role in hepatic steatosis because mitochondrial metabolism produces acetyl-CoA which is the building block for synthesis of lipids and cholesterol. Mitochondria inner membrane is impermeable of metabolites, reducing equivalents, and small molecules such as phosphate, and sulfate. Thus, mitochondrial shuttles and carriers function as the routes of influx and efflux of these metabolites and molecules across the inner membrane. The signal regulation of these shuttles and mitochondrial enzymes could play a key role in coordinating the mitochondrial metabolism to adapt the cytosolic part of metabolic pathways in liver metabolic stress. Intriguingly, the interaction of mitochondria protein SH3 domain-binding protein 5 (SAB/SH3BP5) and c-Jun N-terminal kinase (JNK) was found as a pivotal role in sustained activation of JNK and phosphorylated-JNK (P-JNK) mediated activation of lipogenic pathway in nutritional excess. Knockout or knockdown of SAB prevented or reversed the hepatic steatosis, inflammation, and fibrosis, and improved metabolic intolerance and energy expenditure. Moreover, blocking the SAB peptide prevents palmitic acid-induced P-JNK interaction with SAB and inhibition of mitochondrial bioenergetics, implying the P-JNK effect on mitochondrial metabolism. This review focuses on the flow of mitochondrial metabolites in metabolic stress conditions and the contribution of mitochondria and mitochondrial stress signals in hepatic steatosis.
DOI: https://doi.org/10.37349/edd.2024.00039
This article belongs to the special issue Mitochondria and Lipid Signalling in Liver Diseases

Aim:
Aloysia citrodora has a long history of traditional use in treating various ailments. This study evaluated the in vivo chemopreventive efficacy and systemic toxicity of an extract of A. citrodora in a transgenic mouse model of HPV16 (human papillomavirus type 16)-induced cancer.
Methods:
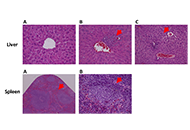
The experiment involved six groups (n = 5): group 1 (G1, wild-type (WT), water), group 2 (G2, HPV, water), group 3 (G3, WT, 0.013 g/mL), group 4 (G4, HPV, 0.006 g/mL), group 5 (G5, HPV, 0.008 g/mL), and group 6 (G6, HPV, 0.013 g/mL). Throughout the assay, humane endpoints, body weight, food, and water consumption were recorded weekly. The internal organs and skin of the mice were collected for analysis after they were sacrificed. Toxicological parameters that were studied included hematological and biochemical blood markers, splenic and hepatic histology, and hepatic oxidative stress.
Results:
A. citrodora extract seems to reduce the incidence of dysplastic and in situ carcinoma skin lesions induced by HPV16 in this model, suggesting that dietary supplementation with concentrations of 0.008 g/mL and 0.013 g/mL may have beneficial chemopreventive effects.
Conclusions:
The extract did not induce any concentration-dependent toxicological effects on any of the parameters included in the study, indicating a favorable toxicological profile under these experimental conditions.
Aim:
Aloysia citrodora has a long history of traditional use in treating various ailments. This study evaluated the in vivo chemopreventive efficacy and systemic toxicity of an extract of A. citrodora in a transgenic mouse model of HPV16 (human papillomavirus type 16)-induced cancer.
Methods:
The experiment involved six groups (n = 5): group 1 (G1, wild-type (WT), water), group 2 (G2, HPV, water), group 3 (G3, WT, 0.013 g/mL), group 4 (G4, HPV, 0.006 g/mL), group 5 (G5, HPV, 0.008 g/mL), and group 6 (G6, HPV, 0.013 g/mL). Throughout the assay, humane endpoints, body weight, food, and water consumption were recorded weekly. The internal organs and skin of the mice were collected for analysis after they were sacrificed. Toxicological parameters that were studied included hematological and biochemical blood markers, splenic and hepatic histology, and hepatic oxidative stress.
Results:
A. citrodora extract seems to reduce the incidence of dysplastic and in situ carcinoma skin lesions induced by HPV16 in this model, suggesting that dietary supplementation with concentrations of 0.008 g/mL and 0.013 g/mL may have beneficial chemopreventive effects.
Conclusions:
The extract did not induce any concentration-dependent toxicological effects on any of the parameters included in the study, indicating a favorable toxicological profile under these experimental conditions.
DOI: https://doi.org/10.37349/emed.2024.00228

Chronic rhinosinusitis with nasal polyps (CRSwNP) is a multifactorial inflammatory disease of the mucous membranes of the nose and paranasal sinuses. Eosinophilic inflammation is described as a common endotype. The anti-interleukin-5 (IL-5) antibody mepolizumab was approved in November 2021 as an add-on therapy to intranasal glucocorticosteroids for the treatment of adults with severe CRSwNP when systemic glucocorticosteroids or surgery do not provide adequate disease control. While national and international recommendations exist for the use of mepolizumab in CRSwNP, therapy monitoring and follow-up documentation are required, and therapy discontinuation has not been adequately established yet. In this paper, recommendations for monitoring the course and efficacy of therapy as well as for reviewing the duration and possible termination of therapy are provided. For this purpose, a literature search was performed to analyze previous data on the treatment of CRSwNP with mepolizumab and to determine the available evidence by searching MEDLINE, PubMed, and the national and international trial and guideline registries and the Cochrane Library. Human studies published in the period up to and including October 2022 were considered. Based on the international literature and previous experience, recommendations for follow-up, adherence to therapy intervals and possible therapy breaks, as well as termination of therapy when using mepolizumab for the indication CRSwNP in the German health care system are given by an expert panel on the basis of a documentation sheet.
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a multifactorial inflammatory disease of the mucous membranes of the nose and paranasal sinuses. Eosinophilic inflammation is described as a common endotype. The anti-interleukin-5 (IL-5) antibody mepolizumab was approved in November 2021 as an add-on therapy to intranasal glucocorticosteroids for the treatment of adults with severe CRSwNP when systemic glucocorticosteroids or surgery do not provide adequate disease control. While national and international recommendations exist for the use of mepolizumab in CRSwNP, therapy monitoring and follow-up documentation are required, and therapy discontinuation has not been adequately established yet. In this paper, recommendations for monitoring the course and efficacy of therapy as well as for reviewing the duration and possible termination of therapy are provided. For this purpose, a literature search was performed to analyze previous data on the treatment of CRSwNP with mepolizumab and to determine the available evidence by searching MEDLINE, PubMed, and the national and international trial and guideline registries and the Cochrane Library. Human studies published in the period up to and including October 2022 were considered. Based on the international literature and previous experience, recommendations for follow-up, adherence to therapy intervals and possible therapy breaks, as well as termination of therapy when using mepolizumab for the indication CRSwNP in the German health care system are given by an expert panel on the basis of a documentation sheet.
DOI: https://doi.org/10.37349/eaa.2024.00027
This article belongs to the special issue Update on Chronic RhinoSinusitis

International Guidelines as well as Cancer Associations recommend a multidisciplinary approach to lung cancer care. A multidisciplinary team (MDT) can significantly improve treatment decision-making and patient coordination by putting different physicians and other health professionals “in the same room”, who collectively decide upon the best possible treatment. However, this is not a panacea for cancer treatment. The impact of multidisciplinary care (MDC) on patient outcomes is not univocal, while the effective functioning of the MDT depends on many factors. This review presents the available MDT literature with an emphasis on the key factors that characterize high-quality patient care in lung cancer. The study was conducted with a bibliographic search using different electronic databases (PubMed Central, Scopus, Google Scholar, and Google) referring to multidisciplinary cancer care settings. Many key elements appear consolidated, while others emerge as prevalent and actual, especially those related to visible barriers which work across geographic, organizational, and disciplinary boundaries. MDTs must be sustained by strategic management, structured within the entity, and cannot be managed as a separate care process. Furthermore, they need to coordinate with other teams (within and outside the organization) and join with the broad range of services delivered by multiple providers at various points of the cancer journey or within the system, with the vision of integrated care.
International Guidelines as well as Cancer Associations recommend a multidisciplinary approach to lung cancer care. A multidisciplinary team (MDT) can significantly improve treatment decision-making and patient coordination by putting different physicians and other health professionals “in the same room”, who collectively decide upon the best possible treatment. However, this is not a panacea for cancer treatment. The impact of multidisciplinary care (MDC) on patient outcomes is not univocal, while the effective functioning of the MDT depends on many factors. This review presents the available MDT literature with an emphasis on the key factors that characterize high-quality patient care in lung cancer. The study was conducted with a bibliographic search using different electronic databases (PubMed Central, Scopus, Google Scholar, and Google) referring to multidisciplinary cancer care settings. Many key elements appear consolidated, while others emerge as prevalent and actual, especially those related to visible barriers which work across geographic, organizational, and disciplinary boundaries. MDTs must be sustained by strategic management, structured within the entity, and cannot be managed as a separate care process. Furthermore, they need to coordinate with other teams (within and outside the organization) and join with the broad range of services delivered by multiple providers at various points of the cancer journey or within the system, with the vision of integrated care.
DOI: https://doi.org/10.37349/etat.2024.00217
This article belongs to the special issue Integrated Approaches for Non-Small-Cell Lung Cancer

Lynch syndrome is a hereditary cancer predisposition syndrome caused by germline alterations in mismatch repair (MMR) genes leading to increased risk of colon cancer as well as other cancer types. Non-small cell lung cancer (NSCLC) is not among typical Lynch syndrome-associated tumors: pembrolizumab, an immune checkpoint inhibitor, is actually approved for the treatment of NSCLC patients and represents a promising treatment option for patients with advanced metastatic MMR-deficient cancer, regardless of tumor origin. This case report describes the clinical presentation and management of a 74-year-old female with a history of rectal adenocarcinoma and ovarian cancer, who has a documented frameshift pathogenic variant in the exon 8 of MSH6 gene and an intronic variant in the BRCA2 gene (classified as a variant of uncertain significance), affected by NSCLC with brain metastases. Despite these premises, the patient was treated with pembrolizumab and she did not benefit from this kind of treatment.
Lynch syndrome is a hereditary cancer predisposition syndrome caused by germline alterations in mismatch repair (MMR) genes leading to increased risk of colon cancer as well as other cancer types. Non-small cell lung cancer (NSCLC) is not among typical Lynch syndrome-associated tumors: pembrolizumab, an immune checkpoint inhibitor, is actually approved for the treatment of NSCLC patients and represents a promising treatment option for patients with advanced metastatic MMR-deficient cancer, regardless of tumor origin. This case report describes the clinical presentation and management of a 74-year-old female with a history of rectal adenocarcinoma and ovarian cancer, who has a documented frameshift pathogenic variant in the exon 8 of MSH6 gene and an intronic variant in the BRCA2 gene (classified as a variant of uncertain significance), affected by NSCLC with brain metastases. Despite these premises, the patient was treated with pembrolizumab and she did not benefit from this kind of treatment.
DOI: https://doi.org/10.37349/etat.2021.00044

Aim:
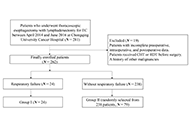
Respiratory failure is common after esophagectomy for esophageal cancer (EC). This study aimed to identify the risk factors associated with postoperative respiratory failure following esophagectomy for EC.
Methods:
A single-center observational study from China was conducted on 262 patients with EC who underwent thoracoscopic esophagectomy between April 2014 and June 2016. The patients were divided into two groups: group I (respiratory failure) and group II (without respiratory failure). Demographic and perioperative variables, tumor-related factors, surgical factors, Acute Physiology and Chronic Health Evaluation II (APACHE II) scores, and clinical course were compared between the groups. Univariable and multivariable logistic regression analyses were performed to assess the risk factors of postoperative respiratory failure after esophagectomy.
Results:
Among the 262 patients, 24 (9.2%) developed respiratory failure. Univariable analysis revealed several risk factors, including age, smoking, comorbidities, partial pressure of oxygen (PO2), partial pressure of carbon dioxide (PCO2), forced vital capacity (FVC), FVC percentage (FVC%), urine volume during surgery, and APACHE II score. Multivariable analysis showed that age, comorbidities of diabetes mellitus (DM), FVC%, urine volume during surgery, and APACHE II score were independent predictors of respiratory failure. Specifically, elderly patients (> 65 years) with comorbidities of DM, lower FVC%, higher urine volume during surgery, and elevated APACHE II score were found to be more susceptible to respiratory failure, resulting in prolonged hospitalization and increased healthcare burden. These findings emphasize the importance of considering these factors in the management and care of patients at risk of respiratory failure.
Conclusions:
As a common complication following esophagectomy for EC. Respiratory failure is significantly associated with age, comorbidities of DM, FVC%, urine volume during surgery, and APACHE II score in the dataset. The findings will contribute to the evaluation of the risk of respiratory failure and guide early intervention strategies in clinical decision-making.
Aim:
Respiratory failure is common after esophagectomy for esophageal cancer (EC). This study aimed to identify the risk factors associated with postoperative respiratory failure following esophagectomy for EC.
Methods:
A single-center observational study from China was conducted on 262 patients with EC who underwent thoracoscopic esophagectomy between April 2014 and June 2016. The patients were divided into two groups: group I (respiratory failure) and group II (without respiratory failure). Demographic and perioperative variables, tumor-related factors, surgical factors, Acute Physiology and Chronic Health Evaluation II (APACHE II) scores, and clinical course were compared between the groups. Univariable and multivariable logistic regression analyses were performed to assess the risk factors of postoperative respiratory failure after esophagectomy.
Results:
Among the 262 patients, 24 (9.2%) developed respiratory failure. Univariable analysis revealed several risk factors, including age, smoking, comorbidities, partial pressure of oxygen (PO2), partial pressure of carbon dioxide (PCO2), forced vital capacity (FVC), FVC percentage (FVC%), urine volume during surgery, and APACHE II score. Multivariable analysis showed that age, comorbidities of diabetes mellitus (DM), FVC%, urine volume during surgery, and APACHE II score were independent predictors of respiratory failure. Specifically, elderly patients (> 65 years) with comorbidities of DM, lower FVC%, higher urine volume during surgery, and elevated APACHE II score were found to be more susceptible to respiratory failure, resulting in prolonged hospitalization and increased healthcare burden. These findings emphasize the importance of considering these factors in the management and care of patients at risk of respiratory failure.
Conclusions:
As a common complication following esophagectomy for EC. Respiratory failure is significantly associated with age, comorbidities of DM, FVC%, urine volume during surgery, and APACHE II score in the dataset. The findings will contribute to the evaluation of the risk of respiratory failure and guide early intervention strategies in clinical decision-making.
DOI: https://doi.org/10.37349/emed.2023.00195

Aim:
The role of tumor burden (TB) for patients with non-small cell lung cancer (NSCLC) receiving immunotherapy is still unknown. The aim of this analysis was to analyze the prognostic value of TB in a real-world sample of advanced NSCLC patients.
Methods:
Sixty-five consecutive patients with advanced NSCLC treated with immunotherapy as first or second line therapy were retrospectively analyzed between August 2015 and February 2018. TB was recorded at baseline considering sites and number of metastases, thoracic vs. extrathoracic disease, measurable disease (MD) vs. not-MD (NMD) and evaluating dimensional aspects as maximum lesion diameter (cut-off = 6.3 cm), sum of the 5 major lesions diameters (cut-off = 14.3 cm), and number of sites of metastases (cut-off > 4). All cut-offs were calculated by receiver operating characteristic curves. Median overall survival (OS) was estimated using Kaplan-Meier method. A Cox regression model was carried out for univariate and multivariate analyses.
Results:
Median age was 70 years and most patients (86.2%) had a good performance status (PS-Eastern Cooperative Oncology Group < 2). No significant difference in OS was noted between subgroups of patients according to TB. Bone metastases (BM) had a negative prognostic impact [median OS (mOS), 13.8 vs. 70.0 months, P = 0.0009; median progression free survival in the second line (mPFS2) 2.97 vs. 8.63 months; P = 0.0037]. Patients with NMD had a poorer prognosis (mOS, 15.9 months vs. not reached, P < 0.0001; mPFS2 3.8 vs. 12.2 months; P = 0.0199). Patients with disease limited to the thorax had a better prognosis compared to patients with involvement of extrathoracic sites (mOS, 70 vs. 17.3 months; P = 0.0136). Having more than 4 metastatic sites resulted as a negative prognostic factor (mOS, 15.9 vs. 25.2 months; P = 0.0106). At multivariate analysis, BM, NMD, extrathoracic disease and number of sites of metastases > 4 were negative prognostic factors (P < 0.0001).
Conclusions:
This study underlines the negative prognostic impact of specific metastatic sites, presence of NMD and extrathoracic disease in advanced NSCLC patients treated with immunotherapy. However, TB does not appear to affect the outcome of these patients.
Aim:
The role of tumor burden (TB) for patients with non-small cell lung cancer (NSCLC) receiving immunotherapy is still unknown. The aim of this analysis was to analyze the prognostic value of TB in a real-world sample of advanced NSCLC patients.
Methods:
Sixty-five consecutive patients with advanced NSCLC treated with immunotherapy as first or second line therapy were retrospectively analyzed between August 2015 and February 2018. TB was recorded at baseline considering sites and number of metastases, thoracic vs. extrathoracic disease, measurable disease (MD) vs. not-MD (NMD) and evaluating dimensional aspects as maximum lesion diameter (cut-off = 6.3 cm), sum of the 5 major lesions diameters (cut-off = 14.3 cm), and number of sites of metastases (cut-off > 4). All cut-offs were calculated by receiver operating characteristic curves. Median overall survival (OS) was estimated using Kaplan-Meier method. A Cox regression model was carried out for univariate and multivariate analyses.
Results:
Median age was 70 years and most patients (86.2%) had a good performance status (PS-Eastern Cooperative Oncology Group < 2). No significant difference in OS was noted between subgroups of patients according to TB. Bone metastases (BM) had a negative prognostic impact [median OS (mOS), 13.8 vs. 70.0 months, P = 0.0009; median progression free survival in the second line (mPFS2) 2.97 vs. 8.63 months; P = 0.0037]. Patients with NMD had a poorer prognosis (mOS, 15.9 months vs. not reached, P < 0.0001; mPFS2 3.8 vs. 12.2 months; P = 0.0199). Patients with disease limited to the thorax had a better prognosis compared to patients with involvement of extrathoracic sites (mOS, 70 vs. 17.3 months; P = 0.0136). Having more than 4 metastatic sites resulted as a negative prognostic factor (mOS, 15.9 vs. 25.2 months; P = 0.0106). At multivariate analysis, BM, NMD, extrathoracic disease and number of sites of metastases > 4 were negative prognostic factors (P < 0.0001).
Conclusions:
This study underlines the negative prognostic impact of specific metastatic sites, presence of NMD and extrathoracic disease in advanced NSCLC patients treated with immunotherapy. However, TB does not appear to affect the outcome of these patients.
DOI: https://doi.org/10.37349/etat.2021.00043
This article belongs to the special issue Immunotherapy in Cancer Patients

Aim:
After the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic and the realization of mass vaccination against the virus, the availability of a reliable, rapid, and easy-to-use system for registering the individual anti-S1 antibody titer could facilitate the personalized assessment of the need for booster vaccine doses and the reduction of social distancing and other measures.
Methods:
The biosensor system is based on immobilized engineered SK-N-SH neuroblastoma cells, bearing the S1 protein, and it can detect immunoglobulin G (IgG) antibodies against the SARS-CoV-2 S1 spike antigen. A disposable electrode strip bearing the engineered mammalian cells is connected to a customized read-out potentiometric device with real-time data transmission to a wireless fidelity (WiFi)-connected smartphone. Blood samples from past-infected individuals and individuals vaccinated against SARS-CoV-2 were used for validation.
Results:
In the present study, a smartphone application (app), capable of analyzing data regarding the levels of anti-S1 antibodies in blood is introduced. The app works in conjunction with a portable, ultra-rapid, and sensitive biosensor transmitting real-time measurements to the smartphone. Both historical and current individual data can be encoded by using the app, resulting in a widely accepted quick response (QR) code, which can then be constantly updated to match a person’s status.
Conclusions:
This novel system could be utilized for the eventual development of a coronavirus disease 2019 (COVID-19) electronic passport, which could be further employed to improve the population-wide, cross-country surveillance of vaccination efficiency, as well as facilitate the implementation of cross-border digital health services in a user-friendly and secure way.
Aim:
After the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic and the realization of mass vaccination against the virus, the availability of a reliable, rapid, and easy-to-use system for registering the individual anti-S1 antibody titer could facilitate the personalized assessment of the need for booster vaccine doses and the reduction of social distancing and other measures.
Methods:
The biosensor system is based on immobilized engineered SK-N-SH neuroblastoma cells, bearing the S1 protein, and it can detect immunoglobulin G (IgG) antibodies against the SARS-CoV-2 S1 spike antigen. A disposable electrode strip bearing the engineered mammalian cells is connected to a customized read-out potentiometric device with real-time data transmission to a wireless fidelity (WiFi)-connected smartphone. Blood samples from past-infected individuals and individuals vaccinated against SARS-CoV-2 were used for validation.
Results:
In the present study, a smartphone application (app), capable of analyzing data regarding the levels of anti-S1 antibodies in blood is introduced. The app works in conjunction with a portable, ultra-rapid, and sensitive biosensor transmitting real-time measurements to the smartphone. Both historical and current individual data can be encoded by using the app, resulting in a widely accepted quick response (QR) code, which can then be constantly updated to match a person’s status.
Conclusions:
This novel system could be utilized for the eventual development of a coronavirus disease 2019 (COVID-19) electronic passport, which could be further employed to improve the population-wide, cross-country surveillance of vaccination efficiency, as well as facilitate the implementation of cross-border digital health services in a user-friendly and secure way.
DOI: https://doi.org/10.37349/edht.2024.00008
This article belongs to the special issue Biosensors for Bioactive Molecules

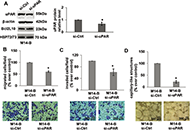
Aim:
B-cell lymphoma-2 (Bcl-2)-like protein-10 (Bcl2L10) is the less studied member of Bcl-2 family proteins, with the controversial role in different cancer histotypes. Very recently, Bcl2L10 expression in melanoma tumor specimens and its role in melanoma response to therapy have been demonstrated. Here, the involvement of Bcl2L10 on the in vitro and in vivo properties associated with melanoma aggressive features has been investigated.
Methods:
Endogenous Bcl2L10 protein expression was detected by western blotting analysis in a panel of patient-derived and commercially available human melanoma cells. In vitro assays to evaluate clonogenicity, cell proliferation, cell migration, cell invasion, and in vitro capillary-like structure formation [vasculogenic mimicry (VM)] have been performed by using human melanoma cells stably overexpressing Bcl2L10 or transiently transfected for loss/gain function of Bcl2L10, grown under two- or three-dimensional (3D) conditions Xenograft melanoma model was employed to evaluate in vivo tumor growth and angiogenesis.
Results:
Results demonstrated that Bcl2L10 acts as an inducer of in vitro cell migration, invasion, and VM, while in vitro cell proliferation, in vivo tumor growth, as well as colony formation properties were not affected. Dissecting different signaling pathways, it was found that Bcl2L10 positively affects the phosphorylation of extracellular-signal-regulated kinase (ERK) and the expression of markers of cell invasion, such as urokinase plasminogen activator receptor (uPAR) and matrix metalloproteinases (MMPs). Of note, Bcl2L10-dependent in vitro migration, invasion, and VM are linked to uPAR. Bcl2L10 also negatively regulates the intracellular calcium level. Finally, reduced invasion capability in 3D spheroid invasion assay of melanoma cells transiently overexpressing Bcl2L10 was observed after treatment with inhibitors of MMPs and uPAR.
Conclusions:
Overall, data reported in this paper provide evidence supporting a positive role of Bcl2L10 in melanoma aggressive features.
Aim:
B-cell lymphoma-2 (Bcl-2)-like protein-10 (Bcl2L10) is the less studied member of Bcl-2 family proteins, with the controversial role in different cancer histotypes. Very recently, Bcl2L10 expression in melanoma tumor specimens and its role in melanoma response to therapy have been demonstrated. Here, the involvement of Bcl2L10 on the in vitro and in vivo properties associated with melanoma aggressive features has been investigated.
Methods:
Endogenous Bcl2L10 protein expression was detected by western blotting analysis in a panel of patient-derived and commercially available human melanoma cells. In vitro assays to evaluate clonogenicity, cell proliferation, cell migration, cell invasion, and in vitro capillary-like structure formation [vasculogenic mimicry (VM)] have been performed by using human melanoma cells stably overexpressing Bcl2L10 or transiently transfected for loss/gain function of Bcl2L10, grown under two- or three-dimensional (3D) conditions Xenograft melanoma model was employed to evaluate in vivo tumor growth and angiogenesis.
Results:
Results demonstrated that Bcl2L10 acts as an inducer of in vitro cell migration, invasion, and VM, while in vitro cell proliferation, in vivo tumor growth, as well as colony formation properties were not affected. Dissecting different signaling pathways, it was found that Bcl2L10 positively affects the phosphorylation of extracellular-signal-regulated kinase (ERK) and the expression of markers of cell invasion, such as urokinase plasminogen activator receptor (uPAR) and matrix metalloproteinases (MMPs). Of note, Bcl2L10-dependent in vitro migration, invasion, and VM are linked to uPAR. Bcl2L10 also negatively regulates the intracellular calcium level. Finally, reduced invasion capability in 3D spheroid invasion assay of melanoma cells transiently overexpressing Bcl2L10 was observed after treatment with inhibitors of MMPs and uPAR.
Conclusions:
Overall, data reported in this paper provide evidence supporting a positive role of Bcl2L10 in melanoma aggressive features.
DOI: https://doi.org/10.37349/etat.2022.00068
This article belongs to the special issue The Role of Bcl-2 Family Proteins in Cancer Progression and Their Relevance to Cancer Therapy

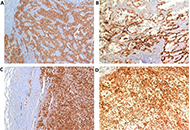
Aim:
In renal cell carcinoma (RCC), tumor heterogeneity generated challenges to biomarker development and therapeutic management, often becoming responsible for primary and acquired drug resistance. This study aimed to assess the inter-tumoral, intra-tumoral, and intra-lesional heterogeneity of known druggable targets in metastatic RCC (mRCC).
Methods:
The RIVELATOR study was a monocenter retrospective analysis of biological samples from 25 cases of primary RCC and their paired pulmonary metastases. The biomarkers analyzed included MET, mTOR, PD-1/PD-L1 pathways and the immune context.
Results:
High multi-level heterogeneity was demonstrated. MET was the most reliable biomarker, with the lowest intratumor heterogeneity: the positive mutual correlation between MET expression in primary tumors and their metastases had a significantly proportional intensity (P = 0.038). The intratumor heterogeneity grade was significantly higher for the mTOR pathway proteins. Combined immunophenotypical expression patterns and their correlations with the immune context were uncovered [i.e., mTOR expression in the metastases positively correlated with PD-L1 expression in tumor-infiltrating lymphocytes (TILs), P = 0.019; MET expression was related to PD-1 expression on TILs (P = 0.041, ρ = 0.41) and peritumoral lymphocytes (RILs; P = 0.013, ρ = 0.49)], suggesting the possibility of predicting drug response or resistance to tyrosine kinase, mTOR, or immune checkpoint inhibitors.
Conclusions:
In mRCC, multiple and multi-level assays of potentially predictive biomarkers are needed for their reliable translation into clinical practice. The easy-to-use immunohistochemical method of the present study allowed the identification of different combined expression patterns, providing cues for planning the management of systemic treatment combinations and sequences in an mRCC patient population. The quantitative heterogeneity of the investigated biomarkers suggests that multiple intralesional assays are needed to consider the assessment reliable for clinical considerations.
Aim:
In renal cell carcinoma (RCC), tumor heterogeneity generated challenges to biomarker development and therapeutic management, often becoming responsible for primary and acquired drug resistance. This study aimed to assess the inter-tumoral, intra-tumoral, and intra-lesional heterogeneity of known druggable targets in metastatic RCC (mRCC).
Methods:
The RIVELATOR study was a monocenter retrospective analysis of biological samples from 25 cases of primary RCC and their paired pulmonary metastases. The biomarkers analyzed included MET, mTOR, PD-1/PD-L1 pathways and the immune context.
Results:
High multi-level heterogeneity was demonstrated. MET was the most reliable biomarker, with the lowest intratumor heterogeneity: the positive mutual correlation between MET expression in primary tumors and their metastases had a significantly proportional intensity (P = 0.038). The intratumor heterogeneity grade was significantly higher for the mTOR pathway proteins. Combined immunophenotypical expression patterns and their correlations with the immune context were uncovered [i.e., mTOR expression in the metastases positively correlated with PD-L1 expression in tumor-infiltrating lymphocytes (TILs), P = 0.019; MET expression was related to PD-1 expression on TILs (P = 0.041, ρ = 0.41) and peritumoral lymphocytes (RILs; P = 0.013, ρ = 0.49)], suggesting the possibility of predicting drug response or resistance to tyrosine kinase, mTOR, or immune checkpoint inhibitors.
Conclusions:
In mRCC, multiple and multi-level assays of potentially predictive biomarkers are needed for their reliable translation into clinical practice. The easy-to-use immunohistochemical method of the present study allowed the identification of different combined expression patterns, providing cues for planning the management of systemic treatment combinations and sequences in an mRCC patient population. The quantitative heterogeneity of the investigated biomarkers suggests that multiple intralesional assays are needed to consider the assessment reliable for clinical considerations.
DOI: https://doi.org/10.37349/etat.2023.00165

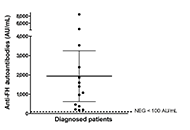
Aim:
To describe the clinical characteristics and frequency of anti-factor H (FH) autoantibody-associated atypical hemolytic uremic syndrome (aHUS) in the first cohort of Argentine patients.
Methods:
The presence of anti-FH autoantibodies in 70 pediatric patients with suspected aHUS was investigated between 2013 and 2022. Clinical and laboratory parameters were collected and compared between patients who were positive and negative for anti-FH antibodies.
Results:
The 70 patients screened for anti-FH autoantibodies presented clinical features of non-immune microangiopathic hemolytic anemia, thrombocytopenia and renal injury. Positive titers were found in 14 children [mean: 1,938 arbitrary units per mL (AU/mL), range 179–8,500]. Due to missing clinical data, two patients who tested positive for anti-FH and 20 patients who tested negative for anti-FH were excluded from the data analysis. The laboratory features and clinical manifestations of anti-FH-positive aHUS cases (n = 12) were very similar to those of subjects with no autoantibodies detected (n = 36). Treatment administration was heterogeneous among the 12 patients analyzed. Dialysis was performed in six patients in total. Five children received plasmapheresis, while three patients were treated with plasma exchange followed by administration of eculizumab. Two patients received eculizumab only and one showed significant improvement solely through supportive care. Eight patients in total received immunosuppressive therapy. Follow-up of three patients showed a significant decrease of anti-FH autoantibody titers in 2/3 after treatment and during clinical remission.
Conclusions:
The cohort of 70 pediatric patients in this study demonstrated that the frequency of anti-FH autoantibody-associated aHUS in Argentina is 20%. The implementation of anti-FH testing in the country can potentially contribute to improved treatment and follow-up for patients with autoimmune aHUS.
Aim:
To describe the clinical characteristics and frequency of anti-factor H (FH) autoantibody-associated atypical hemolytic uremic syndrome (aHUS) in the first cohort of Argentine patients.
Methods:
The presence of anti-FH autoantibodies in 70 pediatric patients with suspected aHUS was investigated between 2013 and 2022. Clinical and laboratory parameters were collected and compared between patients who were positive and negative for anti-FH antibodies.
Results:
The 70 patients screened for anti-FH autoantibodies presented clinical features of non-immune microangiopathic hemolytic anemia, thrombocytopenia and renal injury. Positive titers were found in 14 children [mean: 1,938 arbitrary units per mL (AU/mL), range 179–8,500]. Due to missing clinical data, two patients who tested positive for anti-FH and 20 patients who tested negative for anti-FH were excluded from the data analysis. The laboratory features and clinical manifestations of anti-FH-positive aHUS cases (n = 12) were very similar to those of subjects with no autoantibodies detected (n = 36). Treatment administration was heterogeneous among the 12 patients analyzed. Dialysis was performed in six patients in total. Five children received plasmapheresis, while three patients were treated with plasma exchange followed by administration of eculizumab. Two patients received eculizumab only and one showed significant improvement solely through supportive care. Eight patients in total received immunosuppressive therapy. Follow-up of three patients showed a significant decrease of anti-FH autoantibody titers in 2/3 after treatment and during clinical remission.
Conclusions:
The cohort of 70 pediatric patients in this study demonstrated that the frequency of anti-FH autoantibody-associated aHUS in Argentina is 20%. The implementation of anti-FH testing in the country can potentially contribute to improved treatment and follow-up for patients with autoimmune aHUS.
DOI: https://doi.org/10.37349/ei.2023.00118
This article belongs to the special issue The Complement System in Health and Disease

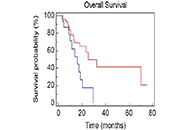
Aim:
Sarcopenia and skeletal muscle density (SMD) have been shown to be both predictive and prognostic marker in oncology. Advanced lung cancer inflammation index (ALI) has been shown to predict overall survival (OS) in small cell lung cancer (SCLC). Computed tomography (CT) enables skeletal muscle to be quantified, whereas body mass index (BMI) cannot accurately reflect body composition. The purpose was to evaluate the prognostic value of modified ALI (mALI) using CT-determined third lumbar vertebra (L3) muscle index beyond original ALI and see the interaction between sarcopenia, SMD, neutrophil-lymphocyte ratio (NLR), ALI and mALI at baseline and post 4 cycles of chemotherapy and their effects on OS and progress free survival (PFS) in patients with advanced non-SCLC (NSCLC).
Methods:
This retrospective study consisted of a total of 285 advanced NSCLC patients. The morphometric parameters such as SMD, skeletal muscle index (SMI) and fat-free mass (FFM) were measured by CT at the L3 vertebra. ALI was defined as BMI × serum albumin/NLR and mALI was defined as SMI × serum albumin/NLR.
Results:
Sarcopenia was observed in over 70% of patients across all BMI categories. Patients having sarcopenia suffered from a higher incidence of chemotherapeutic drug toxicities but this was not found to be statistically significant. Concordance was seen between ALI and mALI in the pre-treatment setting and this was statistically significant. A significant proportion of patients with poor ALI (90.9%), poor pre-chemotherapy mALI (91.3%) and poor post-chemotherapy mALI (89%) had poor NLR and each of them was statistically significant.
Conclusions:
In both univariate and multivariate analyses, this study demonstrated the statistical significance of sarcopenia, SMD, and mALI as predictive factors for OS. Additionally, sarcopenia and SMD were also found to be statistically significant factors in predicting PFS. These biomarkers could potentially help triage patients for active nutritional intervention for better outcomes.
Aim:
Sarcopenia and skeletal muscle density (SMD) have been shown to be both predictive and prognostic marker in oncology. Advanced lung cancer inflammation index (ALI) has been shown to predict overall survival (OS) in small cell lung cancer (SCLC). Computed tomography (CT) enables skeletal muscle to be quantified, whereas body mass index (BMI) cannot accurately reflect body composition. The purpose was to evaluate the prognostic value of modified ALI (mALI) using CT-determined third lumbar vertebra (L3) muscle index beyond original ALI and see the interaction between sarcopenia, SMD, neutrophil-lymphocyte ratio (NLR), ALI and mALI at baseline and post 4 cycles of chemotherapy and their effects on OS and progress free survival (PFS) in patients with advanced non-SCLC (NSCLC).
Methods:
This retrospective study consisted of a total of 285 advanced NSCLC patients. The morphometric parameters such as SMD, skeletal muscle index (SMI) and fat-free mass (FFM) were measured by CT at the L3 vertebra. ALI was defined as BMI × serum albumin/NLR and mALI was defined as SMI × serum albumin/NLR.
Results:
Sarcopenia was observed in over 70% of patients across all BMI categories. Patients having sarcopenia suffered from a higher incidence of chemotherapeutic drug toxicities but this was not found to be statistically significant. Concordance was seen between ALI and mALI in the pre-treatment setting and this was statistically significant. A significant proportion of patients with poor ALI (90.9%), poor pre-chemotherapy mALI (91.3%) and poor post-chemotherapy mALI (89%) had poor NLR and each of them was statistically significant.
Conclusions:
In both univariate and multivariate analyses, this study demonstrated the statistical significance of sarcopenia, SMD, and mALI as predictive factors for OS. Additionally, sarcopenia and SMD were also found to be statistically significant factors in predicting PFS. These biomarkers could potentially help triage patients for active nutritional intervention for better outcomes.
DOI: https://doi.org/10.37349/etat.2023.00172
This article belongs to the special issue Biomarkers for Personalized and Precise Cancer Diagnosis and Treatment
 Previous
Previous