Mitochondria and Lipid Signalling in Liver Diseases
Guest Editor
Prof. Carmen Garcia-Ruiz E-Mail
Professor of the Spanish National Research Council at the Institute of Biomedical Research of Barcelona (IIBB-CSIC); Institute of Biomedical Research August Pi i Sunyer (IDIBAPS); Research Center Network of Hepatic and Digestive Diseases (CIBERehd-ISCIII); Barcelona, Spain
Research Keywords: Mitochondria, lipid metabolism, oxidative stress, cell death, gender differences in NASH driven HCC
About the Special lssue
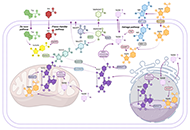
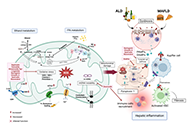
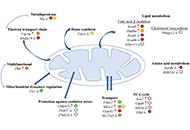
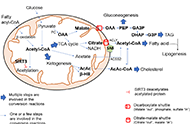
The liver plays a central role in lipid metabolism. Lipids contribute to liver physiology at both the individual and bulk level. Fatty acids (FAs) and cholesterol are the fundamental building blocks of all lipids. Liver steatosis is caused by an increase in FA uptake and de novo lipogenesis exceeding FA oxidation and secretion, and lipid accumulation sensitizes the liver to multiple hits, leading to progressive liver disease. Increased hepatic lipid accumulation is observed in alcohol-induced liver disease (ALD), nonalcoholic fatty liver disease (NAFLD), viral hepatitis (VH), and among individuals suffering from obesity, and diabetes, all of which pose a risk for liver cancer development. In addition to their structural role in shaping the physical properties of the plasma membrane, lipids also play an important signaling role in maintaining plasma membrane integrity.
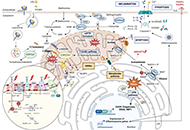
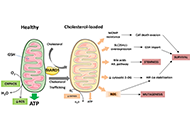
Mitochondria are key organelles, being the main source of energy in hepatocytes and with a central role in normal function of the liver. Impaired mitochondrial functions affect cell survival and contribute to the onset and perpetuation of liver diseases. Altered mitochondrial functions have indeed been documented in a variety of chronic liver diseases including ALD, NAFLD, VH, primary and secondary cholestasis, hemochromatosis, and Wilson's disease. Functional impairment of mitochondria is often accompanied by structural changes, resulting in organelle swelling (e.g. ALD), lowered oxidative capacity, reduced oxidative phosphorylation, decreased ATP production, significant increase in ROS generation, diminished antioxidant defense, and deviations in normal lipid composition can contribute to mitochondrial dysfunction. Adequate mitochondrial functions in hepatocytes are maintained by mitochondrial proliferation and/or increased activity of critical enzymes. The assessment of mitochondrial functions in vivo can be a useful tool in liver diseases for diagnostic and prognostic purposes, and also for the evaluation of (novel) therapeutic interventions. This Special Issue will provide insights into signaling pathways that influence fatty liver disease progression due to changes in lipid composition and linked to altered mitochondrial function, highlighting the importance of targeting them as a novel therapeutic strategy.
Keywords: Lipids, liver cholesterol, mitochondria dysfunction, liver disease
Published Articles