Affiliation:
1Laboratorio de Hemostasia y Trombosis, Instituto de Medicina Experimental-CONICET-Academia Nacional de Medicina, Ciudad Autónoma de Buenos Aires C1425AUM, Argentina
Email: cdossantosimex@gmail.com
ORCID: https://orcid.org/0000-0002-0431-1004
Affiliation:
1Laboratorio de Hemostasia y Trombosis, Instituto de Medicina Experimental-CONICET-Academia Nacional de Medicina, Ciudad Autónoma de Buenos Aires C1425AUM, Argentina
Affiliation:
2Instituto de Investigaciones Hematológicas “Mariano R. Castex”, Academia Nacional de Medicina, Ciudad Autónoma de Buenos Aires C1425AUM, Argentina
Affiliation:
3Unidad de Nefrología Pediátrica, Hospital Interzonal General Dr José Penna, Bahia Blanca, Provincia de Buenos Aires B8000, Argentina
ORCID: https://orcid.org/0000-0002-2130-0307
Affiliation:
4Servicio de Nefrología Pediátrica, Hospital Italiano, Ciudad Autónoma de Buenos Aires C1199, Argentina
ORCID: https://orcid.org/0000-0001-8615-6847
Affiliation:
5Servicio de Hematología, Hospital de Niños “Dr. Orlando Alassia”, Santa Fe, Provincia de Santa Fe 3000, Argentina
ORCID: https://orcid.org/0009-0004-0844-3911
Affiliation:
2Instituto de Investigaciones Hematológicas “Mariano R. Castex”, Academia Nacional de Medicina, Ciudad Autónoma de Buenos Aires C1425AUM, Argentina
Affiliation:
1Laboratorio de Hemostasia y Trombosis, Instituto de Medicina Experimental-CONICET-Academia Nacional de Medicina, Ciudad Autónoma de Buenos Aires C1425AUM, Argentina
2Instituto de Investigaciones Hematológicas “Mariano R. Castex”, Academia Nacional de Medicina, Ciudad Autónoma de Buenos Aires C1425AUM, Argentina
ORCID: https://orcid.org/0000-0002-2971-9217
Explor Immunol. 2023;3:513–524 DOI: https://doi.org/10.37349/ei.2023.00118
Received: April 29, 2023 Accepted: September 13, 2023 Published: November 10, 2023
Academic Editor: Roberta Bulla, University of Trieste, Italy
The article belongs to the special issue The Complement System in Health and Disease
Aim: To describe the clinical characteristics and frequency of anti-factor H (FH) autoantibody-associated atypical hemolytic uremic syndrome (aHUS) in the first cohort of Argentine patients.
Methods: The presence of anti-FH autoantibodies in 70 pediatric patients with suspected aHUS was investigated between 2013 and 2022. Clinical and laboratory parameters were collected and compared between patients who were positive and negative for anti-FH antibodies.
Results: The 70 patients screened for anti-FH autoantibodies presented clinical features of non-immune microangiopathic hemolytic anemia, thrombocytopenia and renal injury. Positive titers were found in 14 children [mean: 1,938 arbitrary units per mL (AU/mL), range 179–8,500]. Due to missing clinical data, two patients who tested positive for anti-FH and 20 patients who tested negative for anti-FH were excluded from the data analysis. The laboratory features and clinical manifestations of anti-FH-positive aHUS cases (n = 12) were very similar to those of subjects with no autoantibodies detected (n = 36). Treatment administration was heterogeneous among the 12 patients analyzed. Dialysis was performed in six patients in total. Five children received plasmapheresis, while three patients were treated with plasma exchange followed by administration of eculizumab. Two patients received eculizumab only and one showed significant improvement solely through supportive care. Eight patients in total received immunosuppressive therapy. Follow-up of three patients showed a significant decrease of anti-FH autoantibody titers in 2/3 after treatment and during clinical remission.
Conclusions: The cohort of 70 pediatric patients in this study demonstrated that the frequency of anti-FH autoantibody-associated aHUS in Argentina is 20%. The implementation of anti-FH testing in the country can potentially contribute to improved treatment and follow-up for patients with autoimmune aHUS.
Atypical hemolytic uremic syndrome (aHUS) is an extremely rare thrombotic microangiopathy (TMA) defined by the triad of non-immune microangiopathic hemolytic anaemia, thrombocytopenia and impaired renal function. Also named complement-mediated TMA, aHUS consists of dysregulation of the alternative complement pathway leading to its uncontrolled activation [1]. Without prompt and adequate treatment, patients are at increased risk of morbidity and mortality. The pathophysiological mechanism is associated with genetic or acquired anomalies. The presence of pathologic gene variants in complement components represents the most frequent form of aHUS and occurs in both adults and children [2, 3]. Anomalies in activators C3 and factor B (FB), or regulators factor H (FH), factor I (FI) and membrane cofactor protein (MCP, also known as CD46) among others [4], are considered as a complement-amplifying condition that, in the presence of a triggering event, predispose patients to developing aHUS. The autoimmune form of the disease is associated with the generation of anti-FH autoantibodies, generally immunoglobulin G (IgG), targeting one of the main regulators of the complement pathway [5]. Depletion of free FH by autoantibodies inhibit the antigen interaction with activated C3, causing complement over activation on cell membrane and endothelial damage. A major frequency of autoimmune aHUS was observed in the pediatric population with an association of complement FH related-1 (CFHR1) homozygous deletion [6, 7]. Localized in the regulators of complement activation (RCA) cluster on chromosome 1, complement FH (CFH) shows strong sequence homology with the five genes CFHR1 to CFHR5. These homologues were probably formed by duplication events of the original CFH gene, as CFHR proteins are composed of complement control protein (CCP) domains with 30–100% homology to CFH [8]. Although, some data in the literature suggests a mechanism to explain the formation of anti-FH antibodies in the context of the aHUS [9] renal pathology, there is no certainty on the matter. Recollection of data and evidence from cohort studies has been of great value to help understand the syndrome better. In this study, the objective was to describe the frequency of anti-FH autoantibodies-associated aHUS in an Argentine pediatric population. We made retrospective comparison of demographic and laboratory parameters among patients who were positive or negative for anti-FH antibodies in a cohort of clinically diagnosed aHUS. Finally, we reported the follow-up of three patients after disease onset and described their treatment and evolution.
The cohort study consists of 70 subjects under the age of 18 with a clinical diagnosis of aHUS. Patients were included in the study if they manifested non-immune microangiopathic hemolytic anemia [haemoglobin < 12 g/dL with schistocytes, elevated lactate dehydrogenase (LDH)], thrombocytopenia (< 150 × 109/L platelets count or a decrease of 25% from baseline) and acute kidney injury. Between January 2013 and December 2022, samples were prospectively collected from outpatients or shipped to our department of Hemostasis and Thrombosis, the only laboratory in Argentina that performs anti-FH autoantibodies assay. Following our laboratory procedure, previously described [10] for patients with clinical suspicion of TMA, we determined that all 70 patients presented normal levels of disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) activity and were negative for anti-ADAMTS13 IgG antibodies. For data analysis, we conducted a retrospective study from clinical records (antecedents, acute phase of first episode) and laboratory parameters (haemoglobin, haematocrit, platelets count, LDH, creatinine, urea).
Autoantibodies anti-FH were detected using enzyme-linked immunosorbent assay (ELISA) method slightly modified from Dragon-Durey et al. [11]. Purified FH (cat#HC2130, Hycult Biotech, Uden, The Netherlands) diluted in phosphate buffered saline buffer [PBS; 137 mmol/L sodium chloride (NaCl), 2.7 mmol/L potassium chloride, 8.1 mmol/L anhydrous sodium phosphate dibasic, 1.76 mmol/L monobasic potassium phosphate] to a final concentration of 3 µg/mL was coated on Nunc™ MaxiSorp™ wells overnight at 4⁰C. After one wash with PBS/0.1% Tween, 200 µL blocking buffer PBS/0.1% Tween/0.5% non-fat dry milk (Powder milk, La Serenísima, Gral. Rodriguez, Provincia de Buenos Aires, Argentina) was added and incubated for 1 h at room temperature. After washing 3 times, plasma from patients was diluted 1/100 in high-salt PBS (0.6 mol/L NaCl)/0.1% Tween and incubated for 1 h at room temperature. After washing 5 times, wells were incubated for 1 h with goat anti-human IgG-peroxidase antibody (cat#A8667, Sigma, St Louis, USA) diluted 1/10,000. After 5 washes, 3,3’,5,5’-tetramethylbenzidine (TMB) substrate was used to reveal peroxidase activity. Data analysis was performed as described previously [11]. Antibody titers were expressed as arbitrary units per mL (AU/mL). The positive threshold (> 100 AU/mL) was calculated in our lab using plasma from 50 individual healthy donors.
Concentration of plasmatic C5b-9 was measured by Complement TCC ELISA Kit (cat#COMPL TCC RUO, Svar Life Science, Malmö, Sweden). The normal range was calculated in our laboratory using 50 blood donors, in accordance with the reference provided by the manufacturer (30–150 ng/mL).
Studies consisted of the determination of copy number of genes CFH, CFHR1, CFHR2, CFHR3 and CFHR5 using SALSA MLPA P236 ARMD mix-1 probemix (cat#P236-100R, MRC-Holland, Amsterdam, The Netherlands) and SALSA MLPA EK1 reagent kit (cat#EK1-FAM, MRC-Holland, Amsterdam, The Netherlands). Sanger method sequencing or next generation sequencing (NGS) was used to explore exonic and intronic flanking regions of genes described in the literature to be associated with aHUS. Results were analyzed from own data, generated in our department or from our collaboration with Molecular Otolaryngology and Renal Research Laboratories (MORL, University of Iowa, USA), but also from outsourced services produced outside the country when genetic studies for aHUS were still partially unavailable in Argentina.
Clinical parameters associated with aHUS manifestations at disease onset in this series of 70 pediatric patients are summarized in Table 1. The age distribution refers to the time of sample collection for anti-FH antibody testing. Therefore, in very few cases (n = 4), the age of the patient did not match the age at disease onset. The number of males and females was very similar. Twenty percent of the 70 patients (n = 14) were investigated for genetic complement anomalies. Five were confirmed to be carriers of pathologic variants in complement genes (data not shown). Fourteen patients were tested positive for anti-FH autoantibodies. Sixty-four percent (9/14) of the patients with anti-FH antibody-associated aHUS were male. The mean titer of anti-FH autoantibodies in the 14 diagnosed patients was 1,938 AU/mL (range 179–8,500 AU/mL) (Figure 1). One of these patients was a young adult male (age 18) studied ten years after the disease first flared up. When he was finally diagnosed with anti-FH antibodies, his clinical condition had critically worsened, and he died from anoxic encephalopathy following two cardiac arrests. An 8-year-old boy was studied during the relapse phase after recovery from onset. A 4-year-old girl was studied one year after her first acute episode, but the clinical data reported in this study are from onset. Copy number variations in CFH-CFHR genes and exon sequencing were analyzed in 5 and 4 patients with anti-FH antibodies, respectively. The results showed that three patients exhibited homozygous deletion of CFHR3-CFHR1 and one presented a rearrangement between CFHR3 and CFHR1 in one allele and a deletion of CFHR3-CFHR1 in the other allele. One patient showed no variation in the number of copies of CFH-CFHR genes. No genetic variants in the complement genes were found in the four patients studied using Sanger sequencing or NGS.
Demographic and clinical parameters during acute phase of the pediatric cohort study (n = 70)
| Parameters | Median (25th–75th percentile) or number of patients (percentage) |
|---|---|
| Age (years old), n = 70 | 6.5 (2–12) |
| Sex—male, n = 70 | 36 (51%) |
| Hemoglobin (g/dL), n = 58 | 8.2 (6.5–9.9) |
| Hematocrit (%), n = 60 | 23 (19–30) |
| Platelets (× 109/L), n = 59 | 51 (28–80) |
| LDH (IU/L), n = 55 | 2,670 (1,366–4,422) |
| Urea (mg/dL), n = 56 | 132 (80–172) |
| Creatinine (mg/dL), n = 56 | 2.60 (1.37–6.08) |
| Complement genetic testing completed | |
| MLPA | 12 (17%) |
| Sanger or NGS | 14 (20%) |
| Anti-FH autoantibodies testing | |
| Positive | 14 (20%) |
| Negative | 56 (80%) |
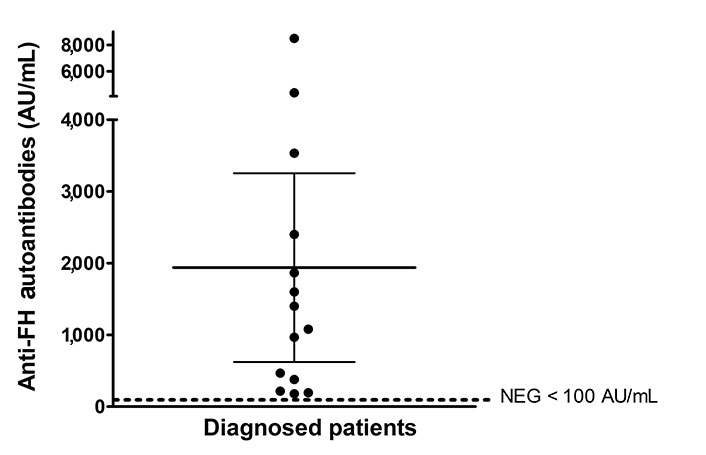
Anti-FH autoantibodies titers at onset expressed in arbitrary units AU/mL. Titers under 100 AU/mL are considered negative
Due to incomplete medical records, we excluded from our data analysis two patients tested positive for anti-FH autoantibodies and 20 patients tested negative for such antibodies. We compared hematological and renal parameters between patients tested positive (n = 12) or negative (n = 36) for anti-FH autoantibodies (Table 2). The majority of patients included in the analysis showed mild-to-life-threatening anemia and thrombocytopenia with severe acute renal failure at disease onset. LDH levels were particularly high and schystocytes were abundant. No significant differences in the severity of the symptoms were observed between the two groups of patients. Gastrointestinal symptom was the major prodrome exhibited in both groups but only patients without anti-FH antibodies presented diarrhea, a common manifestation of hemolytic uremic syndrome (HUS). One patient with polymerase chain reaction (PCR) positive for Shiga toxin-producing Escherichia coli (STEC) presented high titers of anti-FH antibodies. The most reported extrarenal manifestations were neurological and cardiac and occurred almost at the same frequency in both groups.
Laboratory and clinical parameters of patients positive or negative for anti-FH autoantibodies at disease onset
| Parameters | Anti-FH positive, n = 12 | Anti-FH negative, n = 36 | P-value |
|---|---|---|---|
| Age (years old) | 8 ± 3 (6–11) | 7 ± 6 (1–12) | 0.60† |
| Sex—male | 8 (67%) | 16 (44%) | 0.32† |
| Anti-FH antibodies (AU/mL) | 2,165 ± 2,391 (400–3,250) | 4 ± 8 (0–4) | < 0.0001† |
| Hemoglobin (g/dL) | 7.7 ± 2.3 (5.6–10.0) | 7.6 ± 2.4 (6.5–9.1) | 0.96† |
| Hematocrit (%) | 23 ± 7 (17–31) | 22 ± 7 (18–27) | 0.89† |
| Platelets (× 109/L) | 48 ± 26 (24–62) | 58 ± 39 (28–67) | 0.44† |
| LDH (IU/L) | 3,669 ± 1,839 (2,355–4,760) | 3,363 ± 2, 489 (1,530–4,515) | 0.70† |
| Urea (mg/dL) | 148 ± 47 (121–171) | 136 ± 69 (78–189) | 0.49† |
| Creatinine (mg/dL) | 2.50 ± 1.27 (1.53–3.80) | 4.56 ± 3.86 (1.37–7.47) | 0.10† |
| Prodromes | |||
| Gastrointestinal manifestations | |||
| Vomiting | 6 (50%) | 11 (31%) | 0.30¥ |
| Diarrhea | 0 (0%) | 18 (50%) | < 0.01¥ |
| Not specified | - | 3 (8%) | - |
| Fever | 3 (25%) | 4 (11%) | 0.34¥ |
| Petechial rash | 2 (17%) | 3 (8%) | 0.59¥ |
| Infection | |||
| Upper respiratory tract | 2 (17%) | 2 (6%) | 0.26¥ |
| STEC | 1 (8%) | - | - |
| Extrarenal manifestations | |||
| Neurological (severe headache, seizures, sensory alterations) | 4 (33%) | 13 (36%) | 1.00¥ |
| Hypertension | 5 (42%) | 10 (28%) | 0.48¥ |
Values represent mean ± standard deviation and 25th–75th percentile or percentage of the total depending of the analyzed parameter. The data in the last column are the P-values of † t-test or ¥ Fisher’exact test
Treatments administered to the 12 patients diagnosed with anti-FH autoantibody-associated aHUS are summarized in Table 3. Sixty-seven percent of the patients were initially treated with plasmapheresis and received between 3 to 24 daily sessions. Less than 10 sessions were administered to three patients before initiation of eculizumab infusion. Initial immunosuppression consisted of the use of corticosteroids (methylprednisone) while cyclophosphamide and mycophenolate were used as maintenance therapy. One patient was treated with pulses of methylprednisone only. All 12 patients presented positive outcomes with no disease recurrence reported. For comparison, the treatments administered to 30 out of 36 patients without anti-FH autoantibodies were reported in Table 3. The only notable difference observed between the groups was a lower frequency of immunosuppressive drug administration in patients without detected antibodies.
Treatments administered to patients positive or negative for anti-FH autoantibodies at disease onset
| Treatments | Anti-FH positive, n = 12 | Anti-FH negative, n = 30 | P-value (Fisher’exact test) |
|---|---|---|---|
| Dialysis | 6 (50%) | 15 (50%) | 1.00 |
| Plasmapheresis | 5 (42%) | 11 (37%) | 1.00 |
| Plasmapheresis followed by eculizumab | 3 (25%) | 3 (10%) | 0.33 |
| Eculizumab only | 2 (17%) | 1 (3%) | 0.19 |
| Supportive care only | 1 (8%) | 10 (33%) | 0.13 |
| Immunosupression | 8 (67%) | 9 (30%) | < 0.05 |
| Corticosteroids | 6 (50%) | 9 (30%) | 0.29 |
| Mycophenolate, cyclophosphamide | 4 (33%) | 0 (0%) | < 0.005 |
Values in this table represent number of patients and the percentage of the total
Short or long-term follow-up of all the patients was not possible. Monitoring our most recent patients (patient 1, patient 2, patient 3) consisted of monitoring hematologic parameters and renal function, as well as measuring the levels of anti-FH antibodies and free C5b-9 in plasma when adequate samples were available. As observed in Figure 2, anti-FH levels decreased in all patients after treatment but were normalized (< 100 AU/mL) in patient 2 only (Figure 2A).
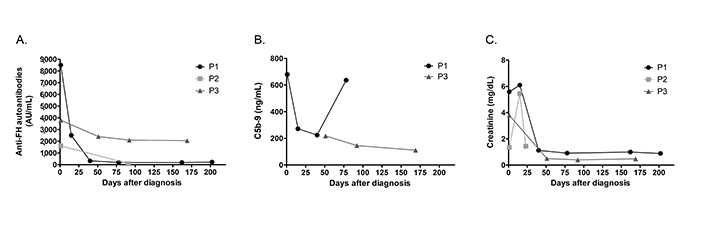
Follow-up of patients P1, P2 and P3. (A) Anti-FH autoantibodies titers; (B) determination of soluble C5b-9; and (C) levels of creatinine during acute phase and after clinical remission
Patient 1 exhibited a decrease in anti-FH levels on day 15 post-diagnosis (8,500 AU/mL vs. 2,500 AU/mL), which correlated with significantly lower levels of circulating C5b-9 (679 ng/mL vs. 272 ng/mL) (Figure 2B). Fourteen days after manifesting aHUS symptoms, patient 1 was infused with eculizumab but treatment was interrupted after PCR for STEC came back positive. When the diagnosis of anti-FH autoantibody-associated aHUS was confirmed, eculizumab was reinitiated in combination with corticosteroids. The patient responded positively to treatment and rapidly recovered renal function (Figure 2C). At day 40 post-diagnosis, anti-FH titers were significantly lower (318 AU/mL). Although levels of C5b-9 were also decreased during remission until the third month after diagnosis, on day 75, patient 1 presented a significant increase in plasma C5b-9 (636 ng/mL), but with anti-FH IgG still low (< 200 AU/mL). After six-month-follow-up, the patient continued to exhibit low levels of antibodies. No sample was available to measure soluble C5b-9. Eculizumab was suspended and the patient is currently receiving mycophenolate.
Patient 2 received methylprednisolone pulses for 3 days and hemodialysis for 15 days after the first symptoms. The renal injury reverted spontaneously, and 23 days after onset, the patient was discharged from hospital. Three months after the acute episode, the anti-FH levels were normal (90 AU/mL). The patient has been in remission for a total of 11 months with normal kidney function and is not receiving any treatment.
Patient 3 received hemodialysis and fresh frozen plasma a couple of days after diagnosis with no significant improvement of hematological parameters or renal injury. At day 14 post-hospitalization, patient 3 was administered with first dose of eculizumab followed by recovery of kidney function. Additionally, patient 3 had received immunosuppressive treatment since disease onset, including administration of mycophenolate and corsticosteroids. At day 28, dialysis was interrupted and at day 40, the patient was discharged with persistent anemia. However, platelet count and LDH levels had returned to normal. C5b-9 levels were not measured at diagnosis, but samples collected on day 51 showed mild complement activation (218 ng/mL) that decreased at day 92 (146 ng/mL) and day 169 (111 ng/mL) to reach normal range. Although patient 3 presented consistently high titers of anti-FH (> 2,000 AU/mL) five months after diagnosis, clinical remission was observed with normal blood and renal laboratory results. Administration of rituximab was tested to inhibit the formation of anti-FH. Currently under mycophenolate and anti-complement therapy, the patient is still carefully monitored and eculizumab suspension is being considered.
Multiple cohort studies, mostly from Western Europe [12–16], followed by Asian countries (India, Korea, and China) [17–19], have described the frequency, symptoms, and outcomes of anti-FH autoantibody-associated aHUS. This is the first report of a pediatric cohort study tested for anti-FH autoantibodies in Argentina. The mean age of our patients (8 years old) at disease onset was similar to that described in previous reports [12–19]. As observed in the literature, our study showed that the prevalence is higher in children between 4 years and 13 years of age. The largest cohort of anti-FH autoantibody-associated aHUS has been reported in India (436 children) [20]. The reason why the prevalence of the disease in that country (56%) is higher than that in other worldwide cohorts (5–29%) [5] remains unclear. However, some evidence, such as age at onset, nature of the symptoms, and higher frequency during the coldest season, supports a possible infectious trigger. A recent study might support this hypothesis, as cases of children with positive anti-FH levels increased significantly following the peak of the SARS-CoV-2 pandemic wave in India [21]. In our study, patients mainly presented gastrointestinal prodrome (n = 6), and a few initiated with upper respiratory tract infection (n = 2), as observed previously [13]. In a smaller cohort like the one presented in this study, no link between the incidence of the illness and cold weather was established. Argentina experiences seasonal variation and average temperatures in the whole country are usually colder in June–July. However, half of the cases reported in this study occurred during the summer months of January and February, suggesting that there is no relation between seasonality or a high frequency of respiratory infections and the incidence of anti-FH antibody-associated aHUS.
As previously reported in both pediatric [12, 14, 18] and adult populations [12], we observed a slight tendency towards male predominance. Data from the literature describes that males are more susceptible than females to most viral infections [22], which might partially explain the sex predominance observed in anti-FH aHUS. In Argentina, we started diagnosing these patients in late 2016 and in 2018, we reported a frequency of 15% in a cohort of 54 pediatric patients [23] experiencing symptoms compatible with aHUS. Present data shows a slight increase in frequency in our pediatric population clinically diagnosed with aHUS, reaching 20% (14/70). This is probably due to an increase in the number of patients tested for the anti-FH antibodies in our laboratory and to a better understanding of differential diagnostic between primary and secondary TMA.
STEC-HUS is a disease considered endemic in Argentina [24] and is generally managed by nephrologists. Complement testing is not requested for diagnosis or follow-up of those patients, which can explain the low numbers seen in our department of Hemostasis and Thrombosis [10]. In our study, one patient who was positive for anti-FH autoantibodies presented STEC infection. This has not been observed frequently in the literature but the French cohort identified one of 45 patients in total [12]. Patients who are diagnosed with STEC infection in Argentina might usually not be tested for anti-FH antibodies. It would be interesting to determine if within the large pediatric population infected by STEC in the country, a higher prevalence of anti-FH antibody-associated aHUS can be observed or if the anti-FH/STEC association is occurring randomly.
Autoimmune forms are mostly diagnosed in children in whom a complete deletion of the CFHR1 gene is frequently detected [6], and usually encompassed deletion of another close homologous gene such as CFHR3 [25], or less frequently CFHR4 [26, 27]. Despite this finding, it should be noted that routine genetic screening of patients with autoimmune aHUS is not necessary for treatment or monitoring. However, given that the clear mechanism underlying the formation of anti-FH autoantibodies remains unknown, genetic studies have shown to be essential for partially understanding the pathology. From our 14 positive anti-FH patients, we identified 3 out of 6 with a complete deletion of CFHR3-CFHR1. One patient presented a rearrangement of CFHR3 and CFHR1 in one allele and a deletion of CFHR3-CFHR1 in the second allele. Although we were unable to perform a comprehensive study to identify the nature of the rearrangement, in this case, the patient is likely to not express FH related-1 (FHR1) protein. Previous data showed that almost 15% of cases can be compound heterozygous (CFHR3-CFHR1/CFHR4-CFHR1) or less frequently (< 1%) presented CFHR3-CFHR1 deletion and a CFHR1 mutation [5]. In a larger cohort (n = 30), patients with anti-FH antibodies associated with FHR1 deficiency were more likely to carry rare possibly pathogenic variants in complement genes than controls selected to be deficient in FHR1 but who never developed aHUS [28]. It has been suggested that patients with deleted CFHR1 are more susceptible to anti-FH when exposed to infectious microorganisms. Components of the latter could indeed be capable of binding to FH and would expose a neoepitope, generating a loss of tolerance for FH, as it presented a similar structure to homologous FHR1 [9]. However, a small percentage of patients who develop anti-FH antibodies carry at least one copy of CFHR1, meaning that more than one mechanism might be involved in the formation of anti-FH antibodies. Regions of FH antigen-antibody interactions have been described along the entire length of the protein, but autoantibodies bind more frequently to the C-terminal domain, with one specific predominant epitope located within a loop. Studies have shown that the presence of autoantibodies impairs complement regulator function by reducing or inhibiting FH binding to C3 components, glycosaminoglycans (heparin), or sialic acid on the cell surface. Recent findings, using a high-affinity recombinant anti-FH antibody, suggested that anti-FH autoantibodies are likely to specifically recognize flexible loops in the C-terminal region, representing a particularity of aHUS pathogenesis [29]. More studies are necessary to understand how these findings are relevant in a “double-hit” model responsible for generating anti-FH antibodies, as previously suggested [28].
Adopting a multidisciplinary approach, including hematologists, nephrologists, and complement specialists, is important for monitoring patients with anti-FH-associated HUS. According to the patient cohorts reported in the literature, disease relapses commonly occur 6–24 months after onset, as summarized by Dragon-Durey et al. [5]. Three of our patients who are still being monitored while writing this work have been followed for 5 months (patient 1), 7 months (patient 3), and 11 months (patient 2). Sustained elevated titers of anti-FH and free-FH during clinical remission have been reported as risk factors for relapse [20, 30]. However, maintenance of immunosuppressive treatment for at least 18–24 months was suggested to lower this risk [30]. Our preliminary investigation measuring free-FH showed an increase of FH levels between acute phase and clinical remission in patient 1 and patient 2. Meanwhile, patient 3 who continued to express high titers of anti-FH during his remission, was characterized by a slight decrease in serum FH during follow-up (data not shown).
Expression of terminal complement activation, C5b-9, in the bloodstream has not been validated as a reliable biomarker of aHUS activity [31] or relapse in patients with anti-FH antibodies [20]. However, Song et al. [32] showed that C5b-9 levels increased during the acute phase and decreased during remission in a cohort of 33 positive for anti-FH patients. Determination of C5b-9 in two of our patients currently monitored to prevent possible relapse showed distinct C5b-9 expression profiles. One patient (patient 3) presented a constant decrease in circulating C5b-9 despite exhibiting high titers of anti-FH antibodies even during remission. In contrast, patient 1 presented a rapid decrease in anti-FH titers from day 15. At day 75 of follow-up, lab results showed a significant increase in plasma C5b-9, exceeding the normal range. Patient monitoring consists of checking a combination of parameters, and while these C5b-9 levels might raise an alarm in current practice, it has been observed that the patient has shown normal clinical values and no specific symptoms. The use of an ex vivo assay to detect C5b-9 formation on the surface of endothelial cells in presence of patient serum, instead of measuring free C5b-9 in plasma, has been described as a promising tool and as a more physiological model of complement activation [33]. Complement activation was indeed described to occur at the cell surface in aHUS pathophysiology compared to C3 glomerulopathy, a disease associated with complement dysregulation in the fluid phase [34]. In a recent study by Valoti et al. [28], samples from patients with anti-FH in remission showed more C5b-9 deposits on the cell surface than samples from blood donors [28]. This result is in accordance with the role of anti-FH antibodies in complement dysregulation on the cell surface by interfering with FH functions.
In conclusions, our study of 14 patients with anti-FH-associated aHUS represent, to the best of our knowledge, the first cohort ever reported in Latin America. The frequency of this autoimmune form was 20% in an Argentine pediatric population with clinical diagnosis of aHUS. While the availability of anti-FH antibodies testing in the country is an essential upgrade to improve diagnosis and therapy management of the patients, our study showed that raising awareness of the illness among health professional might still be necessary, especially when considering ultra-rare diseases like aHUS. Improving monitoring of these patients after disease onset and remission will be a priority in our laboratory, as it can prevent recurrences that are associated with the worst long-term outcomes.
aHUS: atypical hemolytic uremic syndrome
AU/mL: arbitrary units per mL
CFH: complement factor H
CFHR1: complement factor H related-1
FH: factor H
FHR1: factor H related-1
HUS: hemolytic uremic syndrome
IgG: immunoglobulin G
LDH: lactate dehydrogenase
NGS: next generation sequencing
STEC: Shiga toxin-producing Escherichia coli
TMA: thrombotic microangiopathy
We would like to thank the patients and their families for their participation in the study, as well as the physicians and the institutions for their contribution to the acquisition of patients data. We thank James Castaneda, Bespoke English, for his diligent proofreading of this manuscript.
CDS: Conceptualization, Investigation, Visualization, Writing—original draft, Writing—review & editing. JT and SC: Investigation, Formal analysis. LA and PAC: Investigation, Resources, Writing—review & editing. FJM: Investigation, Resources. MFA: Investigation, Formal analysis, Writing—review & editing. ASL: Conceptualization, Investigation, Visualization, Resources, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The study was approved by the Institutional Review Board (or Ethics Committee) of Academia Nacional de Medicina, Buenos Aires (28/22/CEIANM, dated 7/09/2022), and it complies with the Declaration of Helsinki.
Informed consent to participate in the study was obtained from parents/legal guardians.
Not applicable.
The datasets for this manuscript are not publicly available due to privacy and ethical concerns. Requests for accessing the datasets should be directed to Célia Dos Santos (cdossantosimex@gmail.com).
This study was supported by Fundación Baron, CONICET, Agencia I+D+I – FONCYT and Academia Nacional de Medicina de Buenos Aires providing fellowship funding for graduate/PhD students and funding for material and reagents. This study was partially funded by educational grants (2015–2017) from Alexion Argentina and grant for clinical support from Laboratorios Raffo (2018–2022). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Martin Kolev ... Pascal Deschatelets
Mariagiulia Spazzapan ... Roberta Bulla
Jeyaparthasarathy Narayanaperumal, Ganesh Gopal
