









Aim:
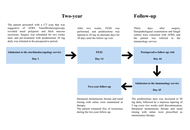
This study aimed to assess the effectiveness of mepolizumab in enhancing asthma control, achieving clinical remission, and alleviating upper airway symptoms in patients with severe eosinophilic asthma (SEA) with comorbid nasal polyps and/or chronic rhinosinusitis (CRS). Additionally, it aimed to identify clinical and laboratory predictors of remission. The findings are based on real-world data from a single center.
Methods:
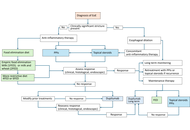
This retrospective, single-center, real-world study included 99 patients diagnosed with SEA. Patients were categorized into three groups based on the presence or absence of nasal polyps and CRS. Treatment response was evaluated using the asthma control test (ACT), spirometry, laboratory biomarkers, computed tomography (CT) scores, and nasal polyp scores. Remission was defined as the absence of asthma exacerbations and systemic corticosteroid use, along with improvements in both forced expiratory volume in 1 second (FEV1) and ACT scores.
Results:
After 12 months of mepolizumab therapy, there were significant improvements in FEV1 values, asthma exacerbation frequency, systemic corticosteroid requirements, and nasal symptom scores. The overall remission rate was 30.6%. Patients with higher baseline FEV1 and no prior exposure to omalizumab were more likely to achieve remission.
Conclusions:
This real-world evidence suggests that mepolizumab provides meaningful clinical, functional, and radiological improvements in patients with SEA, regardless of comorbid nasal polyps or CRS. Furthermore, the study highlights independent predictors associated with treatment-induced remission in this population.
Aim:
This study aimed to assess the effectiveness of mepolizumab in enhancing asthma control, achieving clinical remission, and alleviating upper airway symptoms in patients with severe eosinophilic asthma (SEA) with comorbid nasal polyps and/or chronic rhinosinusitis (CRS). Additionally, it aimed to identify clinical and laboratory predictors of remission. The findings are based on real-world data from a single center.
Methods:
This retrospective, single-center, real-world study included 99 patients diagnosed with SEA. Patients were categorized into three groups based on the presence or absence of nasal polyps and CRS. Treatment response was evaluated using the asthma control test (ACT), spirometry, laboratory biomarkers, computed tomography (CT) scores, and nasal polyp scores. Remission was defined as the absence of asthma exacerbations and systemic corticosteroid use, along with improvements in both forced expiratory volume in 1 second (FEV1) and ACT scores.
Results:
After 12 months of mepolizumab therapy, there were significant improvements in FEV1 values, asthma exacerbation frequency, systemic corticosteroid requirements, and nasal symptom scores. The overall remission rate was 30.6%. Patients with higher baseline FEV1 and no prior exposure to omalizumab were more likely to achieve remission.
Conclusions:
This real-world evidence suggests that mepolizumab provides meaningful clinical, functional, and radiological improvements in patients with SEA, regardless of comorbid nasal polyps or CRS. Furthermore, the study highlights independent predictors associated with treatment-induced remission in this population.
DOI: https://doi.org/10.37349/eaa.2026.1009109
This article belongs to the special issue Update on Chronic Rhinosinusitis

Aim:
Woodworking exposes carpenters to a higher risk of developing asthma or worsening pre-existing asthma. The objective of this study was to determine the prevalence of and factors associated with work-related asthma (WRA) among carpenters in Parakou in 2024.
Methods:
This was a cross-sectional, descriptive, and analytical study with prospective data collection conducted from June to September 2024. Following a voluntary survey, the included carpenters were interviewed using the Occupational Asthma Screening Questionnaire-11 (OASQ-11) to assess the relationship between asthma symptoms and the occupational environment. Peak expiratory flow (PEF) variability and spirometry were also measured. Data were analyzed using R software version 4.4.1. Factors associated with WRA were identified using simple and multiple logistic regression analyses.
Results:
Out of 153 carpenters/apprentices in 117 workshops, 144 (94.1%) were included, all of whom were male. The mean age was 35.9 ± 12.1 years. Among them, 15 (10.4%) had a WRA profile, including 10 (6.9%; 95% CI: 3.8–12.3) with occupational asthma and 5 (3.5%; 95% CI: 1.5–7.9) with work-aggravated asthma. Asthma was confirmed in 7 of the 15 carpenters suspected of having WRA through PEF variability measurement and spirometry. Simple and multiple logistic regression analyses identified a history of allergic rhinitis (aOR = 3.90; p = 0.033) and urticaria (aOR = 8.21; p = 0.002) as factors significantly associated with WRA.
Conclusions:
The prevalence of WRA among carpenters in Parakou is not negligible, particularly occupational asthma. Awareness campaigns, education for carpenters, regular monitoring of working conditions, and systematic medical follow-up of exposed workers could help preserve their respiratory health.
Aim:
Woodworking exposes carpenters to a higher risk of developing asthma or worsening pre-existing asthma. The objective of this study was to determine the prevalence of and factors associated with work-related asthma (WRA) among carpenters in Parakou in 2024.
Methods:
This was a cross-sectional, descriptive, and analytical study with prospective data collection conducted from June to September 2024. Following a voluntary survey, the included carpenters were interviewed using the Occupational Asthma Screening Questionnaire-11 (OASQ-11) to assess the relationship between asthma symptoms and the occupational environment. Peak expiratory flow (PEF) variability and spirometry were also measured. Data were analyzed using R software version 4.4.1. Factors associated with WRA were identified using simple and multiple logistic regression analyses.
Results:
Out of 153 carpenters/apprentices in 117 workshops, 144 (94.1%) were included, all of whom were male. The mean age was 35.9 ± 12.1 years. Among them, 15 (10.4%) had a WRA profile, including 10 (6.9%; 95% CI: 3.8–12.3) with occupational asthma and 5 (3.5%; 95% CI: 1.5–7.9) with work-aggravated asthma. Asthma was confirmed in 7 of the 15 carpenters suspected of having WRA through PEF variability measurement and spirometry. Simple and multiple logistic regression analyses identified a history of allergic rhinitis (aOR = 3.90; p = 0.033) and urticaria (aOR = 8.21; p = 0.002) as factors significantly associated with WRA.
Conclusions:
The prevalence of WRA among carpenters in Parakou is not negligible, particularly occupational asthma. Awareness campaigns, education for carpenters, regular monitoring of working conditions, and systematic medical follow-up of exposed workers could help preserve their respiratory health.
DOI: https://doi.org/10.37349/eaa.2026.1009108

There is unequivocal evidence that the climate is changing, and it is generally accepted that the trend will continue. Climate change is relevant to public health, as it can lead to alterations in the distribution and flowering phenology of plants and to changes in pollen exposure, with subsequent impacts on human health. The primary objective of this paper was to provide a quantitative synthesis of the available literature on the evolution of pollen season intensity and timing in plants with a higher allergenic potential. Six botanical families have been studied: Betulaceae (birch, hazel, alder), Cupressaceae, Oleaceae (olive, ash), Poaceae, Urticaceae, and Asteraceae (mugwort, ragweed). Three main indicators of the potential impact of climate change on pollination have been retained: the pollen integral, the start date of the pollen season, and the duration of the pollen season. The outcome is a dominant trend toward earlier and more abundant pollen seasons, particularly for trees that flower in winter and spring. In contrast, trends for grass or weeds that pollinate later are less consistent and often region-specific. The variations recorded are taxon-, site-, and period-dependent, with some species even showing opposing trends within the same botanical family, illustrating the complex interactions between biological adaptation and climatic variability. While the current influence of climate change on pollen production and phenology is well established, the magnitude of its future impact remains uncertain, and the diversity of methodologies and study durations limits the comparability of available data. Nevertheless, most projections support a continued, though possibly attenuated, increase in pollen intensity and season advancement. In any case, when combined with likely qualitative and quantitative changes in the concentration of allergens in pollen grains, the identified trends may already have, and will very likely continue to have, an impact on both allergic sensitizations, the prevalence of seasonal symptoms, and their severity, thus affecting their diagnosis, prevention, and treatment.
There is unequivocal evidence that the climate is changing, and it is generally accepted that the trend will continue. Climate change is relevant to public health, as it can lead to alterations in the distribution and flowering phenology of plants and to changes in pollen exposure, with subsequent impacts on human health. The primary objective of this paper was to provide a quantitative synthesis of the available literature on the evolution of pollen season intensity and timing in plants with a higher allergenic potential. Six botanical families have been studied: Betulaceae (birch, hazel, alder), Cupressaceae, Oleaceae (olive, ash), Poaceae, Urticaceae, and Asteraceae (mugwort, ragweed). Three main indicators of the potential impact of climate change on pollination have been retained: the pollen integral, the start date of the pollen season, and the duration of the pollen season. The outcome is a dominant trend toward earlier and more abundant pollen seasons, particularly for trees that flower in winter and spring. In contrast, trends for grass or weeds that pollinate later are less consistent and often region-specific. The variations recorded are taxon-, site-, and period-dependent, with some species even showing opposing trends within the same botanical family, illustrating the complex interactions between biological adaptation and climatic variability. While the current influence of climate change on pollen production and phenology is well established, the magnitude of its future impact remains uncertain, and the diversity of methodologies and study durations limits the comparability of available data. Nevertheless, most projections support a continued, though possibly attenuated, increase in pollen intensity and season advancement. In any case, when combined with likely qualitative and quantitative changes in the concentration of allergens in pollen grains, the identified trends may already have, and will very likely continue to have, an impact on both allergic sensitizations, the prevalence of seasonal symptoms, and their severity, thus affecting their diagnosis, prevention, and treatment.
DOI: https://doi.org/10.37349/eaa.2026.1009107
This article belongs to the special issue Climate Change, Allergy, and Immunotherapy

Cow’s milk allergy (CMA) is one of the most common food allergies in infancy, with a prevalence of up to 7.5%. However, accurate diagnosis in primary care remains difficult due to overlapping symptoms with common infant behaviours and limited access to specialist allergy testing. Consequently, CMA is frequently over diagnosed, leading to unnecessary elimination diets, nutritional deficiencies, and increased parental anxiety. This paper introduces the EATERS-X framework, an enhanced, structured approach to obtaining an allergy-focused clinical history (AFCH) that aims to improve diagnostic precision and clinical decision-making in CMA. The tool expands upon the traditional EATERS method—comprising Environment, Allergen, Timing, Exposure, Reproducibility, and Symptoms—by incorporating an additional element, X, denoting treatment and healthcare response. Through clinical scenarios, we demonstrate how EATERS-X can be applied to distinguish between IgE-mediated, non-IgE-mediated, and mixed-type CMA, including subtypes such as food protein-induced enterocolitis syndrome (FPIES). While EATERS-X provides a practical and systematic framework for history-taking, its effectiveness is dependent on clinician training and appropriate clinical interpretation. In addition, the framework has not yet undergone formal validation against gold-standard diagnostic tests such as oral food challenges. Future prospective studies should evaluate the diagnostic reliability, inter-observer consistency, and clinical impact of EATERS-X across different healthcare settings. Integration of the EATERS-X approach in primary care aligns with current BSACI and EAACI guidelines and promotes structured, evidence-based, and patient-centred care. Future research should focus on validating the framework’s diagnostic reliability and exploring its applicability across a wider spectrum of allergic conditions.
Cow’s milk allergy (CMA) is one of the most common food allergies in infancy, with a prevalence of up to 7.5%. However, accurate diagnosis in primary care remains difficult due to overlapping symptoms with common infant behaviours and limited access to specialist allergy testing. Consequently, CMA is frequently over diagnosed, leading to unnecessary elimination diets, nutritional deficiencies, and increased parental anxiety. This paper introduces the EATERS-X framework, an enhanced, structured approach to obtaining an allergy-focused clinical history (AFCH) that aims to improve diagnostic precision and clinical decision-making in CMA. The tool expands upon the traditional EATERS method—comprising Environment, Allergen, Timing, Exposure, Reproducibility, and Symptoms—by incorporating an additional element, X, denoting treatment and healthcare response. Through clinical scenarios, we demonstrate how EATERS-X can be applied to distinguish between IgE-mediated, non-IgE-mediated, and mixed-type CMA, including subtypes such as food protein-induced enterocolitis syndrome (FPIES). While EATERS-X provides a practical and systematic framework for history-taking, its effectiveness is dependent on clinician training and appropriate clinical interpretation. In addition, the framework has not yet undergone formal validation against gold-standard diagnostic tests such as oral food challenges. Future prospective studies should evaluate the diagnostic reliability, inter-observer consistency, and clinical impact of EATERS-X across different healthcare settings. Integration of the EATERS-X approach in primary care aligns with current BSACI and EAACI guidelines and promotes structured, evidence-based, and patient-centred care. Future research should focus on validating the framework’s diagnostic reliability and exploring its applicability across a wider spectrum of allergic conditions.
DOI: https://doi.org/10.37349/eaa.2026.1009106
This article belongs to the special issue Practical Issues in Pediatric Allergy

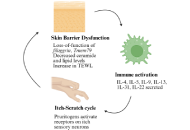
The symptoms of atopic dermatitis (AD), a chronic, recurrent inflammatory skin condition, include immunological dysregulation, severe pruritus, and malfunctioning of the epidermal barrier. Recent developments in our understanding of AD’s molecular and immunological pathways have shed light on the functions of cytokines, including interleukin (IL)-4, IL-13, IL-31, and IL-22, as well as the impact of genetic mutations in filaggrin and other barrier proteins. Inflammation and barrier dysfunction are further aggravated by microbial dysbiosis, especially colonisation of Staphylococcus aureus. Treatment options include topical corticosteroids, topical calcineurin inhibitors, targeted biologics, and small-molecule inhibitors that alter the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) and phosphodiesterase 4 (PDE4) pathways, as well as Mn- and Fe-porphyrin ring-based topical formulations. Even with these advancements, tailored treatment and long-term illness control are still challenging to achieve. New strategies that emphasise gene therapy and microbiome restoration can potentially improve the accuracy and comprehensiveness of AD treatment.
The symptoms of atopic dermatitis (AD), a chronic, recurrent inflammatory skin condition, include immunological dysregulation, severe pruritus, and malfunctioning of the epidermal barrier. Recent developments in our understanding of AD’s molecular and immunological pathways have shed light on the functions of cytokines, including interleukin (IL)-4, IL-13, IL-31, and IL-22, as well as the impact of genetic mutations in filaggrin and other barrier proteins. Inflammation and barrier dysfunction are further aggravated by microbial dysbiosis, especially colonisation of Staphylococcus aureus. Treatment options include topical corticosteroids, topical calcineurin inhibitors, targeted biologics, and small-molecule inhibitors that alter the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) and phosphodiesterase 4 (PDE4) pathways, as well as Mn- and Fe-porphyrin ring-based topical formulations. Even with these advancements, tailored treatment and long-term illness control are still challenging to achieve. New strategies that emphasise gene therapy and microbiome restoration can potentially improve the accuracy and comprehensiveness of AD treatment.
DOI: https://doi.org/10.37349/eaa.2026.1009105
This article belongs to the special issue Atopic Dermatitis – Pathology and Treatment Modalities

Background:
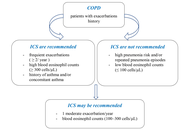
The diversity of physiological actions and pharmacological effects of glucocorticoids (GCs) allows their use in a large group of diseases and pathological conditions. However, this treatment can be accompanied by a multitude of more or less severe side effects. As the mainstay of treatment for asthma and chronic obstructive pulmonary disease (COPD), inhaled corticosteroids (ICS) dramatically reduce morbidity and mortality. This research aims to examine the safety considerations associated with glucocorticoid therapy in patients with COPD and asthma.
Methods:
The search was performed in PubMed, EBSCO, UpToDate, Medline, and Google Scholar for pertinent English-language articles published between 1990 and 2025, using the following keywords: glucocorticoids, asthma, COPD, management, and side effects.
Results:
GCs stand out as one of the most widely prescribed classes of drugs globally, with well-established effectiveness in addressing acute or chronic inflammation, allergic conditions, and acute pathological situations. The undeniable efficacy of GCs, however, comes with a range of reported side effects. These include but are not limited to immunosuppression, cardiovascular issues, manifestation of Cushingoid features, development of diabetes, osteoporosis, suppression of the hypothalamic-pituitary-adrenal (HPA) axis, and adverse effects on the gastrointestinal and dermatologic systems. However, the majority of these events are associated with systemic drug administration, which is less commonly indicated in the treatment of COPD and asthma. There are several factors and specific considerations when deciding on GC treatment in COPD. In the context of corticosteroid treatment for asthma, the overarching impact involves the suppression of inflammatory genes, leading to reduced transcription of genes responsible for cytokines, chemokines, adhesion molecules, inflammatory enzymes, and receptors.
Discussion:
GCs are associated with fewer side effects in both COPD and asthma treatment. It’s crucial to take into account factors such as the patient’s overall health, the severity of symptoms, the presence of comorbidities, and the responsiveness of specific features to GCs therapy.
Background:
The diversity of physiological actions and pharmacological effects of glucocorticoids (GCs) allows their use in a large group of diseases and pathological conditions. However, this treatment can be accompanied by a multitude of more or less severe side effects. As the mainstay of treatment for asthma and chronic obstructive pulmonary disease (COPD), inhaled corticosteroids (ICS) dramatically reduce morbidity and mortality. This research aims to examine the safety considerations associated with glucocorticoid therapy in patients with COPD and asthma.
Methods:
The search was performed in PubMed, EBSCO, UpToDate, Medline, and Google Scholar for pertinent English-language articles published between 1990 and 2025, using the following keywords: glucocorticoids, asthma, COPD, management, and side effects.
Results:
GCs stand out as one of the most widely prescribed classes of drugs globally, with well-established effectiveness in addressing acute or chronic inflammation, allergic conditions, and acute pathological situations. The undeniable efficacy of GCs, however, comes with a range of reported side effects. These include but are not limited to immunosuppression, cardiovascular issues, manifestation of Cushingoid features, development of diabetes, osteoporosis, suppression of the hypothalamic-pituitary-adrenal (HPA) axis, and adverse effects on the gastrointestinal and dermatologic systems. However, the majority of these events are associated with systemic drug administration, which is less commonly indicated in the treatment of COPD and asthma. There are several factors and specific considerations when deciding on GC treatment in COPD. In the context of corticosteroid treatment for asthma, the overarching impact involves the suppression of inflammatory genes, leading to reduced transcription of genes responsible for cytokines, chemokines, adhesion molecules, inflammatory enzymes, and receptors.
Discussion:
GCs are associated with fewer side effects in both COPD and asthma treatment. It’s crucial to take into account factors such as the patient’s overall health, the severity of symptoms, the presence of comorbidities, and the responsiveness of specific features to GCs therapy.
DOI: https://doi.org/10.37349/eaa.2025.1009104
This article belongs to the special issue The Era of Biologics in Allergy

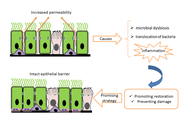
Asthma is one of the most common chronic respiratory diseases worldwide, traditionally defined as airway inflammation, reversible obstruction, and hyperresponsiveness. While this framework has guided decades of research and treatment, it fails to capture asthma as a heterogeneous and systemic condition. This pathology is shaped by complex interactions among genetic, epigenetic, immunological, neuroendocrine, metabolic, and environmental factors. Its coexistence with chronic obstructive pulmonary disease (COPD) in overlapping syndromes further complicates diagnosis and therapeutic decision-making. Asthma etiology involves oxidative stress, genetic susceptibility, and epigenetic regulation, along with age- and sex-dependent hormonal influences that modulate immune responses. Emerging evidence shows that structural and functional changes in the respiratory epithelium, airway smooth muscle (ASM), and alveoli extend the pathology beyond acute inflammation, involving processes such as epithelial barrier dysfunction, airway remodeling, and impaired mucociliary clearance (MCC). Neuro-immune-endocrine interactions have emerged as central contributors to asthma pathogenesis. Endocrine regulation shapes inflammatory activity and treatment responsiveness. Metabolic factors such as obesity introduce additional complexity by generating low-grade systemic inflammation, oxidative stress, adipokine imbalance, and steroid resistance, resulting in a distinct and often more severe asthma phenotype. Parasympathetic and sensory neural pathways amplify bronchoconstriction and inflammation through reciprocal communication with eosinophils, mast cells, innate lymphoid cells, and pulmonary neuroendocrine cells (PNECs). Neurotrophins and neuropeptides further promote airway hyperreactivity and remodeling. Current management integrates inhaled corticosteroids, bronchodilators, and targeted biologics—such as thymic stromal lymphopoietin (TSLP) inhibitors—alongside emerging non-pharmacological strategies. Psychological interventions, particularly mindfulness-based approaches, have demonstrated improvements in quality of life, stress reduction, and patient-reported asthma control, supporting the relevance of addressing the psychosocial dimensions of chronic disease. Understanding asthma as a systemic disorder underscores the need for personalized, multidimensional treatment strategies that integrate pharmacological, behavioral, and lifestyle components. This paradigm provides a more comprehensive framework for improving long-term outcomes and reducing the global burden of asthma.
Asthma is one of the most common chronic respiratory diseases worldwide, traditionally defined as airway inflammation, reversible obstruction, and hyperresponsiveness. While this framework has guided decades of research and treatment, it fails to capture asthma as a heterogeneous and systemic condition. This pathology is shaped by complex interactions among genetic, epigenetic, immunological, neuroendocrine, metabolic, and environmental factors. Its coexistence with chronic obstructive pulmonary disease (COPD) in overlapping syndromes further complicates diagnosis and therapeutic decision-making. Asthma etiology involves oxidative stress, genetic susceptibility, and epigenetic regulation, along with age- and sex-dependent hormonal influences that modulate immune responses. Emerging evidence shows that structural and functional changes in the respiratory epithelium, airway smooth muscle (ASM), and alveoli extend the pathology beyond acute inflammation, involving processes such as epithelial barrier dysfunction, airway remodeling, and impaired mucociliary clearance (MCC). Neuro-immune-endocrine interactions have emerged as central contributors to asthma pathogenesis. Endocrine regulation shapes inflammatory activity and treatment responsiveness. Metabolic factors such as obesity introduce additional complexity by generating low-grade systemic inflammation, oxidative stress, adipokine imbalance, and steroid resistance, resulting in a distinct and often more severe asthma phenotype. Parasympathetic and sensory neural pathways amplify bronchoconstriction and inflammation through reciprocal communication with eosinophils, mast cells, innate lymphoid cells, and pulmonary neuroendocrine cells (PNECs). Neurotrophins and neuropeptides further promote airway hyperreactivity and remodeling. Current management integrates inhaled corticosteroids, bronchodilators, and targeted biologics—such as thymic stromal lymphopoietin (TSLP) inhibitors—alongside emerging non-pharmacological strategies. Psychological interventions, particularly mindfulness-based approaches, have demonstrated improvements in quality of life, stress reduction, and patient-reported asthma control, supporting the relevance of addressing the psychosocial dimensions of chronic disease. Understanding asthma as a systemic disorder underscores the need for personalized, multidimensional treatment strategies that integrate pharmacological, behavioral, and lifestyle components. This paradigm provides a more comprehensive framework for improving long-term outcomes and reducing the global burden of asthma.
DOI: https://doi.org/10.37349/eaa.2025.1009103
This article belongs to the special issue Asthma and its Relationship with Psychological and Psychopathological Factors

Aim:
Angioedema is a common but often underestimated manifestation of chronic spontaneous urticaria (CSU). Its presence may indicate higher disease severity, longer duration, and autoimmune involvement. This study aims to assess the clinical relevance and associations of angioedema in CSU patients with disease severity and duration, treatment response to H1-antihistamines, correlation with autoimmune status, and autologous serum skin test (ASST) positivity.
Methods:
A prospective study was conducted at the Dermatology Department, General Hospital 8th September, Skopje, North Macedonia, from December 2021 to November 2022, including 230 CSU patients. Disease activity was assessed using the Urticaria Activity Score over 7 days (UAS7), and severity was categorized accordingly. Response to H1-antihistamines was defined as achieving UAS7 < 7 for several months. Angioedema was recorded as a symptom regardless of localization. Autoimmune status was based on autoimmune disease history and/or autoantibody (AAb) detection. The ASST was performed, classifying patients as ASST-positive (ASST⁺) or ASST-negative (ASST⁻).
Results:
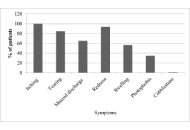
Angioedema was observed in 70% of CSU patients, all with accompanying wheals. It was significantly more common in severe CSU than in moderate (82.02% vs. 65.38%, p = 0.026), mild (82.02% vs. 65.96%, p = 0.036), and well-controlled disease (82.02% vs. 45.45%, p = 0.0004). Patients with a positive autoimmune status more often had angioedema than those with a negative status (75.17% vs. 61.18%, p = 0.025). CSU showed longer duration in patients with angioedema (p = 0.000012), with no association to good antihistamine response (p = 0.55).
Conclusions:
Angioedema in CSU is associated with higher disease activity, autoimmune status, and prolonged disease duration but not with differences in antihistamine response. Its presence marks a more severe phenotype, emphasizing the need for careful monitoring and individualized management.
Aim:
Angioedema is a common but often underestimated manifestation of chronic spontaneous urticaria (CSU). Its presence may indicate higher disease severity, longer duration, and autoimmune involvement. This study aims to assess the clinical relevance and associations of angioedema in CSU patients with disease severity and duration, treatment response to H1-antihistamines, correlation with autoimmune status, and autologous serum skin test (ASST) positivity.
Methods:
A prospective study was conducted at the Dermatology Department, General Hospital 8th September, Skopje, North Macedonia, from December 2021 to November 2022, including 230 CSU patients. Disease activity was assessed using the Urticaria Activity Score over 7 days (UAS7), and severity was categorized accordingly. Response to H1-antihistamines was defined as achieving UAS7 < 7 for several months. Angioedema was recorded as a symptom regardless of localization. Autoimmune status was based on autoimmune disease history and/or autoantibody (AAb) detection. The ASST was performed, classifying patients as ASST-positive (ASST⁺) or ASST-negative (ASST⁻).
Results:
Angioedema was observed in 70% of CSU patients, all with accompanying wheals. It was significantly more common in severe CSU than in moderate (82.02% vs. 65.38%, p = 0.026), mild (82.02% vs. 65.96%, p = 0.036), and well-controlled disease (82.02% vs. 45.45%, p = 0.0004). Patients with a positive autoimmune status more often had angioedema than those with a negative status (75.17% vs. 61.18%, p = 0.025). CSU showed longer duration in patients with angioedema (p = 0.000012), with no association to good antihistamine response (p = 0.55).
Conclusions:
Angioedema in CSU is associated with higher disease activity, autoimmune status, and prolonged disease duration but not with differences in antihistamine response. Its presence marks a more severe phenotype, emphasizing the need for careful monitoring and individualized management.
DOI: https://doi.org/10.37349/eaa.2025.1009101
This article belongs to the special issue Bridging Experimental and Translational Allergology

Intranasal treatments combining corticosteroids with antihistamines are a safe and effective alternative for treating moderate to severe seasonal allergic rhinitis in children over 12 years of age and adults. Evidence for their use in children under 12 years of age is limited and based on four studies: three examining azelastine hydrochloride and fluticasone propionate combination (AzeFlu) (including one placebo-controlled efficacy study, one comparative efficacy study, and one safety study) and one examining olopatadine hydrochloride and mometasone furoate combination (OloMom) (a placebo-controlled study). The recommendations from these studies could be conditional for school children aged 6 to 11 years with seasonal (non-perennial) allergic rhinitis, but only when symptoms cannot be controlled with a single drug.
Intranasal treatments combining corticosteroids with antihistamines are a safe and effective alternative for treating moderate to severe seasonal allergic rhinitis in children over 12 years of age and adults. Evidence for their use in children under 12 years of age is limited and based on four studies: three examining azelastine hydrochloride and fluticasone propionate combination (AzeFlu) (including one placebo-controlled efficacy study, one comparative efficacy study, and one safety study) and one examining olopatadine hydrochloride and mometasone furoate combination (OloMom) (a placebo-controlled study). The recommendations from these studies could be conditional for school children aged 6 to 11 years with seasonal (non-perennial) allergic rhinitis, but only when symptoms cannot be controlled with a single drug.
DOI: https://doi.org/10.37349/eaa.2025.1009102
This article belongs to the special issue Asthma, Allergies, and Respiratory Infections in Pediatric Age

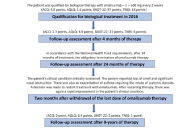
In recent years, biological therapy based on endotyping has become a therapeutic option for patients with type 2 inflammatory process with chronic rhinosinusitis with nasal polyps with or without bronchial asthma. It is also used in the course of a particularly aggressive chronic rhinosinusitis with nasal polyps in patients with bronchial asthma and hypersensitivity to non-steroidal anti-inflammatory drug (NSAID-exacerbated respiratory disease, N-ERD). Identification of the patient’s inflammatory endotype enables targeted biological treatment and optimal results from biological therapy. The search for biomarkers that identify patients who will benefit from biological treatment remains a current topic of investigation. A particularly difficult therapeutic decision concerns patients with a mixed endotype (eosinophilic/allergic), which affects patients with N-ERD. The authors present a case of multidimensional beneficial results in a 39-year-old female patient with N-ERD syndrome during biological treatment with omalizumab. The parameters of inflammation, the results of computed tomography, and the results of questionnaires assessing the patient’s quality of life before, after 4 months and 9 years of biological treatment are presented. Excellent results were found in all criteria of efficacy of biological treatment: reduced nasal polyp size (complete remission of polyps in the paranasal sinuses), reduced need for systemic corticosteroids (no systemic steroids have been used since the start of therapy), improved sense of smell, reduced impact of comorbidities (complete control of bronchial asthma) and in the patient’s opinion, an extremely significant improvement in quality of life. Thus, the patient can be considered as a super-responder. Analysis of the parameters of patients who achieved optimal therapeutic results may also be helpful in selecting type of biological treatment for future patients.
In recent years, biological therapy based on endotyping has become a therapeutic option for patients with type 2 inflammatory process with chronic rhinosinusitis with nasal polyps with or without bronchial asthma. It is also used in the course of a particularly aggressive chronic rhinosinusitis with nasal polyps in patients with bronchial asthma and hypersensitivity to non-steroidal anti-inflammatory drug (NSAID-exacerbated respiratory disease, N-ERD). Identification of the patient’s inflammatory endotype enables targeted biological treatment and optimal results from biological therapy. The search for biomarkers that identify patients who will benefit from biological treatment remains a current topic of investigation. A particularly difficult therapeutic decision concerns patients with a mixed endotype (eosinophilic/allergic), which affects patients with N-ERD. The authors present a case of multidimensional beneficial results in a 39-year-old female patient with N-ERD syndrome during biological treatment with omalizumab. The parameters of inflammation, the results of computed tomography, and the results of questionnaires assessing the patient’s quality of life before, after 4 months and 9 years of biological treatment are presented. Excellent results were found in all criteria of efficacy of biological treatment: reduced nasal polyp size (complete remission of polyps in the paranasal sinuses), reduced need for systemic corticosteroids (no systemic steroids have been used since the start of therapy), improved sense of smell, reduced impact of comorbidities (complete control of bronchial asthma) and in the patient’s opinion, an extremely significant improvement in quality of life. Thus, the patient can be considered as a super-responder. Analysis of the parameters of patients who achieved optimal therapeutic results may also be helpful in selecting type of biological treatment for future patients.
DOI: https://doi.org/10.37349/eaa.2025.1009100
This article belongs to the special issue Update on Chronic Rhinosinusitis

Schnitzler syndrome is a rare acquired autoinflammatory disorder defined by a chronic urticarial rash, monoclonal IgM (or IgG) gammopathy, and systemic features including fever, arthralgia, and elevated inflammatory markers. Interleukin-1 blockade with anakinra is the current treatment of choice. We report the case of a 75-year-old woman who, after seven years of misdiagnosis as chronic spontaneous urticaria, fulfilled the Strasbourg Criteria for Schnitzler syndrome. Treatment with anakinra induced rapid clinical improvement but was complicated by the onset of recall urticaria (RU), characterized by delayed giant wheals at both current and previous injection sites. Laboratory findings suggested an inflammatory response, and the reaction was managed with corticosteroids and antihistamines, followed by colchicine, which achieved stable disease control. RU is a rare hypersensitivity phenomenon previously described with immunotherapy, heparin, NSAIDs, and other biologics, but to our knowledge, this is the first report associated with anakinra. This case broadens the spectrum of anakinra-related adverse effects and highlights the need for further investigation into the immunopathogenesis of RU.
Schnitzler syndrome is a rare acquired autoinflammatory disorder defined by a chronic urticarial rash, monoclonal IgM (or IgG) gammopathy, and systemic features including fever, arthralgia, and elevated inflammatory markers. Interleukin-1 blockade with anakinra is the current treatment of choice. We report the case of a 75-year-old woman who, after seven years of misdiagnosis as chronic spontaneous urticaria, fulfilled the Strasbourg Criteria for Schnitzler syndrome. Treatment with anakinra induced rapid clinical improvement but was complicated by the onset of recall urticaria (RU), characterized by delayed giant wheals at both current and previous injection sites. Laboratory findings suggested an inflammatory response, and the reaction was managed with corticosteroids and antihistamines, followed by colchicine, which achieved stable disease control. RU is a rare hypersensitivity phenomenon previously described with immunotherapy, heparin, NSAIDs, and other biologics, but to our knowledge, this is the first report associated with anakinra. This case broadens the spectrum of anakinra-related adverse effects and highlights the need for further investigation into the immunopathogenesis of RU.
DOI: https://doi.org/10.37349/eaa.2025.100999
This article belongs to the special issue The Era of Biologics in Allergy

Eosinophilic esophagitis (EoE) is an adaptive immune T-cell-mediated type 2 inflammatory disease involving the esophagus. Major advances were made in diagnosis and therapy for EoE in the last decade. A correct diagnosis is important to guide therapy to achieve optimal treatment goals. The diagnostic algorithm eliminated the need for a proton pump inhibitor (PPI) trial, allowing for direct proceeding to disease-specific anti-inflammatory drugs if indicated. PPIs are the first line of therapy in EoE. Two topical steroids and one biologic drug, dupilumab, have been used for the treatment of EoE, with a number of novel therapeutic agents in the pipeline. Because of the chronicity of the disease and nonresponders to PPIs and topical steroids, a subgroup of patients may require long-term therapy with dupilumab. More data also became available on dietary interventions, elimination diets in adults with EoE. A least restrictive diet should be trialed first with milk or milk and wheat elimination. Importantly, EoE patients with fibrostenotic disease often do not respond well to drug therapy and require esophageal dilations. Medical therapy may need to be tailored for each patient depending on the patient’s specific comorbidities, patient preferences, and health status. This review will summarize a current approach to the treatment of adult patients with EoE.
Eosinophilic esophagitis (EoE) is an adaptive immune T-cell-mediated type 2 inflammatory disease involving the esophagus. Major advances were made in diagnosis and therapy for EoE in the last decade. A correct diagnosis is important to guide therapy to achieve optimal treatment goals. The diagnostic algorithm eliminated the need for a proton pump inhibitor (PPI) trial, allowing for direct proceeding to disease-specific anti-inflammatory drugs if indicated. PPIs are the first line of therapy in EoE. Two topical steroids and one biologic drug, dupilumab, have been used for the treatment of EoE, with a number of novel therapeutic agents in the pipeline. Because of the chronicity of the disease and nonresponders to PPIs and topical steroids, a subgroup of patients may require long-term therapy with dupilumab. More data also became available on dietary interventions, elimination diets in adults with EoE. A least restrictive diet should be trialed first with milk or milk and wheat elimination. Importantly, EoE patients with fibrostenotic disease often do not respond well to drug therapy and require esophageal dilations. Medical therapy may need to be tailored for each patient depending on the patient’s specific comorbidities, patient preferences, and health status. This review will summarize a current approach to the treatment of adult patients with EoE.
DOI: https://doi.org/10.37349/eaa.2025.100998
This article belongs to the special issue Beyond Eosinophilic Gastrointestinal Diseases: Pathogenetic Mechanisms and Therapeutic Strategies

Aim:
Eosinophilic gastrointestinal disorders (EGIDs) are chronic inflammatory conditions defined by eosinophilic infiltration of the gastrointestinal tract in the absence of secondary causes. This study aimed to synthesize current evidence on the clinical spectrum, pathogenesis, diagnostic criteria, and prognostic implications of EGIDs, including eosinophilic esophagitis, gastritis, duodenitis, ileitis, and colitis.
Methods:
A retrospective, multi-source observational analysis of published clinical datasets on EGIDs was conducted. Systematic searches of PubMed, Scopus, Web of Science, and EMBASE identified eligible studies that included ≥ 10 patients with EGIDs and provided quantitative data on eosinophil counts, clinical features, endoscopic and histopathological findings. Articles reporting secondary causes of eosinophilia were excluded. Data extraction was done and independently verified by two reviewers. Special emphasis was placed on unresolved diagnostic hurdles, pediatric versus adult presentations, and long-term disease implications.
Results:
A total of eligible datasets highlighted common molecular drivers, including epithelial barrier dysfunction, Th2-skewed immune responses, and genetic susceptibilities (e.g., TSLP, CAPN14). Core symptoms varied by site, with dysphagia predominating in eosinophilic esophagitis, and abdominal pain and diarrhea more frequent in distal EGIDs. Endoscopic findings included rings, furrows, and nodularity, while histology demonstrated patchy eosinophilic infiltration and epithelial damage with site-specific thresholds. Laboratory abnormalities included elevated eosinophil counts, IgE, and biochemical markers of malabsorption. Prognosis was variable, with frequent recurrence and heterogeneity in treatment response.
Conclusions:
Significant knowledge gaps persist in EGID research and practice. Priority areas include establishing consensus-driven histological thresholds and developing non-invasive biomarkers for disease monitoring. Urgent unresolved questions involve the utility of biomarkers in guiding therapy, monitoring response and systematic evaluation of pediatric versus adult differences. Addressing these gaps will require multidisciplinary collaboration, standardized diagnostic protocols, and longitudinal multicenter studies to improve both clinical care and research consistency.
Aim:
Eosinophilic gastrointestinal disorders (EGIDs) are chronic inflammatory conditions defined by eosinophilic infiltration of the gastrointestinal tract in the absence of secondary causes. This study aimed to synthesize current evidence on the clinical spectrum, pathogenesis, diagnostic criteria, and prognostic implications of EGIDs, including eosinophilic esophagitis, gastritis, duodenitis, ileitis, and colitis.
Methods:
A retrospective, multi-source observational analysis of published clinical datasets on EGIDs was conducted. Systematic searches of PubMed, Scopus, Web of Science, and EMBASE identified eligible studies that included ≥ 10 patients with EGIDs and provided quantitative data on eosinophil counts, clinical features, endoscopic and histopathological findings. Articles reporting secondary causes of eosinophilia were excluded. Data extraction was done and independently verified by two reviewers. Special emphasis was placed on unresolved diagnostic hurdles, pediatric versus adult presentations, and long-term disease implications.
Results:
A total of eligible datasets highlighted common molecular drivers, including epithelial barrier dysfunction, Th2-skewed immune responses, and genetic susceptibilities (e.g., TSLP, CAPN14). Core symptoms varied by site, with dysphagia predominating in eosinophilic esophagitis, and abdominal pain and diarrhea more frequent in distal EGIDs. Endoscopic findings included rings, furrows, and nodularity, while histology demonstrated patchy eosinophilic infiltration and epithelial damage with site-specific thresholds. Laboratory abnormalities included elevated eosinophil counts, IgE, and biochemical markers of malabsorption. Prognosis was variable, with frequent recurrence and heterogeneity in treatment response.
Conclusions:
Significant knowledge gaps persist in EGID research and practice. Priority areas include establishing consensus-driven histological thresholds and developing non-invasive biomarkers for disease monitoring. Urgent unresolved questions involve the utility of biomarkers in guiding therapy, monitoring response and systematic evaluation of pediatric versus adult differences. Addressing these gaps will require multidisciplinary collaboration, standardized diagnostic protocols, and longitudinal multicenter studies to improve both clinical care and research consistency.
DOI: https://doi.org/10.37349/eaa.2025.100997
This article belongs to the special issue Beyond Eosinophilic Gastrointestinal Diseases: Pathogenetic Mechanisms and Therapeutic Strategies

Aim:
Olea europaea, an endemic plant of the Mediterranean basin, exhibits a flowering period from April to June, requiring high temperatures and sensitivity to low humidity, rainfall, and windiness. Allergy to O. europaea affects 13.85% of the Southern Italian population. This study investigated O. europaea pollen concentration, morphological and biochemical variations, and clinical symptoms over a 6-year period (2017–2022).
Methods:
Pollen concentration in Southern Italy (Apulia, Bari) was analyzed alongside weather variables (temperature, precipitation, humidity, and windiness) using existing databases (Arpa Puglia; time and date). Optical and fluorescence microscopy techniques were employed to assess pollen morphology and biochemical characteristics. Additionally, the absolute number of prescriptions for various antihistamine drugs (cetirizine, ebastine, bilastine, desloratadine, rupatadine, levocetirizine, fexofenadine, loratadine) was calculated.
Results:
The lowest pollen count occurred in the 2018 (91.1 pollen per m3/week), while the highest was recorded in the 2021 (2,545.3 pollen per m3/week). In 2019, the pollen peak was delayed by 2 weeks. Notably, 2018 exhibited more rainy days in May and June and higher humidity percentages (April 73%, May 70%, June 72%). In contrast, 2021 had lower humidity values (April 68%, May 61%, June 59%) and fewer rainy days (1 day in May and none in June). No changes in pollen size were observed, but modifications in O. europaea pollen fuchsin fluorescence were noted in 2018 and 2021. The number of drug prescriptions was highest in 2021.
Conclusions:
This study highlights that the flowering period, morphology, and pollen production of O. europaea may influence patient symptomatology and the need for antihistamine medications.
Aim:
Olea europaea, an endemic plant of the Mediterranean basin, exhibits a flowering period from April to June, requiring high temperatures and sensitivity to low humidity, rainfall, and windiness. Allergy to O. europaea affects 13.85% of the Southern Italian population. This study investigated O. europaea pollen concentration, morphological and biochemical variations, and clinical symptoms over a 6-year period (2017–2022).
Methods:
Pollen concentration in Southern Italy (Apulia, Bari) was analyzed alongside weather variables (temperature, precipitation, humidity, and windiness) using existing databases (Arpa Puglia; time and date). Optical and fluorescence microscopy techniques were employed to assess pollen morphology and biochemical characteristics. Additionally, the absolute number of prescriptions for various antihistamine drugs (cetirizine, ebastine, bilastine, desloratadine, rupatadine, levocetirizine, fexofenadine, loratadine) was calculated.
Results:
The lowest pollen count occurred in the 2018 (91.1 pollen per m3/week), while the highest was recorded in the 2021 (2,545.3 pollen per m3/week). In 2019, the pollen peak was delayed by 2 weeks. Notably, 2018 exhibited more rainy days in May and June and higher humidity percentages (April 73%, May 70%, June 72%). In contrast, 2021 had lower humidity values (April 68%, May 61%, June 59%) and fewer rainy days (1 day in May and none in June). No changes in pollen size were observed, but modifications in O. europaea pollen fuchsin fluorescence were noted in 2018 and 2021. The number of drug prescriptions was highest in 2021.
Conclusions:
This study highlights that the flowering period, morphology, and pollen production of O. europaea may influence patient symptomatology and the need for antihistamine medications.
DOI: https://doi.org/10.37349/eaa.2025.100996
This article belongs to the special issue Climate Change, Allergy, and Immunotherapy

Aim:
Allergic conjunctivitis (AC) is an inflammatory response of the conjunctiva triggered by exposure to common allergens, including pollen, dust mites, and animal dander. This study aimed to identify probable allergens in Iranian patients with AC.
Methods:
This cross-sectional study included individuals with AC from Southwestern Iran in 2024. Skin prick tests (SPTs) were performed using commercial extracts of various allergens, including tree mix, weed mix, grass mix, dust mite mix, fungi mix, as well as cat and cockroach allergens.
Results:
Among 92 patients with conjunctivitis, with a mean age of 23.66 ± 14.70 years, 80 patients (86.96%) had a positive SPT to at least one of the applied extracts. Sensitization rates detected by SPTs were as follows: weed mix 68.48%, tree mix 58.70%, grass mix 53.26%, dust mite mix 45.65%, cockroach 29.35%, fungi mix 22.83% and cat allergen 17.39%. A significant difference in dust mite sensitization was observed between patients with seasonal and perennial AC (p = 0.023).
Conclusions:
This study highlights the allergic sensitization of patients with conjunctivitis and its connections to other allergic conditions. Allergists can play a crucial role in managing conjunctivitis through comprehensive testing and holistic treatment approaches.
Aim:
Allergic conjunctivitis (AC) is an inflammatory response of the conjunctiva triggered by exposure to common allergens, including pollen, dust mites, and animal dander. This study aimed to identify probable allergens in Iranian patients with AC.
Methods:
This cross-sectional study included individuals with AC from Southwestern Iran in 2024. Skin prick tests (SPTs) were performed using commercial extracts of various allergens, including tree mix, weed mix, grass mix, dust mite mix, fungi mix, as well as cat and cockroach allergens.
Results:
Among 92 patients with conjunctivitis, with a mean age of 23.66 ± 14.70 years, 80 patients (86.96%) had a positive SPT to at least one of the applied extracts. Sensitization rates detected by SPTs were as follows: weed mix 68.48%, tree mix 58.70%, grass mix 53.26%, dust mite mix 45.65%, cockroach 29.35%, fungi mix 22.83% and cat allergen 17.39%. A significant difference in dust mite sensitization was observed between patients with seasonal and perennial AC (p = 0.023).
Conclusions:
This study highlights the allergic sensitization of patients with conjunctivitis and its connections to other allergic conditions. Allergists can play a crucial role in managing conjunctivitis through comprehensive testing and holistic treatment approaches.
DOI: https://doi.org/10.37349/eaa.2025.100995

Allergic fungal rhinosinusitis is typically described as a condition involving nasal polyposis and eosinophilic mucin in which fungal hyphae are entrapped within enlarged sinus cavities, accompanied by an immune hypersensitivity response to fungi. There are rare reports in the pediatric literature. Early diagnosis and management with surgery represent the primary therapeutic approach, complemented by corticosteroid therapy and long-term follow-up to prevent relapse. In addition, novel biologic therapies have been investigated in recent years for the treatment of allergic fungal rhinosinusitis. Here, we report the case of a child with allergic fungal rhinosinusitis and summarize the literature review of data published.
Allergic fungal rhinosinusitis is typically described as a condition involving nasal polyposis and eosinophilic mucin in which fungal hyphae are entrapped within enlarged sinus cavities, accompanied by an immune hypersensitivity response to fungi. There are rare reports in the pediatric literature. Early diagnosis and management with surgery represent the primary therapeutic approach, complemented by corticosteroid therapy and long-term follow-up to prevent relapse. In addition, novel biologic therapies have been investigated in recent years for the treatment of allergic fungal rhinosinusitis. Here, we report the case of a child with allergic fungal rhinosinusitis and summarize the literature review of data published.
DOI: https://doi.org/10.37349/eaa.2025.100994

DOI: https://doi.org/10.37349/eaa.2025.100993

Artificial intelligence (AI) is poised to transform clinical allergy practice by enhancing diagnostic accuracy, personalising treatment, and streamlining healthcare delivery. This narrative review critically examines the current landscape of AI in allergy care, spanning clinical workflows, diagnostics, immunotherapy, and research applications. AI-powered tools such as clinical decision support systems (CDSS), natural language processing (NLP), and conversational agents are being integrated into allergy services, offering improvements in documentation, risk stratification, and remote patient engagement—particularly in paediatric and multilingual settings. Diagnostic innovations include machine learning models that predict oral food challenge outcomes and interpret multi-omics data for personalised allergy phenotyping. AI also supports adaptive immunotherapy dosing, remote monitoring via wearable biosensors, and digital coaching to promote adherence. Federated learning and explainable AI (XAI) emerge as pivotal developments—enabling privacy-preserving collaboration and fostering trust among clinicians and patients. Despite these advancements, significant challenges remain. These include data inequities, algorithmic bias, lack of real-world validation, and regulatory ambiguity. The “black box” nature of many models risks undermining clinician confidence, while over-reliance on alerts could contribute to alarm fatigue. Ethical concerns—particularly around transparency, consent, and liability—require urgent attention. Equitable implementation demands robust governance, diverse training data, and inclusive design that prioritises patient safety. Looking ahead, AI has the potential to power digital twins, support augmented reality training, and enhance allergy surveillance through the integration of environmental and population-level data. With multidisciplinary collaboration, transparent oversight, and patient-centred innovation, AI can help build a more predictive, efficient, and equitable future for allergy care.
Artificial intelligence (AI) is poised to transform clinical allergy practice by enhancing diagnostic accuracy, personalising treatment, and streamlining healthcare delivery. This narrative review critically examines the current landscape of AI in allergy care, spanning clinical workflows, diagnostics, immunotherapy, and research applications. AI-powered tools such as clinical decision support systems (CDSS), natural language processing (NLP), and conversational agents are being integrated into allergy services, offering improvements in documentation, risk stratification, and remote patient engagement—particularly in paediatric and multilingual settings. Diagnostic innovations include machine learning models that predict oral food challenge outcomes and interpret multi-omics data for personalised allergy phenotyping. AI also supports adaptive immunotherapy dosing, remote monitoring via wearable biosensors, and digital coaching to promote adherence. Federated learning and explainable AI (XAI) emerge as pivotal developments—enabling privacy-preserving collaboration and fostering trust among clinicians and patients. Despite these advancements, significant challenges remain. These include data inequities, algorithmic bias, lack of real-world validation, and regulatory ambiguity. The “black box” nature of many models risks undermining clinician confidence, while over-reliance on alerts could contribute to alarm fatigue. Ethical concerns—particularly around transparency, consent, and liability—require urgent attention. Equitable implementation demands robust governance, diverse training data, and inclusive design that prioritises patient safety. Looking ahead, AI has the potential to power digital twins, support augmented reality training, and enhance allergy surveillance through the integration of environmental and population-level data. With multidisciplinary collaboration, transparent oversight, and patient-centred innovation, AI can help build a more predictive, efficient, and equitable future for allergy care.
DOI: https://doi.org/10.37349/eaa.2025.100992
This article belongs to the special issue Allergy and Asthma in the Digital Age

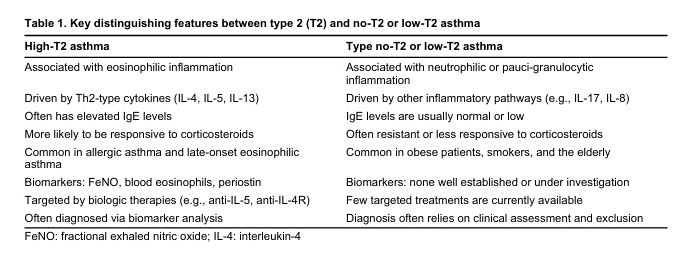
Asthma is a chronic inflammatory airway disorder characterized by recurrent symptoms, airflow obstruction, and bronchial hyperresponsiveness. Approximately 5–10% of asthma cases are classified as severe, requiring high-dose inhaled corticosteroids (ICS) plus additional controllers, often including systemic corticosteroids. Severe asthma imposes a substantial burden on patients due to frequent exacerbations and reduced quality of life. The pathophysiology of severe asthma involves distinct phenotypic and endotypic variations, primarily classified into high-type 2 (T2) and low-T2 inflammatory profiles. While high-T2 asthma, encompassing eosinophilic and allergic subtypes, benefits from targeted biologic therapies such as monoclonal antibodies against interleukin-5 (IL-5), IL-4/IL-13, thymic stromal lymphopoietin (TSLP), and IgE, treatment options for low-T2 asthma remain limited. The advent of precision medicine has facilitated the identification of novel biomarkers for severe asthma, guiding therapeutic decisions and enabling disease stratification. However, key clinical challenges remain, including selecting the most effective biologic therapy, optimal treatment duration, and safe de-escalation strategies upon achieving remission. This review explores the latest evidence on biological therapies, their immunomodulatory effects, and their potential role in reversing bronchial remodelling. Additionally, it discusses emerging biomarkers that may predict treatment response and remission, ultimately contributing to a more personalized approach to asthma management.
Asthma is a chronic inflammatory airway disorder characterized by recurrent symptoms, airflow obstruction, and bronchial hyperresponsiveness. Approximately 5–10% of asthma cases are classified as severe, requiring high-dose inhaled corticosteroids (ICS) plus additional controllers, often including systemic corticosteroids. Severe asthma imposes a substantial burden on patients due to frequent exacerbations and reduced quality of life. The pathophysiology of severe asthma involves distinct phenotypic and endotypic variations, primarily classified into high-type 2 (T2) and low-T2 inflammatory profiles. While high-T2 asthma, encompassing eosinophilic and allergic subtypes, benefits from targeted biologic therapies such as monoclonal antibodies against interleukin-5 (IL-5), IL-4/IL-13, thymic stromal lymphopoietin (TSLP), and IgE, treatment options for low-T2 asthma remain limited. The advent of precision medicine has facilitated the identification of novel biomarkers for severe asthma, guiding therapeutic decisions and enabling disease stratification. However, key clinical challenges remain, including selecting the most effective biologic therapy, optimal treatment duration, and safe de-escalation strategies upon achieving remission. This review explores the latest evidence on biological therapies, their immunomodulatory effects, and their potential role in reversing bronchial remodelling. Additionally, it discusses emerging biomarkers that may predict treatment response and remission, ultimately contributing to a more personalized approach to asthma management.
DOI: https://doi.org/10.37349/eaa.2025.100991

Aim:
Despite the revolutionary impact of biologics (Bx) on severe asthma management, predicting individual treatment responses remains challenging. We aimed to characterize the heterogeneous nature of clinical status and disease activity in patients with severe asthma after biologic therapies through a comprehensive evaluation of real-world clinical outcomes.
Methods:
In this retrospective, multicenter study of 53 patients with severe asthma who received biologic therapies, hierarchical clustering analysis was performed based on three key parameters during treatment: exacerbation, maintenance oral corticosteroid (mOCS) dose, and lung function. Canonical correlation analysis and multinomial logistic regression were used to identify predictors of response patterns.
Results:
Clustering analysis revealed three distinct control groups: well-controlled (n = 23), moderately controlled (n = 22), and poorly controlled (n = 8). Well-controlled patients exhibited minimal exacerbations, no oral corticosteroid (OCS) use, and optimal or stabilized lung function. Moderately controlled patients showed minimal exacerbations and no mOCS use but variable lung function improvements. Poorly controlled patients exhibited persistent exacerbations, mOCS dependence, or both with limited lung function improvement. Baseline forced expiratory volume in 1 second (FEV1) %predicted (percent predicted FEV1) values and blood eosinophil counts independently differentiated well-controlled from moderately controlled patients, whereas baseline mOCS use distinguished moderately controlled from poorly controlled patients.
Conclusions:
Our findings reveal distinct patterns of disease control following biologic therapy in severe asthma, with baseline lung function, eosinophilic inflammation, and OCS use as key predictive factors. These results support the need for personalized treatment approaches in severe asthma management.
Aim:
Despite the revolutionary impact of biologics (Bx) on severe asthma management, predicting individual treatment responses remains challenging. We aimed to characterize the heterogeneous nature of clinical status and disease activity in patients with severe asthma after biologic therapies through a comprehensive evaluation of real-world clinical outcomes.
Methods:
In this retrospective, multicenter study of 53 patients with severe asthma who received biologic therapies, hierarchical clustering analysis was performed based on three key parameters during treatment: exacerbation, maintenance oral corticosteroid (mOCS) dose, and lung function. Canonical correlation analysis and multinomial logistic regression were used to identify predictors of response patterns.
Results:
Clustering analysis revealed three distinct control groups: well-controlled (n = 23), moderately controlled (n = 22), and poorly controlled (n = 8). Well-controlled patients exhibited minimal exacerbations, no oral corticosteroid (OCS) use, and optimal or stabilized lung function. Moderately controlled patients showed minimal exacerbations and no mOCS use but variable lung function improvements. Poorly controlled patients exhibited persistent exacerbations, mOCS dependence, or both with limited lung function improvement. Baseline forced expiratory volume in 1 second (FEV1) %predicted (percent predicted FEV1) values and blood eosinophil counts independently differentiated well-controlled from moderately controlled patients, whereas baseline mOCS use distinguished moderately controlled from poorly controlled patients.
Conclusions:
Our findings reveal distinct patterns of disease control following biologic therapy in severe asthma, with baseline lung function, eosinophilic inflammation, and OCS use as key predictive factors. These results support the need for personalized treatment approaches in severe asthma management.
DOI: https://doi.org/10.37349/eaa.2025.100990
This article belongs to the special issue The Era of Biologics in Allergy
 Previous
Previous