Affiliation:
1Department of Pathology, Microbiology and Forensic Medicine, School of Medicine, The University of Jordan, Amman 11942, Jordan
2Department of Clinical Laboratories and Forensic Medicine, Jordan University Hospital, Amman 11942, Jordan
Email: malik.sallam@ju.edu.jo
ORCID: https://orcid.org/0000-0002-0165-9670
Affiliation:
3Department of Pediatric Surgery, Mediclinic Parkview Hospital, Mediclinic Middle East, Dubai 505004, United Arab Emirates
4College of Medicine, Mohammed Bin Rashid University of Medicine and Health Sciences (MBRU), Dubai 505055, United Arab Emirates
ORCID: https://orcid.org/0009-0007-9454-2503
Affiliation:
5Department of Pediatrics, Mediclinic Parkview Hospital, Mediclinic Middle East, Dubai 505004, United Arab Emirates
6Department of Pediatrics, College of Medicine and Health Sciences, United Arab Emirates University, Al Ain 15551, United Arab Emirates
ORCID: https://orcid.org/0000-0002-6133-2450
Affiliation:
4College of Medicine, Mohammed Bin Rashid University of Medicine and Health Sciences (MBRU), Dubai 505055, United Arab Emirates
7Department of Pharmacy, Mediclinic Parkview Hospital, Mediclinic Middle East, Dubai 505004, United Arab Emirates
8Department of Management, Mediclinic Parkview Hospital, Mediclinic Middle East, Dubai 505004, United Arab Emirates
ORCID: https://orcid.org/0000-0003-3273-524X
Explor Asthma Allergy. 2025;3:100988 DOI: https://doi.org/10.37349/eaa.2025.100988
Received: May 31, 2025 Accepted: July 15, 2025 Published: July 30, 2025
Academic Editor: Michele Miraglia Del Giudice, University of Campania, Italy
The article belongs to the special issue Asthma, Allergies, and Respiratory Infections in Pediatric Age
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract infection (LRTI) burden among infants. Maternal vaccination is a promising preventive strategy, conferring passive immunity through transplacental antibody transfer. The current narrative review was conducted to summarize the current evidence of efficacy and safety of maternal RSV vaccination and assess the practical barriers to its implementation. This review was based on a structured literature search of PubMed/MEDLINE and Google Scholar to identify peer-reviewed studies published between January 2022 and March 2025 using terms such as “maternal RSV vaccine”, “efficacy”, “safety”, “pregnancy”, “Abrysvo”, and “hesitancy”. The review included 5 clinical trials evaluating maternal RSV vaccines and 17 observational and survey studies assessing vaccine acceptance across diverse settings. The bivalent RSVpreF vaccine (Abrysvo) is the only licensed maternal RSV vaccine as of May 2025. In the MATISSE phase 3 trial (n = 7,358), the vaccine demonstrated 81.8% efficacy against medically attended RSV-LRTI at 90 days and 69.4% at 180 days, with 57.1% efficacy against severe RSV-LRTI. No major safety concerns were identified; adverse events and preterm birth rates were comparable between groups. In contrast, trials of GSK’s RSVPreF3-Mat vaccine revealed higher rates of preterm birth (6.8% vs. 4.9%) and a numerical imbalance in infant deaths (0.4% vs. 0.2%), prompting early termination. Across 17 studies (n = 14,959), RSV vaccine acceptance ranged from 39% (France) to 87% (Netherlands), with safety concerns and cultural context influencing attitudes. This review highlights that maternal RSV vaccination with RSVpreF offers effective infant protection with an acceptable safety profile. Future research should focus on long-term infant outcomes, comparative effectiveness in diverse settings, and next-generation vaccines. Implementation will require public trust, cultural sensitivity, and equitable global access.
In the field of pediatric respiratory infections, respiratory syncytial virus (RSV) remains a major threat [1]. First identified in the 1950s, RSV has since been consistently reported as the most common cause of lower respiratory tract infections (LRTIs) in infants and young children globally [2, 3]. Despite decades of clinical experience and incremental advances in supportive care, RSV continues to exert a significant burden both on human lives and economic costs [4–6]. The continued RSV disease burden highlights the limitations of the current management strategies and the urgent need for efficient preventive solutions [7].
The World Health Organization (WHO) estimates that each year RSV leads to more than 3.6 million hospital admissions with 100,000 deaths globally, the vast majority of which occur in low- and middle-income countries (LMICs) [8]. The RSV disease burden falls most acutely on infants in their first months of life [4]. In temperate climates, RSV incidence typically rises in late fall and peaks in the winter months (e.g., between September and January in the Northern Hemisphere; March through June in the Southern Hemisphere) [9, 10]. In contrast, in tropical regions, RSV activity may coincide with the rainy season, though patterns are less uniform [11]. Global Burden of Disease (GBD) 2019 Data revealed that RSV was implicated in approximately 1 in every 50 deaths among children aged 0 to 60 months, and a striking 1 in every 28 deaths among those aged 28 days to six months [12]. Infants born prematurely or with underlying comorbidities such as congenital heart disease, lung disease, or immunodeficiencies are at the highest risk, though previously healthy infants also face substantial morbidity [13–15].
By the age of two, nearly every child becomes infected with RSV at least once, with many experiencing recurrent infections [16]. It is important to recognize that RSV reinfection is common, occurring throughout childhood and beyond [17, 18]. Although subsequent infections are often reported to be less severe, evidence that past RSV infection may not attenuate the risk for reinfection is emerging [19, 20].
The implications of RSV infection stretch beyond the walls of pediatric intensive care units (PICUs). Healthcare systems worldwide are increasingly strained by the seasonal surge of RSV-associated admissions [21, 22]. RSV disease imposes a significant socioeconomic burden worldwide, particularly among young children, due to increased healthcare resource utilization—including hospitalizations, PICUs admissions, and mechanical ventilation—and associated costs, as well as loss of parental work productivity [23]. In LMICs, where access to hospital-based care is often limited, the RSV disease toll is compounded by the absence of supportive interventions and a higher case-fatality rate (CFR) [24]. The economic burden of RSV is thus global, multi-dimensional, and deeply inequitable [25].
Historically, efforts to reduce the RSV burden have leaned heavily on passive immunoprophylaxis [26, 27]. Palivizumab, a monoclonal antibody targeting the RSV fusion (F) protein, represented a significant step forward when introduced over two decades ago [28]. More recently, nirsevimab, a long-acting monoclonal antibody with enhanced potency and extended half-life, has emerged as a promising alternative, offering broader protection with a single dose for all infants during their first RSV season [29]. In addition, clesrovimab, a next-generation monoclonal antibody with an extended half-life, underwent clinical evaluation for its ability to provide durable protection against RSV and was recently approved for use in infants to prevent RSV-related lower respiratory tract disease during their first RSV season [30–33]. Importantly, Gatt et al. [34] emphasized that a combination approach incorporating both maternal vaccination and monoclonal antibody-based passive immunoprophylaxis may offer the most effective strategy for comprehensive RSV prevention across infant populations.
Maternal immunization—the strategic vaccination of pregnant women to confer passive immunity to their newborns—has emerged as one of the most promising frontiers in infectious disease prevention [35]. This strategy utilizes the maternal immune system to produce high titers of pathogen-specific antibodies. In turn, these antibodies are transferred across the placenta to the fetus, thereby providing immediate and early-life protection at a time when the infant’s own immune responses are underdeveloped [36].
Recent years have seen marked progress in advancing maternal RSV immunization [37]. In August 2023, the US Food and Drug Administration (FDA) approved Abrysvo, a bivalent RSV prefusion F protein-based vaccine developed by Pfizer Inc., for use in pregnant women between 32 and 36 weeks’ gestation [38]. The biological rationale for targeting the prefusion conformation of the RSV F protein lies in its superior immunogenicity relative to post-fusion forms, offering higher neutralizing antibody titers and robust protection [39]. The European Medicines Agency (EMA) followed with its own approval, recommending a broader administration window between 24 weeks and 36 weeks of gestation [40]. This discrepancy reflects differing regulatory approaches to balancing maximum infant protection with maternal and fetal safety. Notably, a mathematical model by Willemsen et al. [41] suggested that administering the vaccine earlier in pregnancy (between 24 weeks and 27 weeks assuming vaccine safety is established) could increase the proportion of infants born with protective antibody levels, potentially reducing RSV-related infant mortality by up to 12%. This benefit was most pronounced in LMICs, where preterm birth rates may be higher and access to alternative interventions can be limited. Another maternal RSV vaccine candidate, developed by GlaxoSmithKline (GSK) plc. and based on a similar prefusion F protein design, also demonstrated promising safety and immunogenicity data in early human trials [37]. However, due to concerns over an observed imbalance in preterm births, its clinical trajectory has been paused—highlighting the meticulous scrutiny and rigorous safety thresholds required when intervening during pregnancy [42].
Vaccination offers clear advantages over treatment, particularly in the case of RSV, where antiviral therapies are limited and largely ineffective in clinical practice [43]. However, the introduction of maternal RSV vaccination into routine prenatal care represents both an opportunity and a challenge. Real-world implementation of maternal RSV vaccination must now grapple with questions of cost-effectiveness, public perception, regulatory issues, and vaccine hesitancy [44–49]. Concerns surrounding vaccination during pregnancy require culturally-tailored, evidence-based communication strategies [50, 51]. These challenges are often more pronounced in low-resource settings, where limited access to antenatal care, constrained healthcare infrastructure, and competing public health priorities may hinder maternal RSV vaccine rollout [52, 53].
While previous reviews on maternal RSV vaccination exist [37, 54–56], an updated synthesis is warranted given the recent licensure of maternal RSV vaccines, the availability of new clinical trial data, and emerging real-world evidence on maternal RSV vaccine uptake and hesitancy. Thus, the current narrative review has three primary aims. First, we sought to present a comprehensive overview of the currently approved maternal RSV vaccines. Second, we aimed to review the safety and efficacy data from key clinical trials of approved maternal RSV vaccines to extract actionable insights for clinicians and policy-makers. Third, we aimed to assess the practical challenges facing widespread maternal RSV vaccine implementation, including the issue of possible RSV vaccine hesitancy.
This narrative review was developed in accordance with the Scale for the Assessment of Narrative Review Articles (SANRA), a validated tool for ensuring the quality, transparency, and scientific rigor of non-systematic reviews [57]. Each of the six SANRA criteria—justification of the review importance, statement of review aims, description of literature search, referencing, scientific reasoning, and appropriate presentation of data—was deliberately addressed throughout the review process [57].
The importance of the review topic was established by examining the ongoing global burden of RSV infection in infants, particularly in the critical first months of life. The objective was clearly defined: to synthesize high-quality, recent evidence on the efficacy, safety, and implementation challenges of maternal RSV vaccination as a strategy to protect infants against RSV-related morbidity and mortality.
To guide this review, three central questions were formulated: (1) What are the currently approved maternal RSV vaccines, and which additional candidates are in development? (2) What is the existing evidence on their safety and efficacy based on pivotal clinical trials and regulatory reviews? (3) What practical and policy-level challenges may impede the widespread implementation of maternal RSV vaccination programs?
A structured literature search was conducted across two major databases: PubMed/MEDLINE and Google Scholar. The decision to include Google Scholar search aimed to minimize the likelihood of missing relevant references, as Google Scholar rapidly indexes a wide range of scholarly publications, reports, and survey-based studies [58]. The search was limited to peer-reviewed studies published between January 1, 2022, and March 1, 2025, to ensure that the review included the most current and clinically relevant data following the recent regulatory approvals. The search strategy employed concise and highly specific terms to identify pertinent literature, including the following combinations: “maternal RSV vaccin*” AND “efficacy”, “maternal RSV vaccin*” AND “safety”, “RSV vaccin*” AND “pregnancy”, “Abrysvo” AND “clinical trial”, and “RSV vaccin*” AND “hesitancy”. Filters were applied to include only human studies published in English. The final database search was conducted on March 1, 2025. The structured search identified 49 records from PubMed/MEDLINE and 200 records from Google Scholar, combined for subsequent screening.
Records were examined by the first and senior authors with the aim of extracting the most relevant and informative evidence rather than adhering to strict eligibility screening. Data from key clinical trials, regulatory reviews, observational studies, and systematic reviews were integrated, with emphasis placed on those that addressed the primary themes of vaccine safety, efficacy in preventing infant RSV outcomes, maternal immunogenicity, and barriers to implementation. Particular attention was given to large-scale trials evaluating the bivalent RSV prefusion F protein-based vaccine, Abrysvo, and to policy analyses reflecting regulatory decisions by the US FDA and the EMA.
All findings were synthesized thematically into three domains: the clinical and regulatory status of maternal RSV vaccines, the evidence on efficacy and safety in the maternal-infant dyad, and the logistical and societal challenges associated with maternal immunization programs. Rather than applying rigid exclusion criteria, this review synthesized the most up-to-date evidence to inform clinical practice and maternal immunization policy.
As of May 2025, Pfizer RSVpreF vaccine (ABRYSVO) is the only licensed maternal RSV vaccine, approved to protect infants from RSV-associated LRTIs through six months of age. It is a bivalent protein subunit vaccine containing 60 μg each of RSV-A and RSV-B prefusion F antigens. It is authorized for use between 32–36 weeks’ gestation in the US and 24–36 weeks in the EU. Approval was based on the MATISSE phase 3 trial, which enrolled over 7,000 pregnant individuals and showed 81.8% efficacy against severe RSV-LRTIs at 90 days and 69.4% at 180 days. While efficacy against medically attended RSV-LRTIs was observed, it did not meet statistical significance. Maternal infection was not evaluated. The Novavax RSV F nanoparticle vaccine, targeting the post-fusion F protein, was studied in the PREPARE phase 3 trial (n = 4,636) but did not meet its primary endpoint. However, secondary and exploratory analyses showed reduced RSV-related hospitalizations and severe hypoxemia in infants, supporting the concept of maternal immunization despite the lack of licensure. Maternal RSV infection rates were similar between groups. GSK’s Arexvy and Moderna’s mResvia, though approved for use in older adults, are not approved for use during pregnancy.
Several clinical trials have evaluated the efficacy of maternal RSV vaccination in reducing RSV-related disease in infants (Table 1). Across trials, vaccination during the late second or third trimester was associated with significant reductions in RSV-associated LRTIs, including severe cases and hospitalizations, particularly within the first 90 to 180 days of life. In the phase 3 MATISSE trial, 7,358 pregnant individuals between 24 and 36 weeks of gestation were randomized to receive a single 120 µg dose of Pfizer’s RSVpreF vaccine or placebo. Conducted across 18 countries, the trial demonstrated robust protection, with a vaccine efficacy of 81.8% against medically attended RSV-LRTIs at 90 days and 69.4% at 180 days, and 57.1% efficacy against severe RSV-LRTIs at 180 days. These findings led to regulatory approval of RSVpreF (Abrysvo) for maternal use. A preceding phase 2b trial involving 406 participants across four countries evaluated the same RSVpreF candidate in various formulations, including different doses and adjuvant combinations. Although the vaccine also showed a favorable outcome toward reducing RSV-related disease, the efficacy estimates were based on exploratory, post hoc analyses, limiting definitive interpretation. Nonetheless, the results informed the subsequent formulation and dose selection for phase 3 development. In contrast, a phase 3 trial assessed the efficacy of Novavax’s RSV F nanoparticle vaccine in 4,636 pregnant individuals between 28 and 36 weeks’ gestation. The vaccine, administered as a 120 µg dose adsorbed to 0.4 mg aluminum, did not meet the prespecified efficacy threshold for preventing RSV LRTIs in infants. Compared to RSVpreF, the relative risk (RR) reductions observed for LRTIs, severe LRTIs, and hospitalizations were lower.
Summary of the included clinical trials that assessed maternal RSV vaccine efficacy
| Record | Title | Design | Vaccine | Population | Follow-up period | Vaccine efficacy |
|---|---|---|---|---|---|---|
| Kampmann et al. [59] | Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants | Phase 3, double-blind clinical trial. MATISSE ClinicalTrials.gov number: NCT04424316 | Bivalent RSV prefusion F protein-based (RSVpreF) vaccine funded by Pfizer | Pregnant women at 24 through 36 weeks’ gestation. 3,682 maternal participants received the vaccine, and 3,676 received placebo; 3,570 and 3,558 infants, respectively, were evaluated | 180 days after birth | 81.8% efficacy against severe RSV-LRTI at 90 days (99.5% CI, 40.6–96.3); 69.4% at 180 days (97.58% CI, 44.3–84.1); 57.1% efficacy against medically attended RSV-LRTI at 90 days (99.5% CI, 14.7–79.8) |
| Simões et al. [60] | Prefusion F protein-based respiratory syncytial virus immunization in pregnancy | Phase 2b, double-blind clinical trial. ClinicalTrials.gov number: NCT04032093 | Bivalent RSV prefusion F protein-based (RSVpreF) vaccine funded by Pfizer | 406 women and 403 infants; 327 women (80.5%) received RSVpreF vaccine | 6 months after birth | Post hoc exploratory efficacy analyses showed efficacy of 84.7% (95% CI: 21.6–97.6) and 91.5% (95% CI: –5.6–99.8) for medically attended and severe medically attended RSV-associated lower respiratory tract illness, respectively |
| Madhi et al. [61] | Respiratory syncytial virus vaccination during pregnancy and effects in infants | Phase 3, observer-blind, placebo-controlled clinical trial. ClinicalTrials.gov number: NCT02624947 | Recombinant RSV F-nanoparticle vaccine (RSV-F vaccine) funded by Novavax and the Bill and Melinda Gates Foundation | Pregnant women aged 14–40 years, 28–36 weeks’ gestation; 4,636 randomized; 4,579 live births | 180 days after birth | 39.4% efficacy against RSV-LRTI at 90 days (97.52% CI, –1.0 to 63.7); 48.3% against RSV-LRTI with severe hypoxemia (95% CI, –8.2 to 75.3); 44.4% efficacy against RSV-LRTI hospitalization (95% CI, 19.6 to 61.5) |
| Dieussaert et al. [42] | RSV prefusion F protein-based maternal vaccine—preterm birth and other outcomes | Phase 3, double-blind, randomized, placebo-controlled clinical trial. ClinicalTrials.gov number: NCT04605159 | Subunit vaccine (RSVPreF3-Mat) based on the RSV F protein, funded by GlaxoSmithKline Biologicals | 5,328 pregnant women and 5,233 infants. A total of 3,426 infants in the vaccine group and 1,711 infants in the placebo group were followed from birth to 6 months of age | 6 months after birth | 65.5% efficacy against RSV-LRTI at 6 months (95% CrI, 37.5–82.0); 69.0% efficacy against severe RSV-LRTI (95% CrI, 33.0–87.6) |
| Banooni et al. [62] | Efficacy, immunogenicity, and safety of an investigational maternal respiratory syncytial virus prefusion F protein-based vaccine | Double-blind, randomized, placebo-controlled, phase 3 trial. ClinicalTrials.gov number: NCT04605159 | Subunit vaccine (RSVPreF3-Mat) based on the RSV F protein, funded by GlaxoSmithKline Biologicals | Women 18–49 years old were randomized 2:1 to receive one dose of RSVPreF3-Mat (n = 3,557) or placebo (n = 1,771) at 240/7–340/7 weeks’ gestation | 6 months after birth | 65.5% efficacy against MA-RSV-LRTD (95% CrI, 37.5–82.0); 69.0% against severe MA-RSV-LRTD (95% CrI, 33.0–87.6); 50.1% against hospitalization (95% CrI, –3.6–75.8); efficacy was higher in high-income (75.9%) vs. low-/middle-income (47.8%) countries; efficacy waned after 6 months |
RSV: respiratory syncytial virus; LRTI: lower respiratory tract infection; CI: confidence interval; CrI: credible interval; F: fusion
Across key clinical trials, maternal RSV vaccines have generally demonstrated favorable safety profiles, although some differences between vaccine platforms have emerged, particularly regarding pregnancy-specific outcomes.
In the MATISSE phase 3 trial, the RSVpreF vaccine showed no significant safety concerns in over 7,000 pregnant individuals, with comparable maternal and neonatal adverse event rates between vaccine and placebo groups. Injection-site pain was more common in vaccine recipients (41%) than in placebo recipients (10%). Systemic events were generally similar between groups, apart from slightly higher rates of muscle pain (27% vs. 17%) and headache (31% vs. 28%) in the vaccine group. The overall rate of adverse events within one month after vaccination was comparable (13.8% in vaccine vs. 13.1% in placebo). Among infants, adverse event rates within one month after birth were similar between groups (37.1% in vaccine vs. 34.5% in placebo), as were serious adverse events and medically attended events through 24 months of age. Preterm birth rates were comparable (0.8% in vaccine vs. 0.6% in placebo). Investigators reported a small number of serious maternal events as possibly vaccine-related, including cases of pre-eclampsia, premature labor, and eclampsia. One maternal death due to postpartum hemorrhage occurred in the vaccine group. Infant mortality rates were similar (0.1% in vaccine vs. 0.3% in placebo), with no vaccine-related deaths identified.
Similarly, the phase 2b study reported no unexpected maternal or infant safety issues. Injection-site pain, mostly mild to moderate, was the most common local reaction, reported more frequently in vaccine recipients with aluminum adjuvant. Redness, swelling, and mild fever were also observed only in the vaccine groups. Systemic reactions were generally mild, with muscle pain more frequent in vaccine recipients. Serious adverse events were uncommon and mainly related to pregnancy or delivery, with none attributed to vaccination. Among infants, adverse events within the first month were reported in 42.2% of cases, with similar rates between groups. Common events included neonatal jaundice, delivery complications, and minor infections. Preterm births occurred in 3.7% of infants, mostly near term. No infant events were vaccine-related. Serious adverse events and congenital anomalies were reported at similar rates across groups, with most anomalies mild and within expected background levels. Two RSV infections were identified, both in the placebo group.
The PREPARE trial also found no difference in maternal RSV infection rates between groups (4.9% in vaccine vs. 4.8% in placebo). Injection-site reactions, mostly mild, were more common among vaccine recipients (40.7% vs. 9.9%). Fever within 7 days occurred in 1.2% of vaccine recipients and 1.6% of placebo recipients, with similar rates of systemic reactions overall. Unsolicited adverse events, including adverse events of special interest and delivery outcomes, were comparable between groups. Among infants, the overall rates of common, serious, or protocol-defined adverse events were similar across groups. However, serious adverse events coded as pneumonia were less frequent in infants born to vaccine recipients (2.2%) than in those born to placebo recipients (4.5%) within the first year. In contrast, phase 3 trials of the RSVPreF3-Mat vaccine revealed a higher incidence of preterm birth in the vaccine group (6.8% [95% confidence interval (CI): 6.0–7.7] vs. 4.9% [95% CI: (4.0–6.1)]; RR, 1.37, 95% CI: 1.08–1.75) and a numerically higher rate of infant deaths [0.4% (95% CI: 0.2–0.6) vs. 0.2% (95% CI: 0–0.5); RR, 2.16, 95% CI: 0.62–7.57], particularly in LMICs.
A total of 17 studies assessing maternal and parental attitudes toward RSV vaccination were included, representing 13 countries and encompassing a combined sample of 14,959 participants (Table 2). The dataset spans a wide geographic and socio-economic range. The largest contributions came from China (n = 2,135), Australia (n = 1,992), and multiple cohorts from the US, totaling 5,168 participants. European countries, including France, Ireland, Italy, Greece, and the Netherlands, accounted for over 4,000 participants, while a minority of data came from LMICs: Jordan (n = 404), Nepal (n = 340), and Kenya (n = 24, Table 2).
Summary of selected records that assessed attitudes to maternal RSV vaccination
| Record | Title | Location | Sample size | Maternal/Parental RSV vaccine attitudes |
|---|---|---|---|---|
| Holland et al. [63] | Parental awareness and attitudes towards prevention of respiratory syncytial virus in infants and young children in Australia | Australia | 1,992 parents, pregnant/planning, and future parents | High acceptance of maternal RSV vaccination (79.3%) |
| McClymont et al. [64] | Acceptance and preference between respiratory syncytial virus vaccination during pregnancy and infant monoclonal antibody among pregnant and postpartum persons in Canada | Canada | 723 participants | Acceptance of RSV vaccination during pregnancy is estimated at 77% |
| Cubizolles et al. [65] | Evaluation of intentions to get vaccinated against influenza, COVID 19, pertussis, and to get a future vaccine against respiratory syncytial virus in pregnant women | France | 1,199 pregnant women | Intentions to get vaccinated are estimated at 39.4% |
| Damatopoulou et al. [66] | Prospective attitudes towards respiratory syncytial virus (RSV) vaccine in pregnant women in Greece | Greece | 335 pregnant females | Intention to vaccinate was linked to education, RSV awareness, school-age children, routine vaccine uptake, and previous uptake of COVID-19 vaccines |
| McCormack et al. [46] | Maternal awareness, acceptability, and willingness towards respiratory syncytial virus (RSV) vaccination during pregnancy in Ireland | Ireland | 528 women | 48.5% willing to receive RSV vaccination |
| Miraglia Del Giudice et al. [67] | Respiratory Syncytial Virus: Willingness towards a future vaccine among pregnant women in Italy | Italy | 490 women | 45.9% willing to be vaccinated during pregnancy |
| Wang et al. [68] | Investigating parental perceptions of respiratory syncytial virus (RSV) and attitudes to RSV vaccine in Jiangsu, China: Insights from a cross-section study | China | 2,135 parents of children aged ≤ 14 years old | 70.6% willing to vaccinate their child against RSV |
| Sallam et al. [47] | Attitude to RSV vaccination among a cohort of pregnant women in Jordan: A cross-sectional survey study | Jordan | 404 pregnant women | 77.5% willing to get the RSV vaccination |
| Limaye et al. [69] | RSV awareness, risk perception, causes, and terms: Perspectives of pregnant and lactating women in Kenya to inform demand generation efforts for maternal RSV vaccines | Kenya | 24 pregnant and lactating persons (qualitative study) | Key concerns centered on vaccine safety and side effects |
| Adhikari et al. [70] | Acceptance of new respiratory syncytial virus vaccine among pregnant women in Nepal for future routine immunization: A descriptive crosssectional study | Nepal | 340 pregnant women | 72.4% preferred maternal vaccination over vaccinating their children |
| Harteveld et al. [71] | Respiratory syncytial virus (RSV) prevention: Perception and willingness of expectant parents in the Netherlands | Netherlands | 1,001 pregnant women | 87% likely to accept both maternal and neonatal vaccination |
| Paulson et al. [72] | Protecting against respiratory syncytial virus: An online questionnaire study exploring UK parents’ acceptability of vaccination in pregnancy or monoclonal antibody administration for infants | UK | 1,620 participants | High acceptability (≥ 9/10) for both maternal vaccine and infant antibody if NHS-recommended |
| Beusterien et al. [73] | Healthcare providers’ and pregnant people’s preferences for a preventive to protect infants from serious illness due to respiratory syncytial virus | US | 992 pregnant people | 89.2% chose maternal vaccine or antibodies over no prevention |
| DeSilva et al. [74] | Pregnant persons perceptions and uptake of prenatal RSV vaccine - Minnesota, 2023-2024 | US | 455 pregnant persons participated | 65% intended to vaccinate; 51% received the RSV vaccine |
| Kuntz et al. [75] | Knowledge about respiratory syncytial virus and acceptance of infant monoclonal antibody for RSV and RSV vaccination during pregnancy | US | 1,082 respondents | 70% very likely to accept maternal RSV vaccine |
| Saper et al. [76] | RSV vaccination intention among people who are or plan to become pregnant | US | 1,528 individuals, 18 to 45 years, currently pregnant or planning | 54% very likely to receive RSV vaccine during pregnancy |
| Tucker et al. [77] | Acceptance of the respiratory syncytial virus vaccine in pregnant individuals | US | 111 individuals were eligible for maternal RSV vaccination and were offered the vaccine | 55.9% accepted the RSV vaccine when offered |
RSV: respiratory syncytial virus; COVID-19: coronavirus disease 2019; NHS: National Health Service in England
Across a wide range of geographic and demographic contexts, maternal RSV vaccine acceptance showed substantial variability (Figure 1). In high-income countries, reported willingness to receive maternal RSV vaccination ranged from moderate to high. In the US, several studies demonstrated generally favorable attitudes: 54–70% of respondents across multiple cohorts indicated they were “very likely” to accept the vaccine, with actual uptake among eligible participants reaching 51%. In Canada, 77% reported they would accept RSV vaccination during pregnancy. Similarly, high levels of acceptance were observed in the Netherlands (87%) and Australia (79%). In contrast, lower levels of expressed willingness were documented in parts of Western Europe. In Ireland, only 49% of women stated they would receive the vaccine, while a nearly equal proportion remained undecided. French and Italian participants reported even lower acceptance, at 39% and 46%, respectively. Studies from Jordan (78%), Nepal (72%), and China (71%) revealed strong support for maternal RSV vaccination. Qualitative findings from Kenya emphasized that concerns about safety—especially potential side effects—were central to decision-making regarding maternal RSV vaccination.
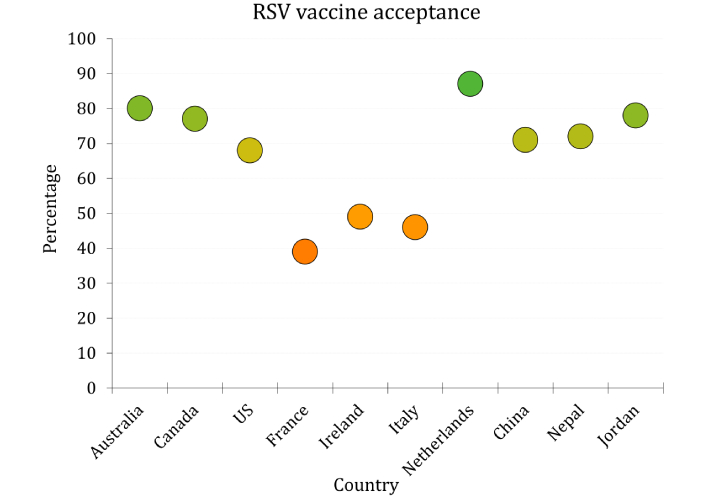
Rates of respiratory syncytial virus (RSV) vaccine acceptance per country based on 17 included records. Each bubble represents a country; bubble color reflects acceptance rate, using a gradient from red (0%) to green (100%) with orange indicating moderate acceptance (60%)
The burden of RSV on infant health, long documented and globally pervasive, represents one of the most persistent challenges in pediatric infectious disease prevention [4, 78–80]. In recent years, the long-sought goal of passive immunization through maternal vaccination—wherein transplacental antibody transfer shields the neonate during the early months of life, where infants are vulnerable—has advanced into a tangible clinical reality [37, 38, 40, 81]. This review, therefore, is intended to describe the currently approved maternal RSV vaccines; to assess the safety and efficacy data emerging from key clinical trials; and to appraise the attitudinal hurdles that are expected to condition the real-world success of maternal RSV immunization strategies.
As of May 2025, only one vaccine—Pfizer’s bivalent RSVpreF (marketed as Abrysvo)—passed the complex regulatory scrutiny to achieve licensure for use during pregnancy [38, 40]. Its approval, first in the US and then in the European Union, was based on the efficacy data generated by the MATISSE trial, which enrolled over 7,000 pregnant individuals across multiple continents [59]. This vaccine, comprised of RSV-A and RSV-B prefusion F antigens, targets the structural conformation most vulnerable to neutralizing antibody attack [82, 83]. Administered in the third trimester (32–36 weeks in the US; 24–36 weeks in the European countries), Abrysvo demonstrated 81.8% efficacy against severe RSV-associated LRTIs at 90 days post-partum and 69.4% at 180 days—a striking achievement given the historical elusiveness of RSV vaccine efficacy [84, 85]. Yet, it is essential to temper the maternal RSV vaccination triumph with critical scrutiny of the remaining challenges.
A critical area for future investigation is the durability of protection conferred by RSVpreF (Abrysvo), particularly its temporal trajectory beyond the first six months of life and the potential modulation of immunogenicity by gestational age at the time of administration [86, 87]. These questions bear directly on the optimization of antenatal vaccination schedules and the consistency of infant protection across populations. In contrast, the RSV F nanoparticle vaccine developed by Novavax, based on a post-fusion conformation of the F protein, did not achieve its prespecified efficacy endpoint [61, 88]. Meanwhile, other promising vaccines—such as GSK’s Arexvy and Moderna’s mResvia—have shown immunogenicity and efficacy in older adults but remain excluded from maternal indications. This lag reflects the need for further data on safety in pregnancy, maternal-fetal immune interactions, and neonatal outcomes [89].
Among pathogens considered for maternal immunization, RSV has remained a high-priority target given its significant burden on infant respiratory health in the early postnatal period, while persistently challenging efforts to meet the dual benchmarks of efficacy and safety required for implementation [84, 90]. The clinical trials included in the current review represent a critical advancement in that pursuit, especially in relation to efficacy profiles. The MATISSE phase 3 trial stands at the forefront, offering robust evidence for the efficacy of Pfizer’s RSVpreF (Abrysvo). Conducted across 18 countries, the trial demonstrated vaccine efficacy of 81.8% against medically attended RSV-associated LRTIs at 90 days, 69.4% at 180 days, and 57.1% against severe disease over the same interval—figures that support its global applicability and clinical value [59]. These findings were preceded by a phase 2b trial, which, while exploratory in design, provided early signals of protection and informed the final formulation and dosing strategy [60]. Although efficacy estimates in that study were derived from post hoc analyses, the consistency in immunogenic trends across both trials reflects the careful, iterative refinement of the vaccine candidate [60]. In contrast, another trial evaluating Novavax’s post-fusion F-protein nanoparticle vaccine did not meet its primary efficacy endpoint [61]. Secondary outcomes, including reductions in RSV-related hospitalizations and severe hypoxemia, suggested partial benefit, but overall protection was modest and variable [61]. These results emphasize the immunologic advantage of the prefusion F protein conformation, now widely recognized as a more effective target for neutralizing antibody responses transmittable via the placenta [91]. Emerging data from GSK’s RSVPreF3-Mat vaccine offer further insights [42]. Two large phase 3 trials reported efficacy ranging from 65.5% to 69.0% against RSV-LRTIs and their severe forms, with notably higher protection in high-income countries compared to LMICs settings [62]. These disparities likely reflect not only differences in healthcare infrastructure and baseline maternal immunity, but also broader structural challenges affecting vaccine delivery and effectiveness. Taken together, the evidence supports maternal RSV vaccination as a scientifically grounded and clinically promising approach that may play an increasingly important role in reducing the burden of severe RSV disease in early infancy. However, ongoing challenges related to optimizing efficacy and ensuring consistent protection across populations warrant further investigation.
An important aspect of this review was an overview of maternal RSV vaccine safety. The safety of any vaccine intended for maternal administration must be assessed with the utmost scrutiny, given its dual imperative to protect the mother without compromising fetal development and health [92–94]. In this context, the safety profile of RSVpreF (Abrysvo) has thus far been reassuring [38, 40, 59]. In the MATISSE phase 3 trial, which enrolled over 7,000 pregnant individuals, rates of maternal and neonatal adverse events were comparable between vaccine and placebo groups, with no signs indicative of increased risk [59]. These findings were consistent with earlier data from a phase 2b study, which similarly reported no concerning safety outcomes, thereby reinforcing confidence in the vaccine’s tolerability during pregnancy [60]. In contrast, data from GSK’s RSVPreF3-Mat vaccine trials warrant a more cautious interpretation [62]. Across two studies, a higher incidence of preterm birth was observed among vaccinated participants [42, 62]. The biological mechanism remains unclear, though the inclusion of an adjuvant in the RSVPreF3-Mat vaccine, maternal immune activation, and context-specific factors such as differing baseline obstetric risks or health system variability can be proposed and should be further investigated [95, 96]. Given the absence of a clear causal link, regulatory agencies have urged enhanced post-marketing surveillance to ensure maternal RSV vaccines meet the highest safety standards [97]. While these differences did not reach definitive statistical significance and causality has not been established, these observations highlight the critical need for rigorous, population-specific safety surveillance in maternal immunization [92]. They also highlight the unique standard to which maternal vaccines must be held—one in which benefit must be unequivocal and harm, even potential, stringently excluded before widespread adoption can be responsibly pursued [93].
While maternal RSV vaccination represents a scientific achievement, its success in public health practice hinges not solely on immunological performance, but on its acceptance and eventual uptake. Vaccine hesitancy—long recognized as a complex, multifactorial phenomenon—is particularly sensitive in the maternal context, where concerns extend beyond self to unborn child [98–101]. Here, perception becomes as influential as data, and the decision to vaccinate is shaped by risk calculation, cultural narratives, and institutional trust [102–104]. Increasingly, deliberate misinformation from unqualified sources flourishes unchecked, suggesting a governmental shortfall in counter-messaging [105, 106].
Quantitative and qualitative survey data across diverse regions present a mosaic of attitudes, ranging from enthusiastic acceptance to deep ambivalence. The role of healthcare providers is particularly decisive since their recommendation has been identified as an influential factor in maternal vaccine acceptance [107, 108]. This underlines the need for interventions including training in empathetic risk communication, standardized counseling tools, and decision aids tailored to maternal populations [106, 109]. Integration of vaccine counseling into routine antenatal care, rather than framing it as an optional or add-on service, could be a helpful approach to normalize maternal RSV vaccine uptake and reinforce institutional trust.
For example, a study in Jordan reported a commendably high willingness among pregnant women to receive maternal RSV vaccination (77.5%), with only 6.2% expressing hesitancy [47]. Similar optimism was observed in Nepal (72.4%) and Jiangsu Province, China, where 70.6% of parents stated they would vaccinate their children against RSV [68, 70]. These figures suggest an encouraging perspective regarding maternal RSV vaccine uptake, especially where maternal health campaigns have precedent and trust in public health remains intact. However, in Western Europe, the picture is more equivocal. For example, in Ireland, McCormack et al. [46] found that while 48.5% of respondents would accept vaccination, an almost equal proportion—45.8%—remained undecided. In France, Cubizolles et al. [65] reported only 39.4% of pregnant women intended to receive the RSV vaccine, and in Italy, Miraglia Del Giudice et al. [67] found willingness at just 45.9%. These data suggest a cautious stance, likely influenced by prior controversies surrounding maternal immunization [e.g., influenza and coronavirus disease 2019 (COVID-19) vaccines] and a lingering skepticism toward pharmaceutical interventions during pregnancy [110–112]. In North America, responses were more favorable, yet not universal.
The US-based survey studies by Beusterien et al. [73] (89.2% preference for preventive measures), Saper et al. [76] (54% “very likely” to accept vaccination), and DeSilva et al. [74] (65% intended to receive RSV vaccine) reflect a population with growing awareness, yet still vulnerable to misinformation and inconsistent provider messaging. In Canada, McClymont et al. [64] observed a relatively strong 77% acceptance rate. Crucially, qualitative studies highlighted that safety—rather than efficacy—is the major area of maternal concern [69, 107, 113]. In Kenya, Limaye et al. [69] documented that pregnant and lactating women prioritized side effect profiles and long-term infant outcomes in their decision-making. Similarly, in Greece, Damatopoulou et al. [66] found that vaccine acceptance correlated positively with education, previous vaccination behavior, and the presence of young children in the household. Collectively, these findings highlight that maternal RSV vaccine hesitancy is related to the socio-cultural aspects surrounding it. Future efforts must therefore couple the clinical assurance with tailored communication in relation to risk perception and potential benefits [114]. This communication should be conducted via trusted sources that integrate vaccine discussions into routine antenatal care and address the specific fears of pregnant women with empathy and scientific clarity [115].
In light of the compelling efficacy and acceptable safety profile of RSVpreF (Abrysvo), maternal RSV vaccination can now be considered a valuable component of antenatal care in regions where regulatory approval and distribution infrastructure permit [116, 117]. However, cost and affordability remain significant considerations for equitable global implementation. In high-income settings, the list price of a single Abrysvo dose is $221 [118], while a recent prospective costing study from Kenya estimated that delivering a maternal RSV vaccine via antenatal platforms would incur an economic cost of $6.60 per vaccinated woman, excluding the commodity price [119]. These estimates, though modest in delivery terms, highlight the importance of donor support and tiered pricing mechanisms to enable access in LMICs. Clinicians should be encouraged to offer the vaccine routinely to eligible pregnant individuals during the recommended gestational window, with emphasis on its protective value for the infant during the first six months of life—a period of high vulnerability and limited therapeutic options [120]. However, implementation must not be merely technical; it must be communicative and culturally appropriate [121], as recently reviewed systematically by Gavaruzzi et al. [54].
Based on the review findings, several priorities emerge. Longitudinal studies are needed to assess the durability of maternal antibody-derived protection, especially beyond six months of age. Further exploration into optimal timing within the gestational window could refine vaccine administration strategies. Additionally, global equity must not be an afterthought: implementation science should guide deployment in LMICs, where both the RSV burden and logistical challenges are greatest. Finally, new vaccine types such as mRNA-based maternal RSV vaccines deserve exploration, particularly for their scalability and potential to co-formulate protection against multiple neonatal pathogens. Importantly, the future of maternal RSV vaccination success will depend not only on scientific and technical aspects, but on the approach that can translate evidence into trust, access, and sustained impact.
At the same time, the RSV prevention domain is evolving with the introduction of long-acting monoclonal antibodies, such as nirsevimab, already approved for broad infant immunoprophylaxis, and clesrovimab, recently approved to prevent RSV-related LRTI during their first RSV season [33]. These monoclonal antibodies provide direct protection to infants shortly after birth, with extended half-lives allowing for season-long efficacy through a single dose [122, 123]. In contrast, maternal RSV vaccination confers passive immunity through transplacental antibody transfer during pregnancy, offering protection for both infants and mothers [37, 124]. Rather than representing competing approaches, maternal vaccination and monoclonal antibody immunoprophylaxis may play complementary roles in RSV prevention programs [34]. Maternal RSV vaccination may be prioritized in settings with high antenatal care coverage and strong vaccine uptake, while monoclonal antibodies could offer critical protection in regions with lower maternal vaccination rates or among high-risk infants, such as those born preterm or with underlying conditions. A combined strategy integrating maternal RSV vaccination with targeted monoclonal antibody use may offer optimal protection, especially in areas with high RSV burden and limited healthcare resources. Further research, including cost-effectiveness and implementation studies, is needed to identify the most effective and sustainable prevention approaches across diverse healthcare systems. A recent expert discussion by Parkinson and Chu [125] highlighted the practical differences between the two strategies. Both maternal RSV vaccination and monoclonal antibodies show similar efficacy in terms of reducing RSV hospitalizations by approximately 60% to 70%, but the two strategies differ in delivery and logistics [125]. Maternal RSV vaccination requires administration during a narrow window late in pregnancy, which may limit uptake, while monoclonal antibodies are given directly to infants after birth but face challenges related to cost and timely administration before hospital discharge [125].
Ultimately, the choice between these strategies will depend on local healthcare infrastructure, reimbursement policies, and parental acceptance, highlighting the need for flexible, context-specific approaches to RSV prevention among vulnerable infants [75, 126]. Taken together, the path forward requires a flexible, integrated approach to RSV prevention—one that aligns innovation with access, and science with social realities. Coordinated action among researchers, clinicians, policymakers, and manufacturers will be essential to ensure that both RSV maternal vaccines and monoclonal antibodies are deployed effectively, equitably, and sustainably to protect infants in their most vulnerable early months.
Despite adherence to the SANRA framework to enhance methodological transparency and rigor, several inherent limitations of this narrative review must be acknowledged. First, the non-systematic nature of narrative reviews introduces a risk of selection bias. Although the literature search was structured and focused, the absence of predefined eligibility criteria and formal study appraisal may have led to the overrepresentation of certain findings or underemphasis of opposing evidence. Second, the exclusive use of English-language sources and the limitation to two databases, PubMed/MEDLINE and Google Scholar, may have excluded relevant research published in other languages or indexed in other repositories, thereby limiting the global representativeness of the findings. This is particularly relevant when evaluating maternal RSV vaccine acceptance across diverse cultural contexts. Third, while the search strategy prioritized recency and relevance, it may have inadvertently excluded earlier foundational studies or grey literature, including health agency reports and conference proceedings, which could offer important context or data on implementation. Finally, the interpretive nature of narrative synthesis introduces subjectivity in data selection and thematic categorization. Although care was taken to ensure balanced reporting, the lack of quantitative meta-analysis restricts the capacity to draw definitive conclusions regarding comparative efficacy or safety profiles across RSV vaccine candidates. These methodological boundaries should be considered when interpreting the review’s findings.
Maternal RSV vaccination represents a notable advance in safeguarding infant health during the earliest and most vulnerable months of life. The approval of RSVpreF (Abrysvo), backed by compelling evidence of safety and efficacy, stands as a landmark achievement in the evolving landscape of perinatal immunization. However, as with any scientific milestone, the translation from clinical trial to real-world benefit demands sustained and multidisciplinary commitment. Continued research is essential to address the emerging clinical, logistical, and immunological challenges facing the rollout of maternal RSV vaccination. Priorities include longitudinal studies extending beyond the six-month window to assess the durability of protection, comparative effectiveness trials across high-income and LMICs to inform context-specific policy, and exploration of next-generation platforms such as mRNA-based maternal RSV vaccines, which may offer enhanced immunogenicity and manufacturing scalability. Equally important are implementation science efforts that investigate how best to integrate maternal RSV immunization into antenatal care pathways, particularly in LMIC settings. Success will ultimately depend not only on biological efficacy but on systemic readiness, provider engagement, and public trust. Clinicians, researchers, policymakers, and global health leaders must act in concert to close coverage gaps, strengthen delivery infrastructure, and communicate the life-saving potential of this intervention with clarity and compassion. With such coordinated resolve, maternal RSV vaccination may well become a cornerstone of equitable and effective neonatal care.
CFR: case-fatality rate
CI: confidence interval
COVID-19: coronavirus disease 2019
CrI: credible interval
EMA: European Medicines Agency
F: fusion
FDA: Food and Drug Administration
GBD: Global Burden of Disease
LMICs: low- and middle-income countries
LRTI: lower respiratory tract infection
NHS: National Health Service in England
PICUs: pediatric intensive care units
RR: relative risk
RSV: respiratory syncytial virus
SANRA: Scale for the Assessment of Narrative Review Articles
WHO: World Health Organization
Malik S: Conceptualization, Methodology, Formal analysis, Validation, Investigation, Resources, Data curation, Writing—original draft, Writing—review & editing, Visualization, Supervision, Project administration, Software. HN: Methodology, Formal analysis, Validation, Investigation, Data curation, Writing—review & editing. AAS: Methodology, Formal analysis, Validation, Investigation, Data curation, Writing—review & editing. Mohammed S: Conceptualization, Methodology, Formal analysis, Validation, Resources, Investigation, Data curation, Writing—review & editing, Supervision, Project administration, Software. All authors have read and agreed to the published version of the manuscript.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Cristiana Indolfi ... Michele Miraglia del Giudice
Sonila Borici ... Ilir Akshija
Alberto Vidal, Pedro Cortez
