Affiliation:
Department of Pathology, All India Institute of Medical Sciences (AIIMS), New Delhi- 110029, Delhi, India
ORCID: https://orcid.org/0000-0002-9594-6709
Affiliation:
Department of Pathology, All India Institute of Medical Sciences (AIIMS), New Delhi- 110029, Delhi, India
Email: rimdutt369@gmail.com; rimleedutta@aiims.edu
ORCID: https://orcid.org/0000-0002-4338-7781
Affiliation:
Department of Pathology, All India Institute of Medical Sciences (AIIMS), New Delhi- 110029, Delhi, India
Affiliation:
Department of Pathology, All India Institute of Medical Sciences (AIIMS), New Delhi- 110029, Delhi, India
Affiliation:
Department of Pathology, All India Institute of Medical Sciences (AIIMS), New Delhi- 110029, Delhi, India
Explor Asthma Allergy. 2025;3:100997 DOI: https://doi.org/10.37349/eaa.2025.100997
Received: July 12, 2025 Accepted: September 21, 2025 Published: November 06, 2025
Academic Editor: Eleonora Nucera, Catholic University of the Sacred Heart, Italy
The article belongs to the special issue Beyond Eosinophilic Gastrointestinal Diseases: Pathogenetic Mechanisms and Therapeutic Strategies
Aim: Eosinophilic gastrointestinal disorders (EGIDs) are chronic inflammatory conditions defined by eosinophilic infiltration of the gastrointestinal tract in the absence of secondary causes. This study aimed to synthesize current evidence on the clinical spectrum, pathogenesis, diagnostic criteria, and prognostic implications of EGIDs, including eosinophilic esophagitis, gastritis, duodenitis, ileitis, and colitis.
Methods: A retrospective, multi-source observational analysis of published clinical datasets on EGIDs was conducted. Systematic searches of PubMed, Scopus, Web of Science, and EMBASE identified eligible studies that included ≥ 10 patients with EGIDs and provided quantitative data on eosinophil counts, clinical features, endoscopic and histopathological findings. Articles reporting secondary causes of eosinophilia were excluded. Data extraction was done and independently verified by two reviewers. Special emphasis was placed on unresolved diagnostic hurdles, pediatric versus adult presentations, and long-term disease implications.
Results: A total of eligible datasets highlighted common molecular drivers, including epithelial barrier dysfunction, Th2-skewed immune responses, and genetic susceptibilities (e.g., TSLP, CAPN14). Core symptoms varied by site, with dysphagia predominating in eosinophilic esophagitis, and abdominal pain and diarrhea more frequent in distal EGIDs. Endoscopic findings included rings, furrows, and nodularity, while histology demonstrated patchy eosinophilic infiltration and epithelial damage with site-specific thresholds. Laboratory abnormalities included elevated eosinophil counts, IgE, and biochemical markers of malabsorption. Prognosis was variable, with frequent recurrence and heterogeneity in treatment response.
Conclusions: Significant knowledge gaps persist in EGID research and practice. Priority areas include establishing consensus-driven histological thresholds and developing non-invasive biomarkers for disease monitoring. Urgent unresolved questions involve the utility of biomarkers in guiding therapy, monitoring response and systematic evaluation of pediatric versus adult differences. Addressing these gaps will require multidisciplinary collaboration, standardized diagnostic protocols, and longitudinal multicenter studies to improve both clinical care and research consistency.
Eosinophils, first described by Paul Ehrlich in the late 19th century, are specialized granulocytes that play a multifaceted role in immune function and disease pathology. They have bilobed nuclei and granules containing cytotoxic proteins and inflammatory mediators, contributing to both defense and pathogenesis. Though a minor fraction of leukocytes (0–500 cells/µL), their numbers rise in allergies, parasitic infections, and inflammation [1, 2]. Beyond helminth immunity, eosinophils modulate bacterial and viral infections, tumor immunity, and tissue remodeling [3, 4]. They release cytokines (IL-4, IL-5, IL-13), chemokines (CCL5, CCL11), and lipid mediators that regulate immune responses [5]. As antigen-presenting cells, they integrate into adaptive immunity. Eosinophilic inflammation plays a key role in various conditions like asthma, chronic rhinosinusitis, eosinophilic gastrointestinal disorders (EGIDs), and hypereosinophilic syndromes [6, 7]. It leads to tissue damage, fibrosis, and remodeling, particularly in the gastrointestinal (GI) tract [8]. While eosinophils aid mucosal immunity, dysregulation contributes to conditions like eosinophilic esophagitis (EoE), gastritis, gastroenteritis, and colitis [9].
Biologic therapies targeting IL-5 (e.g., mepolizumab, benralizumab, reslizumab) have transformed treatment for eosinophil-driven diseases [10]. Despite advancements, eosinophils’ full physiological role remains unclear, as they exist in non-inflammatory states also in tissues like the GI tract, thymus, and uterus [11].
EGIDs clinically present as GI dysfunction and histopathologically constitute eosinophil-rich inflammation [12]. Eosinophils are naturally present in the gut and are densest in the terminal ileum and cecum [13]. Based on the segment of the GI tract involved, EGIDs have been variously termed as EoE, eosinophilic gastritis (EoG), eosinophilic duodenitis (EoD), eosinophilic jejunitis (EoJ), eosinophilic ileitis (EoI), eosinophilic colitis (EoC), or as eosinophilic gastroenteritis when the entire tract is involved. Amongst them, EoE is the most common (> 90%) [14].
Diagnosis is based on the correlation of clinical symptoms, imaging, and histopathological findings. Though chronic, EGIDs are manageable, with spontaneous remission in 40% of cases [15]. Treatment includes diet changes and corticosteroids, with delayed diagnosis increasing surgical risks.
Accurate diagnosis demands systematic exclusion of secondary causes of tissue eosinophilia—such as inflammatory bowel disease (IBD), parasitic infections, drug reactions, and vasculitis—through clinical, laboratory, and radiologic evaluation. Despite this growing burden, diagnostic thresholds remain inconsistent, molecular drivers and site-specific pathology are incompletely defined, pediatric-adult differences are underexplored, and long-term outcomes such as relapse and fibrosis remain poorly characterized.
EGIDs are increasingly recognized worldwide, with EoE now affecting an estimated 20–90 per 100,000 individuals in North America and Europe, while EoG and enteritis remain less common but are being diagnosed with growing frequency [15]. Regional differences suggest an interplay between genetic susceptibility and environmental exposures, with a higher prevalence in Western populations compared to Asia. In India, the true prevalence of EGIDs remains poorly defined due to limited population-based data. Hospital-based endoscopy studies report EoE in 3–6% of patients undergoing evaluation for dysphagia, with a male predominance and peak onset between the second and fourth decades of life [16]. Pediatric EGIDs are also increasingly recognized, though large-scale prevalence data are lacking. EGIDs frequently affect children and young adults, though demographic patterns vary by subtype. These epidemiological trends underscore the growing clinical and public health importance of EGIDs.
Several reviews have summarized the clinical and pathological features of EGIDs; however, most have focused primarily on EoE or provided broad overviews without systematically addressing diagnostic inconsistencies, site-specific pathology, or emerging molecular mechanisms. What distinguishes the present work is its emphasis on unresolved diagnostic challenges, critical appraisal of consensus criteria, and integration of recent insights into molecular drivers and histopathological variability across GI sites. This focus is particularly timely given the publication of updated international guidelines, the development of novel biologics targeting eosinophil pathways, and the increasing recognition of paediatric–adult differences in disease presentation and management. Accordingly, this manuscript not only consolidates current knowledge but also highlights unmet needs and outlines research priorities, thereby offering a forward-looking perspective to refine diagnosis and advance the field. This manuscript aims to address the gaps by synthesizing updated diagnostic criteria, integrating emerging molecular insights, and highlighting site-specific histopathological distinctions, thereby providing a framework for refining diagnosis and guiding future research.
This study is a retrospective, multi-source observational analysis of published clinical datasets on EGIDs. The primary objective was to characterize site-specific pathogenesis, clinical features, diagnosis, histopathologic features, and prognosis across EGID subtypes using standardized extraction from all published literature from 2000 to 2024. Earlier studies were excluded because consensus diagnostic criteria for EGIDs and standardized histopathological definitions only emerged in the early 2000s.
We conducted a structured literature search using PubMed, Scopus, Web of Science, and EMBASE up to the first quarter of 2025. The search strategy combined Boolean operators with MeSH terms to ensure both precision and comprehensiveness in retrieving relevant literature. Search terms included were: “eosinophilic gastrointestinal disorders”, “eosinophilic esophagitis”, “eosinophilic gastritis”, “eosinophilic duodenitis”, “eosinophilic jejunitis”, “eosinophilic ileitis”, “eosinophilic colitis”, “histopathology”, “diagnostic criteria”, “biomarkers”, and “molecular drivers”.
Articles were included if they:
Reported original data (not review or opinion pieces).
Included ≥ 10 patients with EGID (any subtype).
Provided quantitative information on eosinophil counts, clinical features, endoscopic, and histologic features.
Clearly reported diagnostic definitions and site-specific findings.
Articles were excluded if:
Only abstract data was available.
Non-English language (unless translation was available).
Cases were secondary eosinophilia from non-EGID etiologies (e.g., IBD, parasitic infections).
Two reviewers independently screened and selected articles, resolving conflicts by consensus or senior author adjudication.
Data were extracted using a standardized template, including: pathogenesis, symptoms, physical examination, laboratory tests, endoscopy, histology, and prognosis.
Data on genetic markers (e.g., TSLP, CAPN14), cytokine profiles (e.g., IL-5, eotaxin-3), and environmental triggers (e.g., food allergens) were extracted.
Core symptoms (e.g., dysphagia, abdominal pain, diarrhea) were harmonized by frequency and severity. Symptom patterns were stratified by EGID subtype.
Findings such as abdominal tenderness, growth delay, and malabsorption signs were standardized.
Peripheral eosinophil counts, IgE, C-reactive protein (CRP), iron, and albumin levels were prioritized, and inter-lab variations were documented.
Endoscopic findings (e.g., rings, furrows, nodularity) and histological data [eosinophils/high-power field (HPF), epithelial damage] were standardized per current consensus criteria. Prognostic variables—treatment response, recurrence, and complications—were mapped to ordinal outcome scales.
All extracted data were independently verified by a second reviewer.
Where available, site-specific eosinophil thresholds were normalized using a common per-HPF measurement. Histopathologic patterns were stratified by GI site. Studies with overlapping cohorts (e.g., same institution, years) were de-duplicated.
EoE is a chronic, immune-mediated disorder characterized by esophageal dysfunction and eosinophil-predominant inflammation (≥ 15 eosinophils per HPF) [12]. It is the most common EGID, with a rising incidence of 34 per 100,000 children and 42 per 100,000 adults [16, 17]. EoE predominantly affects males (3:1 ratio) and follows a bimodal age distribution, peaking in childhood and the third to fourth decades of life [18]. The esophagus is normally devoid of eosinophils, and their presence in the absence of other pathologies distinguishes EoE from conditions such as eosinophilic gastroenteritis, gastroesophageal reflux disease (GERD), and IBD [19].
EoE arises from genetic and environmental factors. Genetic risk (~20%) involves TSLP and CAPN14 gene variants driving Th2 inflammation and epithelial dysfunction. Environmental triggers (pet exposure, C-section, low H. pylori) support the hygiene hypothesis. Rising EoE prevalence in developed nations has been linked to sanitation, diet, and microbial exposure shifts. Seasonal allergens (e.g., Aspergillus) suggest airborne antigen involvement. EoE integrates Th2 cytokines (IL-5, IL-13), drivers of fibrogenesis (TGF-β, periostin), and epithelial barrier defects. Eosinophil degranulation releases cytotoxic proteins, causing tissue damage and motility issues.
EoE is diagnosed based on clinical semiology, endoscopic findings, and histological findings. Dysphagia is the most common symptom, though patients may describe it variably [12, 20]. Many unconsciously adapt by altering eating habits, leading to compensated dysphagia, which may only be revealed through detailed history-taking [21]. Other frequent symptoms include heartburn, chest pain, and epigastric discomfort [17, 22].
Physical examination is generally not useful for diagnosing EoE. However, since nearly 50% of patients have coexisting allergic conditions, findings indicative of bronchial asthma, allergic rhinitis, or atopic dermatitis may be present [12].
Laboratory testing has limited diagnostic value for EoE. Peripheral eosinophilia occurs in ~30% of patients but is typically mild and counts pertain to just above the 500 eosinophils/μL threshold [12, 18]. Serum IgE is often elevated, but no specific allergen IgE has been consistently identified [17]. Attempts to establish reliable blood biomarkers, such as eotaxin-3 and IL-5, have been unsuccessful due to poor correlation with disease severity [23]. The esophageal string test, which collects esophageal secretions, is being explored as a non-invasive diagnostic tool [24]. Emerging non-invasive diagnostic methods, such as Cytosponge for esophageal cell collection and Raman spectroscopy-based eosinophil peroxidase detection, are under investigation [25, 26].
Endoscopic findings in EoE include concentric rings (trachealization), linear furrows, white plaques, and strictures, present in 70% of cases, but they are not pathognomonic [12, 20]. Additional features, such as fragile “crepe paper” mucosa and tissue firmness (tug sign), may aid diagnosis [22]. However, 30% of patients have a normal-appearing esophagus, making multiple biopsies essential [17]. The EoE Endoscopic Reference Score (EREFS) standardizes evaluation, thereby improving diagnostic consistency [27]. Given the patchy nature of inflammation, at least 5–6 biopsies from different esophageal sites are recommended [28]. Strictures can be missed on endoscopy, highlighting its diagnostic limitations [29].
EoE is confirmed by identifying ≥ 15 eosinophils per HPF in esophageal mucosal biopsies (Figure 1). Due to patchy distribution, at least five samples from different regions are recommended for accurate diagnosis [21]. Eosinophil infiltration is typically denser in distal esophageal regions with the presence of white plaques or furrows [12, 20]. Additional histological features include eosinophil degranulation, eosinophilic microabscess, basal cell hyperplasia, spongiosis, dilated intercellular spaces, and papillary elongation [30, 31]. Eosinophil counts vary widely due to differences in biopsy sampling, staining methods, and HPF definitions. Inter-observer variability among pathologists further complicates borderline cases. Endoscopic practices also differ, with inconsistent biopsy numbers and sites affecting diagnostic yield. Standardized protocols and training are essential to improve diagnostic reproducibility across centers.
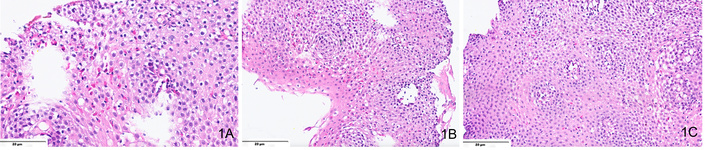
A microphotograph shows multiple features of eosinophilic esophagitis. Including (A) intraepithelial eosinophilia > 15 eosinophils in one high-powered field (H&E, ×400); (B) basal cell hyperplasia, with basal layer comprising > 15% of the thickness of the squamous epithelium (H&E, ×200), and (C) intercellular edema or “spongiosis” (H&E, ×200).
In EoE, 30–40% of untreated patients develop strictures. EoE requires long-term treatment to prevent fibrosis and strictures [32]. The association between EoE and Barrett’s esophagus, especially in GERD patients, is debatable [33]. Patients also have an elevated risk of other EGIDs, necessitating routine surveillance [34].
EoG is a rare, chronic inflammatory disorder characterized by eosinophilic infiltration of the gastric wall. The etiology is multifactorial and related to environmental triggers in genetically predisposed individuals.
The precise etiology of EoG remains unclear, but it involves genetic predisposition, immune dysregulation, and environmental triggers such as food allergens and infections. It is frequently associated with atopic disorders, including asthma, allergic rhinitis, and EoE, suggesting an underlying immunologic mechanism. EoG can affect the mucosal, muscular, or serosal layers of the stomach.
EoG symptoms range from mild nausea, vomiting, early satiety, and bloating to severe hematemesis, weight loss, and malnutrition, especially with extensive mucosal involvement. Non-responsiveness to proton pump inhibitors (PPIs) may suggest EoG. Antral or pyloric narrowing from inflammation or fibrosis can cause delayed gastric emptying. Coexistence with EoE or gastroenteritis complicates diagnosis. The mucosal form is associated with inflammation and ulceration, while the muscular form may cause gastric outlet obstruction due to hypertrophy. The serosal form is the rarest but can lead to ascites and peritoneal involvement [35].
Physical examination findings are generally nonspecific but may include epigastric tenderness, particularly in cases with active inflammation or ulceration. Signs of malnutrition, such as weight loss or hypoalbuminemia, may be present. Allergic manifestations, including eczema, asthma, or urticaria, can be seen in patients with a systemic atopic predisposition.
Laboratory findings support EoG suspicion but are not diagnostic. Peripheral eosinophilia (> 500 eosinophils/µL) occurs in ~50% of cases, often with elevated serum IgE in atopic patients. Hypoalbuminemia may indicate protein-losing enteropathy. Negative stool studies help exclude parasitic infections. CT/MRI may show gastric wall thickening, nodular mucosa, and mural stratification, but these findings overlap with Ménétrier disease and gastric lymphoma.
Endoscopic findings are often nonspecific and may include thickened gastric folds, erythema, friability, and nodularity. Pseudo polyps occur in nearly 25% of cases [36]. Mucosal ulcerations, particularly in the antrum, may lead to GI bleeding.
Histological examination of gastric mucosal biopsies is critical for diagnosis (Figure 2). Findings include eosinophilic infiltration (> 25–30 eosinophils per HPF), which is a commonly used diagnostic threshold [17]. There is crypt hyperplasia and mucosal edema, reflecting chronic inflammation. Fibrosis and smooth muscle hypertrophy are seen, particularly in the muscular form of the disease. As eosinophils can also be seen in other conditions (e.g., parasitic infections, IBD), diagnosis should be made in conjunction with clinical and endoscopic findings.
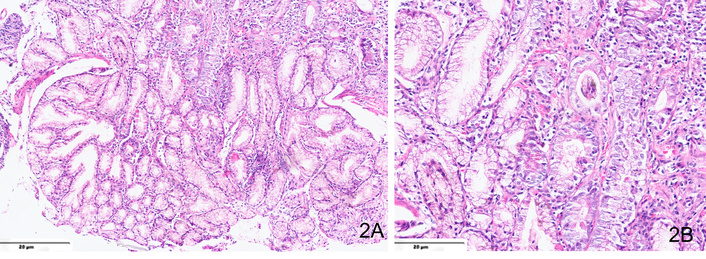
Hematoxylin and eosin-stained sections from a case of eosinophilic gastritis showing (A) moderate mixed inflammatory cell infiltrate in the lamina propria, comprising predominantly of neutrophils (×200) and (B) > 25–30 eosinophils per high-power field (×400).
EoG prognosis depends on severity and treatment response. While some achieve remission with dietary changes or corticosteroids, others develop chronic relapsing disease. Complications include gastric strictures, fibrosis, dysmotility, and protein-losing enteropathy, leading to hypoalbuminemia and edema. Rarely, severe, untreated cases may result in gastric perforation [34]. Early diagnosis and treatment with elimination diets, corticosteroids, or biologicals improve outcomes and prevent complications. EoG shows relapse rates above 50% after treatment discontinuation. Beyond structural complications, persistent symptoms and dietary limitations significantly impair quality of life, underscoring the need for long-term management strategies.
EoD is a chronic duodenal inflammation caused by eosinophilic infiltration, often triggered by hypersensitivity reactions to food antigens, allergens, or microbiota in genetically predisposed individuals. It is commonly linked to atopic disorders but can also occur independently.
The pathogenesis of EoD is incompletely understood, but it is thought to result from immune-mediated hypersensitivity reactions. Food allergens and environmental triggers induce an eosinophilic inflammatory response, leading to epithelial damage, smooth muscle dysfunction, and fibrosis. Patients with EoD frequently have comorbid atopic disorders such as asthma, food allergies, and atopic dermatitis, suggesting a systemic allergic component.
EoD causes nonspecific GI symptoms like pain, nausea, bloating, and diarrhea. Severe cases lead to weight loss, anemia, and protein-losing enteropathy. Extensive eosinophilic infiltration can cause strictures and motility issues. Associated with allergies, EoD affects mucosal (malabsorption, ulcers), muscular (fibrosis, obstruction), and serosal (inflammation, ascites) layers.
Physical examination in EoD is often nonspecific. Some patients may have epigastric or periumbilical tenderness, while severe cases show pallor, weight loss, edema, or muscle wasting from malabsorption. Rarely, palpable masses suggest fibrosis or strictures, and atopic signs may be present [37]. Diagnosis relies on endoscopic and histological evaluation.
Laboratory tests in EoD assess eosinophilia, allergic markers, and malabsorption, but again are not diagnostic. Peripheral eosinophilia (> 500 cells/μL) may be present but does not always reflect severity [17]. Elevated IgE suggests an allergic component, while normal/mildly elevated CRP and erythrocyte sedimentation rate (ESR) help distinguish EoD from Crohn’s disease. Hypoalbuminemia, anemia, and vitamin deficiencies indicate malabsorption. Low fecal calprotectin differentiates EoD from IBD [37]. Diagnosis requires histological confirmation via duodenal biopsy.
Endoscopic findings in EoD are often nonspecific and may include mucosal erythema, edema, nodularity, granularity, white exudates, and erosions, while severe cases can present with ulcerations, mucosal atrophy, or strictures. However, the duodenal mucosa may appear normal in some patients, making multiple biopsies essential for diagnosis [38].
Since EoD lacks specific clinical and endoscopic features, histological examination of duodenal biopsies is essential for diagnosis. However, no universally accepted eosinophil threshold exists for defining EoD. Histological confirmation requires ≥ 20 eosinophils per HPF, often accompanied by crypt abscesses, degranulation, and villous atrophy (Figure 3). Submucosal or muscular involvement is seen in refractory cases. Since EoD can mimic celiac disease, Crohn’s disease, and H. pylori-associated duodenitis, biopsies from multiple segments of the duodenum are necessary for accurate assessment and elimination of other differential diagnoses [37, 39].
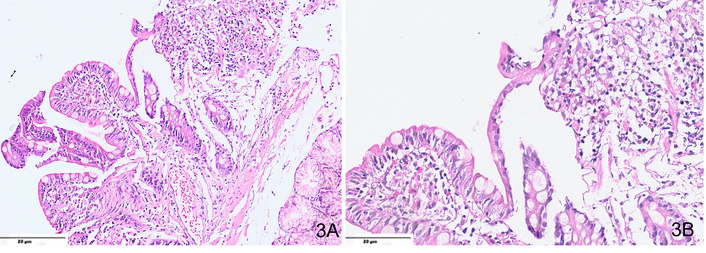
Microphotograph from a case of EoD, showing (A) mild villous atrophy and crypt hyperplasia (H&E, ×200); (B) ≥ 20–25 eosinophils per high-power field, along with the presence of degranulation (H&E, ×400).
EoD prognosis depends on severity and treatment response. Some achieve remission with dietary changes or steroids, while others develop chronic or relapsing disease requiring long-term therapy [39]. Early diagnosis and treatment help prevent complications like fibrosis, strictures, and malabsorption.
EoJ is a rare disorder marked by chronic eosinophilic infiltration and inflammation of the jejunum. It mimics IBDs like Crohn’s disease but differs in pathogenesis and treatment. The disease follows a relapsing-remitting course, with symptoms varying based on the depth and extent of intestinal involvement. While primarily affecting the small intestine and stomach, it can involve any part of the GI tract. Eosinophils contribute directly to tissue damage, worsening inflammation and injury [39].
EoJ is classified as a subtype of EGID, an immune-mediated disorder driven by an exaggerated Th2-type immune response. The infiltration of eosinophils into the jejunal mucosa is often triggered by food allergens in genetically predisposed individuals, leading to chronic inflammation, tissue remodeling, and epithelial barrier dysfunction. The absence of infectious or autoimmune triggers suggests a primary dysregulation of mucosal immunity, with increased intestinal permeability allowing allergens to provoke an inappropriate immune response [40].
EoJ is diagnosed by symptoms, eosinophilic infiltration, and exclusion of systemic causes. Peripheral eosinophilia is absent in ~20%, complicating diagnosis. Symptoms mimic inflammatory or obstructive disorders. EoJ can be classified into mucosal (malabsorption, bleeding), muscularis (obstruction, cramping), and serosal (ascites, eosinophilia) forms as in EoG [40].
Despite advances in diagnostic techniques, EoJ remains a largely retrospective and histopathological diagnosis. Laboratory tests, including complete blood counts and stool analysis, may not always confirm the disease. In cases with ascites, infectious cultures, cell counts, and cytological analysis are necessary to exclude other causes. While no universally accepted diagnostic criterion exists for eosinophilic ascites, eosinophil counts as high as 88% have been reported in some cases [40–42].
Physical examination in patients with EoJ typically reveals diffuse abdominal tenderness without guarding or rebound tenderness. Bowel sounds are usually present in all quadrants, and mild abdominal distension may be observed, depending on the severity of the disease. However, vital signs are generally normal, making clinical detection difficult in the absence of further diagnostic workup.
Endoscopic findings in EoJ are often nonspecific, with common features including erythema, erosions, and ulcerations. Upper GI endoscopy with biopsy confirms the diagnosis in approximately 80% of cases involving the mucosal layer [40]. However, in patients with deeper muscular or serosal involvement, endoscopic biopsies may appear normal, necessitating a laparoscopic full-thickness biopsy for definitive diagnosis [41].
Histological analysis plays a critical role in diagnosis (Figure 4). While eosinophils are present throughout the GI tract (except the esophagus) under normal conditions, significant eosinophilic infiltration in the jejunum suggests EoJ. No universally accepted eosinophil threshold per HPF exists for diagnosis, though some studies suggest that counts exceeding 100 eosinophils per HPF are indicative of disease [42]. Variability in biopsy interpretation underscores the importance of expert pathological opinion.
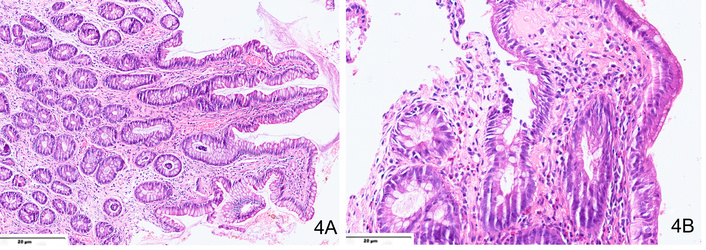
Hematoxylin and eosin-stained sections from a case of EoJ showing (A) significant eosinophilic infiltration in the lamina propria (×200) with (B) presence of eosinophilic abscesses at places (×400).
The prognosis for EoJ is generally favorable, with most patients responding well to dietary modifications and corticosteroid therapy. However, chronic cases may require long-term management with immunosuppressive agents to prevent recurrent inflammation and complications. Severe cases involving intestinal obstruction or perforation may necessitate surgical intervention.
EoI is a rare form of EGID, characterized by abnormal eosinophilic infiltration of the ileal wall. The exact cause remains unclear, but a combination of various factors like genetic predisposition, immune dysregulation, and environmental triggers (e.g., allergens, infections, or dietary antigens) is suspected. Interleukin-5 (IL-5) plays a key role in eosinophil recruitment, and increased levels of eotaxins, while IL-13 and IL-4 contribute to chronic inflammation and tissue damage [43, 44].
EoI involves excessive eosinophil infiltration of the ileum, causing chronic inflammation and tissue damage. It arises from an exaggerated Th2 response to allergens, environmental factors, or microbiota in genetically predisposed individuals. Elevated IL-5 and eotaxin-3 drive eosinophil recruitment, leading to barrier disruption, fibrosis, and motility issues. Depending on the affected layer, it can cause malabsorption (mucosal), strictures (muscular), or eosinophilic ascites (serosal). The absence of infectious or autoimmune causes classifies it as a primary immune-mediated disorder.
EoI presents with nonspecific GI symptoms, often mimicking Crohn’s disease. These include abdominal pain (commonly in the right lower quadrant), diarrhea, nausea, bloating, and weight loss [45]. Severe cases may lead to intestinal obstruction, perforation, or strictures due to transmural inflammation and fibrosis.
Findings vary depending on disease severity. Many patients appear normal, but in symptomatic cases, abdominal tenderness (often in the right lower quadrant), distension, and signs of malnutrition (weight loss, pallor, edema from protein-losing enteropathy) may be noted. In severe cases, palpable masses may indicate fibrosis, stricture formation, or thickened bowel loops.
Peripheral eosinophilia is present in about 50% of cases but is not diagnostic [46]. Elevated serum IgE may indicate an allergic component. Mildly elevated CRP and ESR can help differentiate EoI from Crohn’s disease, which typically shows a stronger inflammatory response. Hypoalbuminemia and iron-deficiency anemia suggest malabsorption and chronic inflammation. Fecal calprotectin is typically low, unlike in IBD [47, 48].
Endoscopic findings in EoI are nonspecific and may include ileal mucosal erythema, edema, friability, nodularity, or erosions. Ulcerations or strictures may occur in severe cases. White exudates or thickened folds, resembling eosinophilic gastroenteritis, may be present [46, 47]. Since endoscopic abnormalities may be absent, multiple biopsies are crucial for diagnosis.
Histology confirms the diagnosis with increased eosinophilic infiltration (> 30 eosinophils per HPF) in the ileal mucosa, muscularis, or submucosa (Figure 5). Other features include crypt abscesses, eosinophilic degranulation, and fibrosis, which may contribute to stricture formation [47–49].
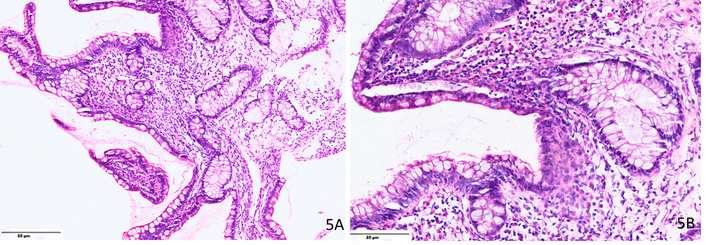
Microphotograph from a case of eosinophilic ileitis showing (A) significant eosinophilic infiltration in the lamina propria (H&E, ×200); (B) shows > 50 eosinophils per high power field in the ileal mucosa (H&E, ×400).
EoI shows relapse rates above 50% after treatment discontinuation. The prognosis varies based on severity and response to treatment. Mild cases may resolve spontaneously or with dietary modifications, while chronic cases require steroids, immunomodulators, or biologicals. Severe cases with strictures, perforation, or obstruction may need surgical intervention. Early diagnosis and management are key to preventing complications.
EoC is a pathological entity associated with abnormal infiltration of colonic mucosa by eosinophils. It is a rare form of primary eosinophilic GI disease with a bimodal peak of prevalence in neonates and young adults. EC remains a vaguely understood condition in contrast to the increasingly recognized EoE. EoC may be primary, with no known etiology of eosinophilic infiltration, or secondary to an identified cause.
The exact cause is unclear, though ~75% of patients have a history of allergy or atopy [27]. Cow’s milk and soy proteins are common triggers in infants, but cases have also occurred in exclusively breastfed infants. In adults, the role of IgE is debated, with some studies suggesting mast cell involvement while others indicate a non-IgE-mediated mechanism via STAT6-dependent T-cell activation [48]. Eotaxins and IL-5 contribute to eosinophil recruitment. Experimental models suggest GI exposure leading to widespread disease, whereas lung sensitization may cause isolated esophageal involvement [49, 50].
Symptoms depend on the affected intestinal layer: mucosal involvement leads to malabsorption, diarrhea, and protein-losing enteropathy; transmural disease causes colonic wall thickening and obstruction; serosal involvement manifests as eosinophilic ascites (up to 95% eosinophils). Acute cases may present with intestinal obstruction, cecal volvulus, intussusception, or perforation.
Clinical findings vary by severity. Infants may have distension and irritability, while older patients show abdominal tenderness. Severe cases present with dehydration, weight loss, and malnutrition. Rarely, fibrosis causes palpable masses. As symptoms often overlap with other GI diseases, the gold standard diagnostic modality remains biopsy evaluation.
Diagnosing EoC requires both clinical and histological confirmation, ruling out other causes of colonic eosinophilia like infections or IBD. Peripheral eosinophilia (> 500 cells/µL) is absent in ~20% of cases, and stool tests help exclude parasitic infections. Allergic skin tests (AST) and radioallergosorbent test (RAST) can detect IgE-mediated allergies but lack sensitivity [51–54]. CT findings in children often show predominant involvement of the cecum and ascending colon, with minimal small bowel involvement. In adults, nonspecific CT findings in 60–70% of cases include colonic wall thickening, nodularity, and the “araneid-limb-like” sign due to diffuse mucosal thickening [46, 55]. These findings must be differentiated from inflammatory and infectious colitis.
The colonic mucosa is endoscopically normal in about 70% of cases [55]. In a retrospective series encompassing a study of 37 patients with EoC, mucosal involvement was noted in 33% of cases, most of which were segmental, while pancolitis was observed in only 11% of cases. The right and left hemi-colon were the most commonly affected (44% of cases). Mucosal lesions were non-specific: erythema in 88% of cases, edema with a decrease in mucosal vascularization in 50% of cases, erosions or even ulcerations in 63% and 50% of cases, respectively [56].
EoC lacks standardized diagnostic criteria, with interobserver variability complicating histological assessment. A threshold of > 40 eosinophils per HPF in at least two colonic segments is suggested (Figures 6, 7), though sensitivity (60%) and specificity (50%) remain low. Histological features include lamina propria eosinophilic infiltrates, intraepithelial eosinophils (76%), eosinophilic microabscesses (21%), submucosal involvement (6.6%), and degranulation (51%) [57]. A standardized protocol should assess eosinophil count, distribution pattern, presence of activation markers, fibrosis, and inflammatory changes.
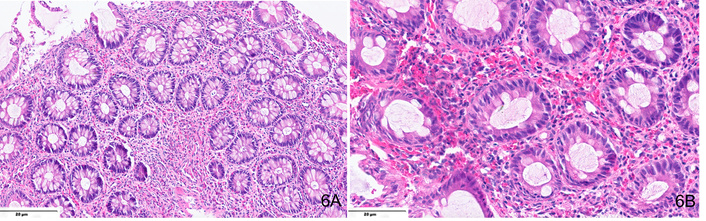
H&E-stained sections from a transverse colon mucosal biopsy in a case of EoC showing dense mixed inflammatory cell infiltrate enriched in eosinophils in the lamina propria (A, ×400), including > 80 eosinophils per high-power field and presence of eosinophilic microabscesses at places (B, ×400).
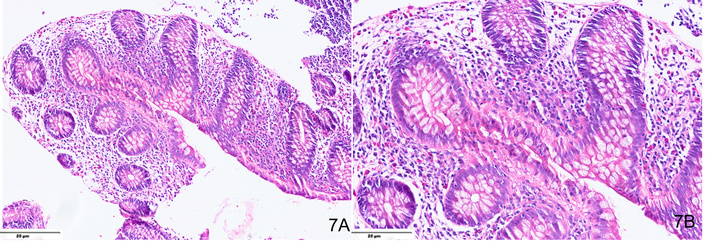
H&E-stained sections from a case of eosinophilic proctitis showing numerous eosinophils (> 60) in the lamina propria (A, ×200), including intra-epithelial eosinophils at places (B, ×400).
EoC in infancy generally has a favorable prognosis, often resolving spontaneously within days. Over time, affected infants may even tolerate previously implicated foods. In contrast, EoC in young adults tends to follow a chronic course, characterized by alternating periods of activity and remission [58, 59]. EGIDs represent a spectrum of chronic, immune-mediated conditions characterized by dysregulated Th2 responses triggered by genetic susceptibility factors such as TSLP and CAPN14, in concert with environmental exposures including food allergens and microbes. Central cytokines—IL-5, IL-13, and eotaxins—mediate eosinophil recruitment, driving tissue injury and remodeling. Clinically, EGIDs frequently coexist with atopic diseases, with nearly three-quarters of patients reporting asthma, allergic rhinitis, or eczema. Among subtypes, EoE predominates, accounting for more than 90% of cases. Symptoms and complications vary by anatomic site and depth of involvement, ranging from mucosal inflammation to transmural or serosal disease. Endoscopic findings are often subtle or nonspecific, and peripheral eosinophilia is absent in up to half of patients, limiting the diagnostic utility of blood tests. Currently, histopathology remains the gold standard, though biopsy thresholds for defining eosinophilia differ across GI segments. Management typically begins with dietary elimination and corticosteroids, with biologics such as anti-IL-5 agents (mepolizumab, benralizumab) reserved for refractory cases, and surgical intervention indicated only for complications like obstruction or perforation. Prognosis is variable, spanning spontaneous resolution to the need for chronic maintenance therapy. High clinical suspicion remains essential, particularly in patients with persistent GI symptoms and an atopic background despite normal endoscopic appearances. Because EGIDs remain diagnoses of exclusion, systematic evaluation—including targeted laboratory tests, parasite and H. pylori screening, celiac serologies, and careful elimination of mimics such as GERD and IBD—together with biopsy-based confirmation, is critical to ensuring accurate diagnosis and appropriate management.
A brief summary of the various subtypes of eosinophilic GI diseases, including clinical semiology, endoscopic findings, diagnostic features, key differential diagnoses with distinguishing features, and management protocols, has been tabulated in Table 1. A comprehensive overview of their reported long-term outcomes is provided in Table 2.
Features of EGIDs.
| EGID type | Diagnostic threshold (eosinophils/HPF) | Clinical semiology | Endoscopic findings | Key differential diagnoses & distinguishing features | Management protocol |
|---|---|---|---|---|---|
| EoE (esophagitis) | ≥ 15 (Normally absent) | Dysphagia, food impaction | Rings, furrows, white exudates (30% normal) |
| Topical steroids, diet elimination, and anti-IL-5 biologics |
| EoG (gastritis) | > 25–30 | Early satiety, vomiting, bloating | Thickened folds, antral ulcers, pseudo polyps (nonspecific) |
| Corticosteroids, allergen-free diet |
| EoD (duodenitis) | ≥ 20 | Malabsorption, bloating, and anemia | Mucosal erythema, edema, nodularity, erosions (often normal) |
| Steroids, elemental diet |
| EoJ (jejunitis) | > 100 (Suggested) | Obstruction, ascites | Mucosal friability (deep disease: normal) |
| Immunosuppressants, surgery in case of obstruction |
| EoI (Ileitis) | > 30 | Right lower quadrant pain, diarrhea | Mucosal erythema, edema, friability, nodularity, strictures |
| Biologics, stricturoplasty |
| EoC (Colitis) | > 40 (Low specificity) | Diarrhea, malabsorption, eosinophilic ascites | Often normal (70%); patchy erythema |
| Infant: watchful waiting. Adult: steroids |
EGIDs: eosinophilic gastrointestinal disorders; HPF: high-power field; GERD: gastroesophageal reflux disease; PPI: proton pump inhibitor.
Reported long-term outcomes in EGIDs.
| EGID subtype | Long-term complications | Relapse risk | Impact on quality of life |
|---|---|---|---|
| Eosinophilic esophagitis (EoE) | Fibrostenosis and strictures in 30–40% of untreated patients; food impaction risk | High relapse is common after treatment withdrawal | Dysphagia, dietary restrictions, impaired social functioning |
| Eosinophilic gastritis | Gastric wall thickening, protein-losing enteropathy (rare) | 50% recurrence after corticosteroid tapering | Chronic abdominal pain, nausea, nutritional compromise |
| Eosinophilic enteritis | Malabsorption, anemia, and occasional strictures | Frequent relapse, especially after drug cessation | Fatigue, weight loss, reduced work capacity |
| Eosinophilic colitis | Chronic diarrhea, occasional bleeding; less risk of strictures | Relapsing-remitting course in many patients | Impact on daily activities, anxiety related to chronic symptoms |
EGIDs: eosinophilic gastrointestinal disorders.
EGIDs represent a growing clinical challenge, with expanding recognition across both paediatric and adult populations. This manuscript highlights the major diagnostic hurdles, including inconsistent histological thresholds, variable endoscopic findings, and the limited utility of peripheral eosinophilia in clinical practice. Site-specific histopathological features and emerging molecular insights provide important clues for disease classification but remain incompletely validated.
From a clinical perspective, the lack of standardized diagnostic protocols and validated biomarkers continues to impede timely recognition and effective management. Paediatric–adult differences in presentation, disease course, and response to therapy emphasize the need for tailored approaches. Long-term complications, including strictures, fibrosis, and relapse, further underscore the impact of EGIDs on patient quality of life and health care utilization.
Significant gaps in knowledge persist. Priority areas for research include multicentre efforts to validate eosinophil thresholds across subtypes, the development of non-invasive biomarkers for disease monitoring, and systematic comparisons of paediatric and adult disease phenotypes. Standardization of histopathological criteria and consensus-driven diagnostic algorithms (Figure 8) is essential to reduce variability and improve reproducibility in real-world settings.
In conclusion, EGIDs represent an evolving spectrum of disorders that require multidisciplinary collaboration and methodological rigor to advance the field. Establishing standardized diagnostic thresholds and developing reliable biomarkers should be considered the most urgent next steps. A clear research agenda focused on these priorities will be critical to improving outcomes, reducing diagnostic delays, and guiding precision-based therapeutic strategies.
AST: allergic skin tests
CRP: C-reactive protein
EGIDs: eosinophilic gastrointestinal disorders
EoC: eosinophilic colitis
EoD: eosinophilic duodenitis
EoE: eosinophilic esophagitis
EoG: eosinophilic gastritis
EoI: eosinophilic ileitis
EoJ: eosinophilic Jejunitis
EoP: eosinophilic proctitis
ESR: erythrocyte sedimentation rate
GERD: gastroesophageal reflux disease
GI: gastrointestinal
HPF: high-power field
IBD: inflammatory bowel disease
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/100997_sup_1.pdf.
NN: Investigation, Writing—original draft. RD: Conceptualization, Investigation, Writing—review & editing. LM: Writing—review & editing. RY: Writing—review & editing. PD: Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Ethical review was waived by the local ethics committee since this study was a secondary analysis of published anonymized data and a retrospective analysis of anonymized histopathology slides available in the departmental records.
Informed consent to participate was obtained from relevant participants.
Not applicable.
Relevant data may be available from the corresponding author upon reasonable request.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3422
Download: 44
Times Cited: 0
Adrianna Wierzbicka, Andrew Ukleja
