Affiliation:
1Department of Medicine, Kingston Health Sciences Center, Queen’s University, Kingston, ON K7L2V7, Canada
ORCID: https://orcid.org/0009-0000-8374-5355
Affiliation:
2Department of Medicine, McGill University Health Center (MUHC), McGill University, Montreal, QC H4A 3S9, Canada
3Department of Medicine, Lausanne University Hospital, Lausanne University, Lausanne, VD CH-1011, Switzerland
4The Research Institute of the McGill University Health Center, McGill University Health Center (MUHC), McGill University, Montreal, QC H4A 3J1, Canada
ORCID: https://orcid.org/0000-0003-1687-7811
Affiliation:
2Department of Medicine, McGill University Health Center (MUHC), McGill University, Montreal, QC H4A 3S9, Canada
4The Research Institute of the McGill University Health Center, McGill University Health Center (MUHC), McGill University, Montreal, QC H4A 3J1, Canada
5Department of Infectious Diseases, The Peter Doherty Institute for Infection and Immunity, University of Melbourne, Melbourne, VIC 3000, Australia
ORCID: https://orcid.org/0000-0002-9183-5032
Affiliation:
2Department of Medicine, McGill University Health Center (MUHC), McGill University, Montreal, QC H4A 3S9, Canada
4The Research Institute of the McGill University Health Center, McGill University Health Center (MUHC), McGill University, Montreal, QC H4A 3J1, Canada
Email: ghislaine.isabwe@mcgill.ca
ORCID: https://orcid.org/0000-0002-5557-2415
Explor Asthma Allergy. 2025;3:100989 DOI: https://doi.org/10.37349/eaa.2025.100989
Received: March 26, 2025 Accepted: July 09, 2025 Published: August 05, 2025
Academic Editor: Cristiano Caruso, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Italy
Hypersensitivity reactions (HSRs) to paclitaxel, particularly those mediated by the solubilizer Cremophor® EL, are common, occurring in approximately 10% of patients despite premedication. Nab-paclitaxel, a newer formulation using human serum albumin as the vehicle, is generally considered a safer alternative due to a lower rate of HSRs. We present the case of a 44-year-old woman with breast cancer who developed severe HSRs following multiple doses of paclitaxel and carboplatin. Despite standard premedication, she experienced fever, erythematous skin eruptions, arthralgias, and systemic symptoms following her fourth and fifth cycles of treatment. Subsequent administration of nab-paclitaxel also elicited a similar severe reaction. Skin testing revealed a positive reaction to paclitaxel, but not to carboplatin, suggesting sensitization to paclitaxel. In the context of the similar reaction to nab-paclitaxel, this suggests sensitization to the taxane moiety itself rather than to the solubilizer. The combination of features consistent with both type IV hypersensitivity and cytokine release syndrome further complicates the presentation as well. To our knowledge, this is the first reported case of cross-reactivity between paclitaxel and nab-paclitaxel, challenging the assumption that nab-paclitaxel is always a safe alternative. This emphasizes the need for vigilance and thorough evaluation in patients experiencing atypical chemotherapy reactions, as cytokine release reactions may play a role even in the absence of immunotherapy. It also raises the concern that alternative formulations like nab-paclitaxel may not always be safe in patients with atypical or severe reactions, as they could possibly be sensitized to the taxane moiety itself.
Paclitaxel is an anti-neoplastic agent, originally isolated from the Pacific yew tree (Taxus brevifolia), used to treat multiple cancers, including breast, ovarian, and non-small cell lung cancer. The regular formulation associates paclitaxel with the solubilizer Cremophor® EL (polyoxyethylene glycerol triricinoleate 35). Immediate hypersensitivity reactions (HSRs) to regular paclitaxel formulations are common, occurring in around 10% of patients despite premedication with steroids and/or antihistamines [1, 2]. Nab-paclitaxel (nanoparticle albumin-bound paclitaxel) is a more recent formulation that uses human serum albumin as the vehicle instead. As opposed to the regular paclitaxel formulation, it does not require premedication, as it is associated with a lower rate of immediate HSRs [2]. Hence, in immediate HSRs to regular paclitaxel, patients can usually be safely switched to nab-paclitaxel.
We report an unusual case of paclitaxel hypersensitivity. The patient is a 44-year-old woman who had a history of right-sided stage III breast cancer and no other significant past medical or atopic history. She started weekly chemotherapy treatments with paclitaxel, carboplatin (on Day 1, 8, 15), and immunotherapy with pembrolizumab every three weeks (on Day 1). The patient was premedicated before receiving treatment with dexamethasone 10 mg intravenous (IV), famotidine 20 mg IV, diphenhydramine 25 mg IV, and ondansetron 16 mg per os (PO). She received pembrolizumab followed by paclitaxel, then carboplatin on Day 1 of the cycle. She received paclitaxel followed by carboplatin on Days 8 and 15. She took dexamethasone 8 mg PO for two days following each treatment. Her first three doses of chemotherapy were uneventful.
After her fourth dose of paclitaxel, carboplatin, and the second dose of pembrolizumab in April 2023, she developed a fever of 38.3°C, chills, and an erythematous macular skin eruption on her entire torso within two to three hours of receiving the chemotherapy (Figure 1). Her symptoms were mild and resolved within 24 h with oral acetaminophen and antihistamines.
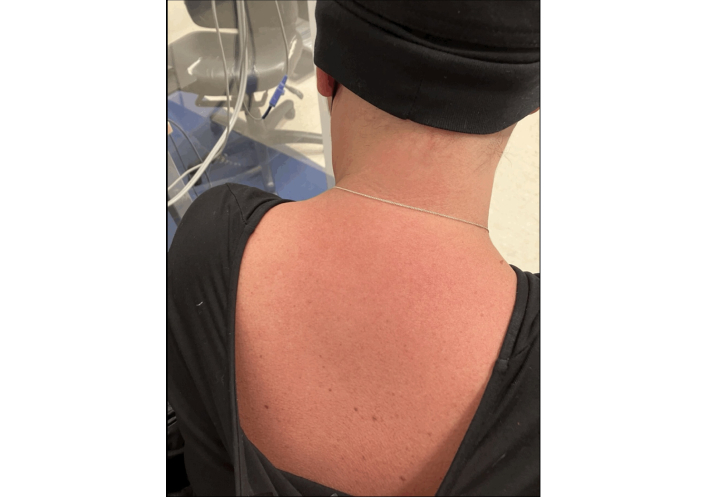
Macular rash appeared on the patient’s torso two to three hours after receiving her fourth dose of paclitaxel and carboplatin
After her fifth dose of paclitaxel and carboplatin, without receiving pembrolizumab, she had a similar reaction two to three hours after her chemotherapy infusion. Her symptoms were worse at this point, including a fever of 39.6°C, erythematous diffuse patchy skin eruption over her upper chest wall with facial flushing, arthralgia, tachycardia (up to 125 bpm), and hypotension (91/54 mmHg). She received IV fluids, oral cetirizine, oral dexamethasone, oral acetaminophen, and one dose of IV ceftriaxone in the emergency department. Her symptoms resolved 24 to 30 h later. She once again developed similar symptoms after her sixth dose of paclitaxel and carboplatin. At this point, the oncology team decided to switch her paclitaxel to nab-paclitaxel.
She started her third cycle with nab-paclitaxel (1st dose), carboplatin (7th dose), and pembrolizumab (3rd dose). Within three hours, she developed a fever of 39.0°C, facial edema, chest tightness, and hypotension (83/43 mmHg). She required hospital admission for monitoring. She improved with IV fluids and IV antihistamines and was discharged 2 days later. However, a few days later, she required re-hospitalization for acute hepatitis secondary to pembrolizumab, which was treated with IV solumedrol followed by a PO prednisone taper. Following these two hospitalizations, her previous chemotherapy regimen was discontinued, and she was switched to doxorubicin and cyclophosphamide (Figure 2).
We conducted skin prick and intradermal testing with paclitaxel and carboplatin for this patient in September 2023, 2 months after she completed her prednisone taper. Non-irritant concentrations were used. Paclitaxel 6 mg/mL was used for skin prick, and 0.006 mg/mL and 0.06 mg/mL were used for intradermal testing [3]. Carboplatin 10 mg/mL was used for skin prick, and 1 mg/mL and 10 mg/mL were used for intradermal testing [4]. The patient had negative results at 20 min for both agents. She developed a positive delayed intradermal reaction to paclitaxel 6 h later, which resolved without treatment in 24–48 h (Figure 3). The carboplatin test remained negative.
Our working diagnosis is a rare case of combined type IV delayed HSR and cytokine release syndrome in this patient. The type IV features include a diffuse maculopapular rash and the positive skin testing (ST), upon delayed reading. As per the International Consensus on Drug Allergy, as in this case, delayed HSRs may occur as soon as one hour after drug administration and are associated with a T-cell mediated mechanism [5]. The positivity of ST does not evoke a type III reaction. Meanwhile, fever, chills, and arthralgias are features of cytokine release [6, 7]. Unfortunately, IL-6 testing is not readily available at our institution and could not be performed to confirm this assumption.
Another particularity of this case is the occurrence of a reaction to both formulations (paclitaxel and nab-paclitaxel). Unfortunately, nab-paclitaxel could not be skin tested in the absence of a validated protocol, but we can still hypothesize that the HSR was secondary to the taxane moiety itself (as opposed to the usual culprit, the preservative Cremophor® EL), as this is the only common point between both formulations. Another point for this hypothesis is the description of cross-reactivity between paclitaxel and hazelnut, which evokes the implication of the taxane moiety itself [8, 9].
There have been two previously reported cases of positive delayed ST for paclitaxel. Both involved female patients undergoing chemotherapy for the treatment of breast cancer seem to involve T-cell-mediated HSRs. In the first case, the patient presented with flushing and maculopapular exanthem 48 h after receiving paclitaxel and carboplatin. She then had a negative skin test to carboplatin and a positive delayed skin test for both paclitaxel and docetaxel at 96 h and 144 h post-testing, respectively, which could indicate cross-reactivity between taxanes and a reaction resulting from the taxane moiety [10]. In the second case, the patient presented with widespread cutaneous reaction 24 h after receiving paclitaxel and carboplatin. The patient also underwent ST. She had a negative skin test to carboplatin but a positive skin test 24 h post-testing to paclitaxel. This patient was subsequently switched to docetaxel, which she was able to tolerate [11]. Hence, the culprit remains unclear.
There has also been a combined type I HSR and cytokine release syndrome to paclitaxel described in an observational prospective study where the authors used ST as a marker of type I reaction and IL-6 as a marker of cytokine release [12]. None of these articles commented on the patients receiving nab-paclitaxel, which is usually expected to be well tolerated despite taxol hypersensitivity [2].
To our knowledge, our case is the first case of combined type IV/cytokine release reaction to paclitaxel, with cross reactivity to nab-paclitaxel. When a patient has an HSR to paclitaxel, it is usually safe to administer nab-paclitaxel as an alternative. Nonetheless, our case highlights that this might not always be the case, and the taxane moiety could be the culprit. Caution is therefore needed in cases of severe or atypical reactions.
HSRs: hypersensitivity reactions
IV: intravenous
PO: per os
ST: skin testing
FS received funding for his fellowship at McGill University and the McGill University Health Center from the Center Hospitalier Universitaire Vaudois (CHUV), Lausanne, Switzerland and from the Société Industrielle et Commerciale de Produits Alimentaires (SICPA) Foundation, Prilly, Switzerland.
STL: Conceptualization, Data curation, Writing—original draft, Writing—review & editing. FS: Conceptualization, Writing—review & editing. AMC: Writing—review & editing. GI: Conceptualization, Writing—review & editing, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The study was approved by the MUHC Ethics Committee (MUHC REB number—MP-37-2022-8458) and complies with the Declaration of Helsinki (2013 version).
Informed consent to participate for the study was obtained from the patient.
Informed consent to publication was obtained from the patient.
The data can be available from the corresponding author upon reasonable request.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3884
Download: 49
Times Cited: 0
