Affiliation:
1Department of Paediatric Allergy and Respiratory Medicine, University Hospital Southampton, SO16 6YD Southampton, UK
Email: melvin.qiyu@uhs.nhs.uk
ORCID: https://orcid.org/0000-0003-2476-960X
Affiliation:
3Department of Paediatric Surgery, University Hospital Southampton, SO16 6YD Southampton, UK
Affiliation:
4College of Medicine and Health, University of Birmingham, Edgbaston, B15 2TT Birmingham, UK
5NIHR Birmingham Biomedical Research Centre, Institute of Translational Medicine, B15 2TH Birmingham, UK
ORCID: https://orcid.org/0000-0002-2066-2349
Explor Asthma Allergy. 2025;3:100992 DOI: https://doi.org/10.37349/eaa.2025.100992
Received: August 11, 2025 Accepted: September 10, 2025 Published: September 25, 2025
Academic Editor: Juan Carlos Ivancevich, Universidad del Salvador, Argentina
The article belongs to the special issue Allergy and Asthma in the Digital Age
Artificial intelligence (AI) is poised to transform clinical allergy practice by enhancing diagnostic accuracy, personalising treatment, and streamlining healthcare delivery. This narrative review critically examines the current landscape of AI in allergy care, spanning clinical workflows, diagnostics, immunotherapy, and research applications. AI-powered tools such as clinical decision support systems (CDSS), natural language processing (NLP), and conversational agents are being integrated into allergy services, offering improvements in documentation, risk stratification, and remote patient engagement—particularly in paediatric and multilingual settings. Diagnostic innovations include machine learning models that predict oral food challenge outcomes and interpret multi-omics data for personalised allergy phenotyping. AI also supports adaptive immunotherapy dosing, remote monitoring via wearable biosensors, and digital coaching to promote adherence. Federated learning and explainable AI (XAI) emerge as pivotal developments—enabling privacy-preserving collaboration and fostering trust among clinicians and patients. Despite these advancements, significant challenges remain. These include data inequities, algorithmic bias, lack of real-world validation, and regulatory ambiguity. The “black box” nature of many models risks undermining clinician confidence, while over-reliance on alerts could contribute to alarm fatigue. Ethical concerns—particularly around transparency, consent, and liability—require urgent attention. Equitable implementation demands robust governance, diverse training data, and inclusive design that prioritises patient safety. Looking ahead, AI has the potential to power digital twins, support augmented reality training, and enhance allergy surveillance through the integration of environmental and population-level data. With multidisciplinary collaboration, transparent oversight, and patient-centred innovation, AI can help build a more predictive, efficient, and equitable future for allergy care.
Allergic diseases are among the most prevalent chronic health conditions globally, affecting an estimated 20–30% of the population [1]. The World Allergy Organization (WAO) reports a rising trend in the prevalence of allergic diseases, including asthma, allergic rhinitis, food allergy, eczema, and drug allergies, particularly in industrialised nations and urbanising low- and middle-income countries [1, 2]. The burden of these conditions extends beyond morbidity and mortality in asthma, contributing to significant reductions in quality of life, school and work absenteeism, and sleep disturbance.
From a health systems perspective, allergic diseases account for substantial economic costs [1, 3]. Allergic diseases represent a major public health issue in the UK, with nearly 40% of children and 30% of adults diagnosed with at least one atopic condition such as asthma, eczema, and allergic rhinitis [3]. Asthma, often co-occurring with other atopic diseases, is responsible for the majority of emergency admissions, and food allergies are associated with growing healthcare utilisation for anaphylaxis and specialist referrals [4].
Clinical allergy practice in the UK is hampered by a critical shortage of specialist allergists and inadequate allergy training for general practitioners, which potentially increases underdiagnosis and misdiagnosis and inconsistent patient care [5]. Access to expert services is highly unequal, creating a postcode lottery for patients and exacerbating health inequities—especially in underserved areas where poor health outcomes are further compounded by social determinants such as inadequate housing. As allergy prevalence and severity continue to rise [6], particularly among children, the growing gap between the availability of specialist paediatric allergists and the increasing number of cases has rendered current workforce and training provisions increasingly unsustainable [5]. The absence of national coordination and underinvestment in allergy services have further compounded these issues, leading to preventable health risks, inefficiency, and unnecessary National Health Service (NHS) costs [5]. The growing NHS budget deficit has further compounded the issue, leading to longer waiting times and a rise in preventable, worsening health outcomes. Urgent, coordinated national action is needed to address these systemic challenges.
Artificial intelligence (AI), including machine learning (ML), natural language processing (NLP), deep learning (DL), and related technologies, has emerged as a potentially transformative tool in addressing these challenges in healthcare, where there have been traditional barriers to implementing comprehensive health care screening programmes [7, 8]. In allergy and immunology, AI is increasingly being used to predict oral food challenge (OFC) outcomes, analyse large-scale electronic health record (EHR) data, personalise immunotherapy protocols, and automate clinical documentation [9–12]. There is also growing potential for AI to support asynchronous patient communication and tailored advice, improving access to specialist input and streamlining follow-up care [11]. These tools offer potential pathways to enhance efficiency, reduce diagnostic delays, improve patient safety, and enable proactive disease monitoring [10, 11].
However, the integration of AI into clinical allergy care remains limited. Most published AI medical devices are proof-of-concept or retrospective validations, with few tools successfully implemented in real-world settings [13]. Key barriers include the limited availability of high-quality, diverse datasets (particularly paediatric-specific data), hindering the robust development, validation, and generalisability of AI tools in allergy and immunology. The “black box” nature of many AI models raises concerns about transparency, interpretability, and explainability, leading to clinician hesitation [13]. Ethical and regulatory issues, such as patient privacy, data security, and algorithmic bias, are significant, compounded by the lack of clear governance frameworks [13, 14]. There are also concerns about how AI-enabled medical tools may influence clinician behaviour, including the risk of algorithmic aversion, highlighting the need for thoughtful integration that supports, rather than undermines, clinical judgment [13, 14].
This review aims to provide a comprehensive assessment of current AI medical devices in clinical allergy practice, including outpatient workflows, diagnostics, immunotherapy, and allergy research. We highlight the opportunities and limitations of current tools and highlight areas for future development and innovation. By synthesising evidence from multiple domains, we provide insights into how AI can support a more personalised, efficient, and sustainable approach to allergy care.
This section outlines the applications of AI in clinical allergy practice, with Figure 1 providing a summary of the key themes.
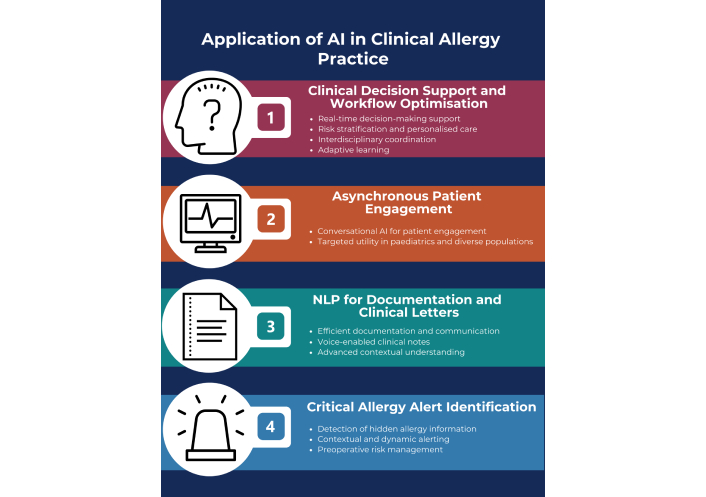
Summary of the applications of AI in clinical allergy practice. This diagram outlines four key areas where AI technologies are integrated into clinical allergy care. AI: artificial intelligence; NLP: natural language processing. Created using a template from Venngage.com.
AI-driven clinical decision support systems (CDSS), tools that assist clinicians in making evidence-based decisions at the point of care, have significant potential to transform allergy care [15]. For example, systems like HELIOT have demonstrated near-perfect accuracy in managing drug allergy alerts, highlighting AI’s capacity to improve clinical safety and efficiency [15, 16]. Integrated into EHRs, these tools provide real-time decision support during consultations and ward rounds, reducing alert fatigue, enhancing prescription safety, and improving adherence to evidence-based guidelines [16].
Beyond drug allergy management, CDSS in allergy settings can evolve to offer real-time asthma control recommendations, immunotherapy initiation criteria, and diagnostic guidance such as specific IgE or component-resolved testing. For example, asthma-focused CDSS that integrate patient data into the existing Global Initiative for Asthma (GINA) framework or National Asthma Education and Prevention Program (NAEPP) guidelines have shown improvements in standardised severity scoring and treatment step recommendations [17]. However, the study revealed suboptimal utilisation of the CDSS and limited adherence to the provided recommendations [17].
Advanced CDSS capabilities include dynamic risk stratification [18]. In food allergy clinics, for instance, systems can estimate allergen thresholds by analysing prior reactions and co-factors like infections or exertion, informing decisions about food challenges or home introduction, especially critical in paediatric settings.
CDSS also supports interdisciplinary care [18]. For example, an AI tool might flag PEG-asparaginase allergy before scheduling contrast-enhanced imaging. In dermatology and gastroenterology, CDSS can pre-emptively alert clinicians to risks before initiating biologics or immunomodulators.
With embedded NLP, CDSS can analyse patient histories to extract data on patterns of allergic reactions, including potential triggers and precipitants. For example, recurrent idiopathic flares in chronic urticaria may trigger recommendations for omalizumab evaluation.
Ultimately, as CDSS platforms evolve with ML, they can learn from outcome data, refining recommendations based on prior intervention success. This adaptive capability is amongst the promising strengths for CDSS and AI more broadly, training itself on patient data to fine-tune the foundation model in readiness for future patient interactions.
Conversational AI tools, including chatbots, mobile applications, and AI-enabled medical devices, offer significant potential for supporting asynchronous patient engagement and disease monitoring in asthma and allergic rhinitis. While distinct in their design and purpose, these technologies can incorporate advanced AI functionalities to enhance usability and clinical relevance. Studies have demonstrated that telemedicine approaches improve treatment adherence and reduce exacerbations [19], outcomes that AI tools may help replicate or amplify through intelligent automation and real-time feedback [20]. These tools are particularly valuable in paediatric care and multilingual environments, where continuous, tailored communication is critical to patient outcomes.
NLP algorithms enable transcription, summarisation, and automated clinical letter generation, offering significant value in allergy clinics where thorough histories and consistent follow-up are essential [21, 22]. NLP tools can extract critical medical information, such as allergen triggers, symptom patterns, and past immunotherapy responses, from unstructured clinical notes [23]. When paired with ambient voice transcription, clinicians can dictate during or after consultations, with the NLP engine transcribing and formatting the content into structured clinical letters or discharge summaries. This reduces documentation time and enables immediate information sharing with referring physicians, enhancing communication and care coordination. More broadly, such tools can support clinician engagement with patients, particularly in specialties like paediatrics, where frequent in-person interactions benefit from streamlined, AI-assisted workflows.
Advanced NLP models trained on allergy-specific data can identify subtle distinctions, for example, differentiating IgE- from non-IgE-mediated reactions or recognising contextual indicators of anaphylaxis risk [23, 24]. In research, NLP supports high-throughput screening of EHRs to identify patients eligible for clinical trials, using allergy diagnosis codes, medication histories, and lab results [23, 24]. Clinically, NLP enables real-time decision support by detecting missing elements in documentation, such as incomplete immunotherapy schedules or omitted rescue medications. Some systems now offer live prompts during note-taking to ensure accuracy and completeness.
In allergy clinics, the implementation of AI-driven documentation tools such as ambient scribing systems has been shown to significantly enhance clinical workflow efficiency. For example, one study found that ambient scribe tools reduced clinicians’ time in notes per appointment by about 20.4% (from 10.3 to 8.2 minutes), increased same-day appointment closure by 9.3%, and decreased after-hours documentation by 30% [25]. Clinicians also reported lower mental burden and greater engagement with patients during consultations.
AI systems can extract structured and unstructured data from EHRs to detect historical allergy events and flag potential triggers [26]. These tools use NLP to identify past reactions recorded in clinician notes, prescription histories, or scanned documents that may not be immediately visible in allergy fields. For instance, a prior reaction to an antibiotic may be recorded in a discharge summary or old correspondence but not coded in the structured allergy list. AI can flag such cases and prompt clinician review, potentially preventing serious medication errors. However, over-reliance on automated alerts may contribute to alarm fatigue, highlighting the need for carefully calibrated systems that prioritize clinical relevance.
Beyond static alerts, AI platforms are evolving to offer contextual and dynamic allergy alerts. These systems consider factors like current medications, lab results (e.g., elevated eosinophil counts), or recent diagnoses (e.g., new-onset asthma in the context of pollen season) to infer possible allergenic risk [24]. In paediatrics, where caregiver reports and evolving symptom profiles are key, AI can reconcile inconsistent entries and build a longitudinal allergy profile that updates in real time.
Hospitals are beginning to deploy AI tools for preoperative assessment to automatically flag patients with perioperative anaphylaxis risk based on past exposure to dyes, latex, or anaesthetic agents [27]. These alerts can help streamline perioperative planning and incorporate allergist input into complex perioperative plans.
AI is reshaping diagnostic strategies in allergy by enabling early recognition, enhanced phenotyping, and non-invasive evaluation, all of which contribute to improved safety and efficiency in clinical decision-making (Figure 2) [28].
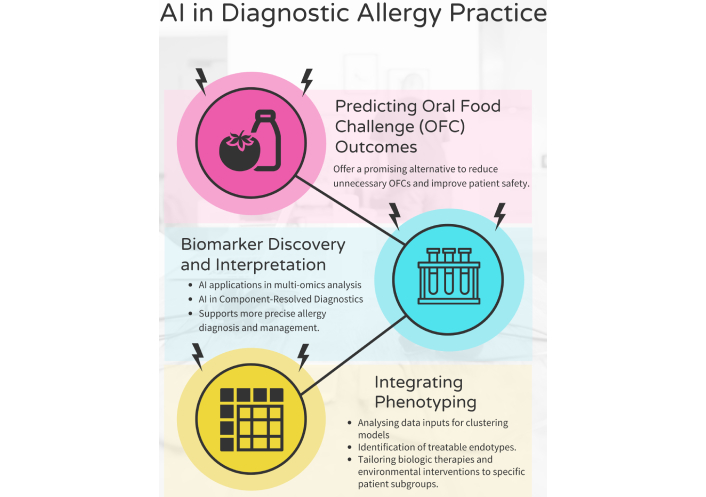
Applications of AI in diagnostic allergy practice. This figure illustrates three key areas where AI enhances diagnostic practices in allergy care. AI: artificial intelligence. Created using a template from Venngage.com.
OFCs remain the gold standard for confirming food allergies, but they are resource-intensive, costly, and anxiety-inducing—particularly in paediatric patients—and carry a significant risk of anaphylaxis. To mitigate these concerns, AI models such as logistic regression, support vector machines, and ensemble learning have been trained on combinations of skin prick test wheal sizes, specific IgE levels, component-resolved diagnostics (CRD), and clinical history to predict OFC outcomes. Support vector machines, which are algorithms that separate patients into groups based on patterns in test results, and ensemble learning approaches, which combine multiple models to improve accuracy, have been used to predict OFC outcomes [9]. These models consistently report area under the curve (AUC) between 0.85 and 0.96 for peanut, egg, and milk allergy prediction [9].
Multi-omics data, ranging from genomics and transcriptomics to metabolomics, can now be processed by unsupervised clustering and DL models to discover molecular signatures [29]. For example, latent class analysis and neural networks have helped define distinct inflammatory phenotypes in eosinophilic esophagitis and severe asthma. CRD, which analyse sensitisation to individual allergen proteins, can be interpreted using AI to differentiate primary from cross-reactive sensitisation [29].
ML also plays a role in phenotype clustering for conditions like asthma and chronic rhinosinusitis with nasal polyposis [30]. Using data from spirometry, FeNO levels, eosinophil counts, and symptom scores, unsupervised learning algorithms (e.g., k-means clustering, hierarchical clustering) can identify endotypes that may respond differently to biologics or environmental interventions [30].
Traditionally, immunotherapy schedules have been developed based on population-level data and generalised protocols. AI-driven systems now allow for the stratification of patients using ML algorithms trained on a combination of clinical phenotypes, specific IgE patterns, CRD, comorbidities, environmental exposures, and even genomic information [31, 32]. These models can predict which patients are likely to benefit from sublingual vs. subcutaneous immunotherapy, determine optimal allergen selection, and guide the timing and duration of treatment.
Predictive analytics further identifies patients at risk of poor response or adverse events. One study used historical adherence and immunologic markers to forecast non-adherence in subcutaneous immunotherapy with 60–84% accuracy via sequential models [33].
For example, combining AI with pharmacogenomics opens new possibilities for tailoring biologic therapy in autoimmune diseases. TNF-α polymorphisms can influence response to etanercept in psoriatic arthritis, highlighting the role of genomic profiling in treatment choice [34]. Given the variable infection risks linked to TNF-α inhibitors, integrating genomic, clinical, and AI-driven insights may enable more precise decisions that maximize efficacy while reducing adverse events.
AI has also made real-time monitoring during immunotherapy feasible and scalable. Wearable biosensors that track heart rate, respiratory rate, oxygen saturation, and skin conductance can feed data to AI systems that continuously evaluate for early signs of systemic reactions [35]. Integration with patient-facing apps allows for two-way communication: patients can log symptoms or side effects, and AI algorithms can automatically flag concerning trends for clinician review.
These platforms are particularly useful in home-based sublingual immunotherapy, where timely feedback from patients may be limited. In such settings, AI tools can act as virtual safety nets, enhancing patient autonomy while maintaining clinical oversight, but they also require robust regulatory frameworks to ensure patient safety remains paramount.
One proof-of-concept study utilized AI techniques—such as artificial neural networks and genetic algorithms—to develop a decision support system for allergen-specific immunotherapy [36]. This system achieved a 72% success rate in predicting symptom progression and therapy outcomes, illustrating how AI can meaningfully inform personalized treatment decisions [36].
Reinforcement learning and Bayesian optimisation are increasingly adopted to dynamically adjust dosing based on immunologic feedback (e.g., IgG4 or basophil activation) and clinical response. While much work has focused on cancer immunotherapy [37], the framework is potentially extensible to allergen dosing for venom or food immunotherapy.
Digital tools supported by AI, such as virtual nurse assistants, daily adherence prompts, and behaviour prediction models, can improve patient engagement [38]. These systems not only remind patients to take doses but also track patterns in missed doses and side effects, enabling clinicians to intervene early. Some platforms use gamification or psychological behaviour modelling to enhance motivation, particularly in adolescents and young adults.
Despite its promise, the integration of AI into allergy care faces substantial technical, ethical, and regulatory issues (Figure 3). A primary limitation is the lack of interoperability between AI tools and EHR systems, many of which lack standardised data structures or application programming interfaces (APIs) to support seamless exchange. This is compounded by the prevalence of bias in training datasets, often drawn from homogeneous or incomplete populations, where inequity in data can only perpetuate bias and disparity, particularly for underrepresented demographic groups [13].

Limitations, challenges, and ethical recommendations for AI in diagnostic allergy practice. This figure outlines four major categories of limitations and challenges associated with implementing AI in allergy diagnostics, along with corresponding recommendations for ethical AI adoption. EHR: electronic health record; AI: artificial intelligence. Created using a template from Venngage.com.
For instance, models trained primarily on European paediatric cohorts may underperform in predicting food allergy risk in Asian or African populations, where sensitisation patterns differ. Similarly, asthma phenotyping algorithms built on adult datasets often fail to capture unique paediatric endotypes, leading to reduced accuracy and potential misclassification.
The opaque, “black box” nature of many DL models can undermine clinical trust, particularly in high-stakes scenarios like predicting anaphylaxis, where a lack of explainability may perpetuate mistrust and contribute to algorithmic aversion. While interpretability tools like SHapley Additive exPlanation (SHAP) and Local Interpretable Model-agnostic Explanations (LIME) offer partial insights, their regulatory acceptance remains limited [39].
Few AI models in allergy medicine have been prospectively validated in real-world settings, raising questions about generalisability and robustness. Implementation is further constrained by infrastructural and financial limitations, particularly in resource-poor settings. Effective deployment also requires clinician training, governance structures, and organisational change management, elements often overlooked.
However, one prospective study of an AI tool predicting peanut OFC outcomes showed reliable performance in a clinical setting with paediatric patients [9]. Similarly, CDSS platforms for asthma care integrated into primary care practices improved adherence to guideline-based treatment, although uptake by clinicians was variable [17]. These examples illustrate both the potential promise and the challenges of moving from proof-of-concept to real-world application.
There are significant ethical considerations at the core of deploying AI in healthcare. AI systems rely on access to large volumes of sensitive health data, raising concerns around consent, secondary data use, and privacy. While de-identification techniques, such as pseudonymisation and federated learning, help reduce data privacy risks, robust governance frameworks and transparent consent processes remain essential [14, 40].
Accountability and explainability are paramount. Clinicians must understand how AI generates recommendations, particularly for high-impact decisions like initiating immunotherapy or food challenges. Without transparency, clinical uptake will falter, and medicolegal liability may rise. Currently, there is a lack of clarity on whether liability rests with developers, institutions, or clinicians.
Bias and fairness continue to demand scrutiny. Unrepresentative training data can lead to inequitable outcomes, worsening existing health disparities. Regulatory agencies such as the FDA, MHRA, and NICE stress the need for subgroup performance benchmarks, diverse training datasets, and continuous post-deployment market surveillance [13, 14].
Foundational frameworks like the GDPR and NHS Digital guidance provide a starting point, while WHO’s ethical AI guidelines offer global direction [41–43]. However, allergy-specific policies and standards are notably absent. For example, the UK’s NHS Digital guidance requires explicit patient consent for secondary data use, while the FDA’s Good Machine Learning Practice framework mandates documentation of algorithmic performance across diverse patient groups [44]. In allergy practice, this could mean requiring subgroup analyses (e.g., paediatric vs. adult, different ethnic groups) before deployment.
To ensure ethical, safe, and effective AI adoption in allergy practice, equity, transparency, and accountability must be prioritised. This includes patient and public involvement in algorithm development, the establishment of ethical oversight boards, and continuous real-world performance monitoring [42]. Speciality-led advocacy is essential to develop tailored guidelines that safeguard equity and trust while enabling responsible innovation.
Several emerging areas are set to define the next phase of AI integration in allergy care. Foremost is the advancement of explainable AI (XAI), which will be crucial not only for fostering clinician trust but also for empowering patients and supporting broader adoption. Interpretability tools such as SHAP and LIME can provide transparent, clinician-friendly explanations of model outputs, facilitating their use in real-world decision-making and clinical workflows [39, 45].
Federated learning also holds significant promise, enabling collaborative AI model development across multiple healthcare institutions without compromising patient privacy [40]. This is particularly relevant in allergy research, where multi-centre data pooling is essential yet often constrained by privacy regulations. Moreover, federated learning can support the development of personalised digital twins by leveraging diverse, distributed datasets while maintaining data security.
The incorporation of multi-omics data, genomic, proteomic, and metabolomic profiles, alongside environmental exposure records, will enable more accurate allergy risk prediction and further individualise treatment strategies [10]. Simultaneously, AI-driven augmented and virtual reality technologies are poised to transform education and training by simulating high-risk scenarios such as anaphylaxis or complex immunotherapy protocols, offering immersive, hands-on learning experiences [46].
At the population level, AI will support allergy surveillance by analysing environmental data (e.g., pollen, pollution) and identifying emerging sensitisation trends. This capability will allow public health authorities to anticipate seasonal surges and deploy targeted interventions efficiently.
Collectively, these developments signal a shift toward more predictive, proactive, and precision-driven allergy care powered by AI innovation.
AI is reshaping clinical allergy practice, from diagnostics and documentation to personalised treatment and public health surveillance. Despite ongoing technical, ethical, and operational challenges, the potential for transformative impact is clear. Realising this potential will require multidisciplinary collaboration, robust governance, continuous evaluation, and inclusive design. With thoughtful implementation, the allergy community stands on the brink of a digital revolution that could redefine how allergic diseases are understood, treated, and prevented.
AI: artificial intelligence
APIs: application programming interfaces
AUC: area under the curve
CDSS: clinical decision support systems
CRD: component-resolved diagnostics
DL: deep learning
EHR: electronic health record
GINA: Global Initiative for Asthma
LIME: Local Interpretable Model-agnostic Explanations
ML: machine learning
NAEPP: National Asthma Education and Prevention Program
NHS: National Health Service
NLP: natural language processing
OFC: oral food challenge
SHAP: SHapley Additive exPlanation
WAO: World Allergy Organization
XAI: explainable artificial intelligence
MLQ: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. WH: Writing—review & editing. PH: Writing—review & editing. QM: Writing—review & editing, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3429
Download: 57
Times Cited: 0
