Affiliation:
1Key Clinical Laboratory of Henan Province, Department of Clinical Laboratory, The First Affiliated Hospital of Zhengzhou University, Henan 450052, Zhengzhou, P. R. China
†
Affiliation:
2Department of Clinical Laboratory, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, P. R. China
3Beijing Key Laboratory for Mechanisms Research and Precision Diagnosis of Invasive Fungal Diseases, Beijing 100730, P. R. China
4Key Laboratory of Pathogen Infection Prevention and Control (Ministry of Education), National Institute of Pathogen Biology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, P. R. China
5Key Laboratory of Pathogen Infection Prevention and Control (Peking Union Medical College), Ministry of Education, Peking Union Medical College, Beijing 100730, P. R. China
†
Affiliation:
2Department of Clinical Laboratory, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, P. R. China
3Beijing Key Laboratory for Mechanisms Research and Precision Diagnosis of Invasive Fungal Diseases, Beijing 100730, P. R. China
4Key Laboratory of Pathogen Infection Prevention and Control (Ministry of Education), National Institute of Pathogen Biology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, P. R. China
5Key Laboratory of Pathogen Infection Prevention and Control (Peking Union Medical College), Ministry of Education, Peking Union Medical College, Beijing 100730, P. R. China
†
Affiliation:
2Department of Clinical Laboratory, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, P. R. China
3Beijing Key Laboratory for Mechanisms Research and Precision Diagnosis of Invasive Fungal Diseases, Beijing 100730, P. R. China
Affiliation:
2Department of Clinical Laboratory, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, P. R. China
3Beijing Key Laboratory for Mechanisms Research and Precision Diagnosis of Invasive Fungal Diseases, Beijing 100730, P. R. China
4Key Laboratory of Pathogen Infection Prevention and Control (Ministry of Education), National Institute of Pathogen Biology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, P. R. China
Affiliation:
2Department of Clinical Laboratory, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, P. R. China
3Beijing Key Laboratory for Mechanisms Research and Precision Diagnosis of Invasive Fungal Diseases, Beijing 100730, P. R. China
4Key Laboratory of Pathogen Infection Prevention and Control (Ministry of Education), National Institute of Pathogen Biology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, P. R. China
Affiliation:
2Department of Clinical Laboratory, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, P. R. China
3Beijing Key Laboratory for Mechanisms Research and Precision Diagnosis of Invasive Fungal Diseases, Beijing 100730, P. R. China
Affiliation:
2Department of Clinical Laboratory, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, P. R. China
3Beijing Key Laboratory for Mechanisms Research and Precision Diagnosis of Invasive Fungal Diseases, Beijing 100730, P. R. China
Affiliation:
2Department of Clinical Laboratory, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, P. R. China
3Beijing Key Laboratory for Mechanisms Research and Precision Diagnosis of Invasive Fungal Diseases, Beijing 100730, P. R. China
4Key Laboratory of Pathogen Infection Prevention and Control (Ministry of Education), National Institute of Pathogen Biology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, P. R. China
Affiliation:
2Department of Clinical Laboratory, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, P. R. China
3Beijing Key Laboratory for Mechanisms Research and Precision Diagnosis of Invasive Fungal Diseases, Beijing 100730, P. R. China
4Key Laboratory of Pathogen Infection Prevention and Control (Ministry of Education), National Institute of Pathogen Biology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, P. R. China
Email: xycpumch@139.com
Affiliation:
2Department of Clinical Laboratory, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, P. R. China
3Beijing Key Laboratory for Mechanisms Research and Precision Diagnosis of Invasive Fungal Diseases, Beijing 100730, P. R. China
4Key Laboratory of Pathogen Infection Prevention and Control (Ministry of Education), National Institute of Pathogen Biology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, P. R. China
5Key Laboratory of Pathogen Infection Prevention and Control (Peking Union Medical College), Ministry of Education, Peking Union Medical College, Beijing 100730, P. R. China
Email: yangqiwen81@vip.163.com
Explor Med. 2025;6:1001363 DOI: https://doi.org/10.37349/emed.2025.1001363
Received: January 29, 2025 Accepted: May 27, 2025 Published: October 11, 2025
Academic Editor: Lindsay A. Farrer, Boston University School of Medicine, USA
The article belongs to the special issue Global Perspectives on the Clinical Diagnosis, Treatment, and Functional Cure of HIV Infection in the Post-ART Era
Aim: Because of severe immunosuppression, coinfections continue to be a major cause of morbidity and mortality in HIV-infected patients. As the primary method currently used for diagnosing coinfections in HIV-infected patients, conventional microbiological tests (CMTs) often suffer from limitations such as prolonged processing times and low sensitivity, which may delay the initiation of appropriate treatment. This retrospective study aims to explore the applicability of metagenomic next-generation sequencing (mNGS) as a diagnostic tool compared with CMT.
Methods: A retrospective study was conducted on HIV-infected patients with coinfections admitted to Peking Union Medical College Hospital between November 2022 and November 2024. A receiver operating characteristic (ROC) curve was generated to evaluate the predictors and determine their sensitivities and specificities. The comprehensive final clinical diagnosis (FCD) was used as the reference standard for evaluating the diagnostic performance of CMT and mNGS. Then, treatment adjustments and outcomes after mNGS and CMT of the HIV-infected patients were also assessed.
Results: The areas under the ROC curve (AUCs) for CMT and mNGS were 0.600 and 0.775, respectively. When mNGS was combined with CMT, the AUC was 0.833. The sensitivity and specificity of mNGS were 80% and 75%, whereas those of CMT were 20% and 100%. When mNGS was combined with CMT, the sensitivity increased to 86.67%. Among the 15 coinfected HIV patients, 8 patients underwent treatment adjustments on the basis of mNGS results and achieved effective treatment, whereas only 1 patient underwent treatment adjustments solely on the basis of CMT and achieved effective treatment.
Conclusions: Compared with CMT, mNGS has a better detection rate. mNGS provides an alternative and promising method for identifying coinfections in HIV-positive patients. Thus, the combination of mNGS and CMT is a better diagnostic strategy for coinfections in HIV patients.
The global burden of human immunodeficiency virus (HIV) remains a significant challenge to public health worldwide. According to the Joint United Nations Programme on HIV/AIDS (UNAIDS), approximately 1.3 million new HIV infections were reported in 2023, culminating in an estimated 39.9 million individuals living with HIV by the end of the year. Additionally, 630,000 deaths were attributed to HIV-related illnesses globally in the same year [1]. A considerable proportion of HIV-related morbidity and mortality is driven by opportunistic coinfections, which exploit the compromised immune systems of HIV-positive individuals [2–4]. Therefore, early and accurate identification of coinfecting pathogens is critical for optimizing antimicrobial therapy and improving clinical outcomes [5].
Conventional microbiological tests (CMTs), including culture, parasitological microscopy, quantitative polymerase chain reaction (qPCR), serological tests, and biochemical assays, remain the cornerstone of diagnostic approaches for HIV-related coinfections. However, these methodologies have significant limitations. For example, microbial isolation through culture often requires prolonged incubation periods and demonstrates low sensitivity, particularly for slow-growing or unculturable pathogens such as Mycobacterium tuberculosis (MTB) and Pneumocystis jirovecii (P. jirovecii), both of which are common in HIV coinfections [6, 7]. Similarly, the performance of qPCR is constrained by its reliance on prior knowledge of target pathogens, limiting its utility for detecting emerging, rare, or unexpected pathogens. On the other hand, serological tests are prone to false-negative results in HIV patients due to weakened immune responses. Furthermore, the inability of CMTs to reliably detect mixed infections or atypical pathogens frequently delays appropriate therapeutic interventions.
In recent years, metagenomic next-generation sequencing (mNGS) has emerged as a transformative diagnostic tool in clinical laboratories. Unlike CMTs, mNGS is culture-independent, unbiased, high-throughput, and capable of detecting a broader range of pathogens with higher accuracy and speed. This technology has shown particular promise in diagnosing HIV-related coinfections, including pulmonary and central nervous system (CNS) infections. Nevertheless, despite growing evidence supporting the utility of mNGS, comparative analyses of its diagnostic performance relative to CMTs across diverse infection types in HIV patients remain limited.
This study aims to evaluate the diagnostic utility of mNGS for coinfections in HIV-positive individuals and to explore its implications for personalized therapy and clinical decision-making.
This retrospective study included HIV-positive patients with suspected coinfections who were admitted to Peking Union Medical College Hospital between November 2022 and November 2024. All enrolled patients underwent both mNGS and CMT. mNGS was performed on the basis of the comprehensive clinical judgment of the infectious disease physicians, who also determined the appropriate type of specimen to be submitted. According to a published study, the final clinical diagnosis (FCD) was established by consensus among three infectious disease physicians [8]. This study was approved by the Ethics Committee of Peking Union Medical College Hospital (Approval Number: I-24PJ0589), and all participants provided written informed consent.
Patients > 18 years old;
HIV-positive diagnosis confirmed by laboratory screening and CDC confirmatory testing;
Hospitalized patients suspected of coinfection, meeting at least one criterion: (i) new-onset fever unresponsive to antibiotics; (ii) afebrile with imaging suggestive of coinfection;
Patients who had completed mNGS and CMT testing.
Patients with repeated mNGS testing.
Clinical data were collected from electronic medical records and patient interviews, including the following:
General patient information: sex, age, antiretroviral therapy (ART) status, clinical presentation (e.g., fever, organ-specific symptoms), and treatment outcomes (recovery, death, discharge).
Routine blood and inflammatory marker tests: Blood tests were performed within 24 h of sample collection for mNGS and included total white blood cell (WBC) count, neutrophil percentage (N%), C-reactive protein (CRP), lactate dehydrogenase (LDH), interleukin (IL-6, IL-8, and IL-10), tumor necrosis factor-alpha (TNF-α), and procalcitonin (PCT) tests.
HIV-related immunological markers, such as the HIV viral load and CD4+/CD8+ T-cell ratio, were collected to evaluate the immune status of patients.
CMT results: The results included culture, parasitological microscopy, qPCR, serological tests [e.g., antigen-antibody reactions, galactomannan (GM) test, and (1,3)-β-D-glucan (G) test], Xpert-MTB, and the T-cell spot test for MTB (T-spot test).
Specimen collection was guided by the suspected site(s) of infection and followed standardized protocols established by the Pathogen Microbiology Testing Laboratory at Peking Union Medical College Hospital.
Cerebrospinal fluid (CSF): A minimum of 1.5 mL was collected in sterile, additive-free sealed tubes.
Bronchoalveolar lavage fluid (BALF): At least 5 mL of BALF was collected.
Pleural and peritoneal fluids: A minimum of 10 mL was collected in sterile sealed tubes.
Pathological tissues: Tissues from lymph nodes, the brain, or other sites were surgically obtained, placed in sterile containers, and moistened with 1 mL of saline. A sample volume greater than 1 cubic centimeter was preferred.
Blood and bone marrow samples: A volume of 3–5 mL was collected via cell-free DNA blood collection tubes, which were gently inverted 10 times to ensure thorough mixing with the preservative.
The samples were transported promptly: Blood and bone marrow samples were maintained at room temperature, whereas non-blood samples were stored and transported at 2–8°C within 2 h. Repeated freeze-thawing and vigorous shaking of the samples during transportation were strictly avoided.
The samples were processed according to the laboratory’s SOP. Genomic DNA was extracted via the Micro Sample Genomic DNA Kit (Tiangen Biotech, Beijing, China), and DNA fragmentation was performed via the Genomic DNA Fragmentation Kit (VISION MEDICALS, Guangzhou, China). Libraries were prepared via the Nextera XT DNA Library Preparation Kit (Illumina, USA) following standard PCR cycling conditions. Library quality was assessed with a Qsep1 Bio-Fragment Analyzer, and qualified libraries were sequenced on an Illumina NextSeq CN500 sequencer with 75 cycles of single-end sequencing, ensuring a minimum of 20 M data per library. Sequencing data were processed with Trimmomatic to remove low-quality sequences, short reads, and adaptors, and analyzed via platforms from Guangzhou VISION MEDICALS and PUMCH’s in-house pipeline for microbial sequence identification. The mNGS results were interpreted by a multidisciplinary team, which included bioinformaticians, clinical laboratory specialists, and infectious disease experts at PUMCH.
All the statistical analyses and figures were generated via SPSS Statistics 27.0 and GraphPad Prism 10 software. Numerical variables are presented as medians with interquartile ranges (IQRs), whereas categorical variables are summarized by counts and percentages. A receiver operating characteristic (ROC) curve was used to evaluate the predictors and determine their sensitivities and specificities.
A total of 19 HIV-positive patients (median age: 37 years; range: 19–57 years) who met the inclusion criteria were included in the study. All patients were male (100%). The clinical and laboratory characteristics of these patients are presented in Table 1. Despite receiving ART, 18 patients had a CD4/CD8 ratio significantly below the normal range, indicating incomplete immune reconstitution. One patient (5.3%) had a CD4/CD8 ratio of 2.03, which was close to the reference range for healthy individuals (1.4–2.0).
Clinical features of the enrolled HIV-positive patients.
| Characteristic, n = 19 | Clinical symptoms, n (%) | Sites of infection, n (%) | Underlying diseases, n (%) | ||||
|---|---|---|---|---|---|---|---|
| Age (years), median (Q1, Q3) | 37 (32, 50) | Fever | 12 (63.2) | Central nervous system | 6 (31.6) | Syphilis | 5 (26.3) |
| Gender (male), n (%) | 19 (100%) | Headache | 4 (21.1) | Lung | 3 (15.8) | Hepatitis B | 2 (10.5) |
| WBC ( 109/L), median (Q1, Q3) | 5.19 (3.13, 11.41) | Abdominal | 5 (26.3) | Liver | 3 (15.8) | Lymphoma | 2 (10.5) |
| Lymphocyte ( 109/L), median (Q1, Q3) | 1.17 (0.59, 1.86) | Cough | 1 (5.3) | Peritoneal cavity | 1 (5.3) | Hypertension | 2 (10.5) |
| CD4/CD8, median (Q1, Q3), n | 0.21 (0.09, 0.37), 18 | Fatigue or limb weakness | 3 (15.8) | Pleural cavity | 1 (5.3) | Coronary atherosclerosis | 1 (5.3) |
| LDH (U/L), median (Q1, Q3), n | 226.00 (162.00, 378.50), 13 | Consciousness disorder | 1 (5.3) | Retina | 1 (5.3) | Tuberculous meningitis | 1 (5.3) |
| CRP (mg/L), median (Q1, Q3), n | 23.60 (1.76, 75.91), 17 | Decreased vision | 1 (5.3) | No-coinfection | 4 (21.1) | Abdominal infection | 1 (5.3) |
| PCT (ng/mL), median (Q1, Q3), n | 0.39 (0.20, 1.04), 10 | mNGS positive | 10 (52.6) | ||||
| HIV viral load (copies/mL), median (Q1, Q3), n | 81.00 (20.00, 620.00), 19 | Both mNGS and CMT positive | 2 (10.5) | ||||
| Anti-HIV treatment, n (%) | 19 (100%) | Both mNGS and CMT negative | 3 (15.8) | ||||
CRP: C-reactive protein; HIV: human immunodeficiency virus; LDH: lactate dehydrogenase; mNGS: metagenomic next-generation sequencing; PCT: procalcitonin; Q1: first quartile (25th percentile); Q3: third quartile (75th percentile); WBC: white blood cell.
The specimen types from the 19 patients were analyzed (Table 2). The most commonly collected specimen type was CSF (7/19, 36.8%), followed by blood samples (4/19, 21.1%), liver puncture fluid (3/19, 15.8%), and tissue samples (2/19, 10.5%). Additionally, pleural fluid, bone marrow, and BALF were each collected from one patient (1/19, 5.3% each). A schematic of the study profile is shown in Figure 1.
Distributions of sample types.
| Specimen | n (%) |
|---|---|
| CSF | 7 (36.8) |
| Plasma | 4 (21.1) |
| Liver puncture fluid | 3 (15.8) |
| Tissue | 2 (10.5) |
| Pleural fluid | 1 (5.3) |
| Bone marrow | 1 (5.3) |
| BALF | 1 (5.3) |
CSF: cerebrospinal fluid; BALF: bronchoalveolar lavage fluid.
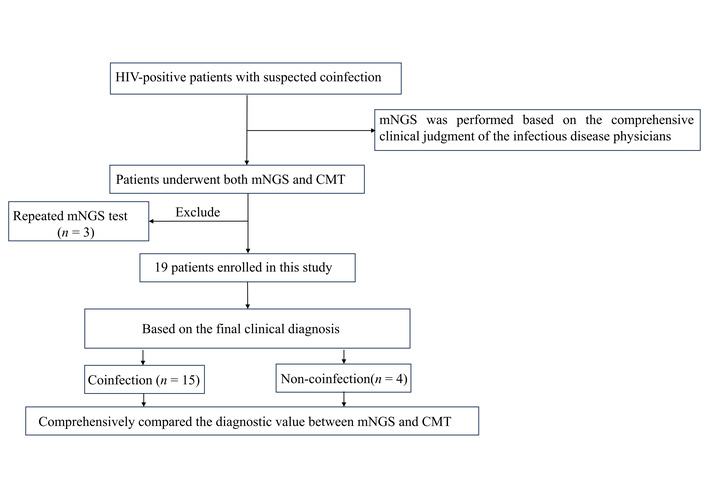
Schematic of the study profile. CMT: conventional microbiological test; HIV: human immunodeficiency virus; mNGS: metagenomic next-generation sequencing.
On the basis of the FCD, 15 patients (78.9%, 15/19) were confirmed to have coinfections. Among these, 14 had confirmed microbiological findings, which included 13 single infections and 1 mixed infection (2 pathogens, fungi and viruses). The remaining patient had a viral infection, and the patient exhibited decreased blood counts and bone marrow proliferation, which was attributed to both viral infection and medication-related effects. The remaining four patients (21.1%) did not have confirmed coinfections: one was diagnosed with severe mitral regurgitation (MR) and heart failure, one with leukoaraiosis, one with limb weakness and atrophy, and one with an unclear cause of dural enhancement.
Using FCD as the standard, 16 strains from 10 types of pathogens were identified, including 3 types (4 strains, 25%) of bacteria, 2 types (5 strains, 31.3%) of fungi, 3 types (3 strains, 18.8%) of viruses, and 2 types (4 strains, 25%) of parasites. mNGS identified 8 types of pathogens, whereas CMT identified only 3 types. Both mNGS and CMT detected Penicillium marneffei (a fungus) in one patient. Seven types were detected only by mNGS: bacteria (Mycobacterium gordonae and Mycobacterium tuberculosis), fungi (Aspergillus flavus), viruses (Epstein-Barr virus, cytomegalovirus, parvovirus B19), and parasites (Entamoeba histolytica). A consistency assay for pathogen detection between mNGS and CMT is shown in Figure 2.
Among the 15 coinfected HIV-positive patients, 2 (10.5%, 2/19) were positive by both mNGS and CMTs, while 3 (15.8%, 3/19) were negative by both methods among the 4 noncoinfected patients; the consistency of mNGS and CMTs was 26.3% (5/19). Overall, 15 patients were FCD-positive, with 12 positive mNGS detections; the true-positive (TP) rate was 80% (12/15), and 3 patients (20%, 3/15) were false negatives (FNs). In CMT, 3 patients (3/15, 20%) had consistent FCD, and the FN rate was 80% (12/15). ROC curve analysis revealed that mNGS had better diagnostic performance. The sensitivity, specificity, and area under the ROC curve (AUC) for mNGS were 80%, 75%, and 0.775, respectively. In contrast, the corresponding values for CMT were 20%, 100%, and 0.600. When the combination of mNGS and CMT was used as a diagnostic strategy, the sensitivity, specificity, and AUC increased to 86.67%, 75%, and 0.833, respectively. These values are shown in Figure 3.
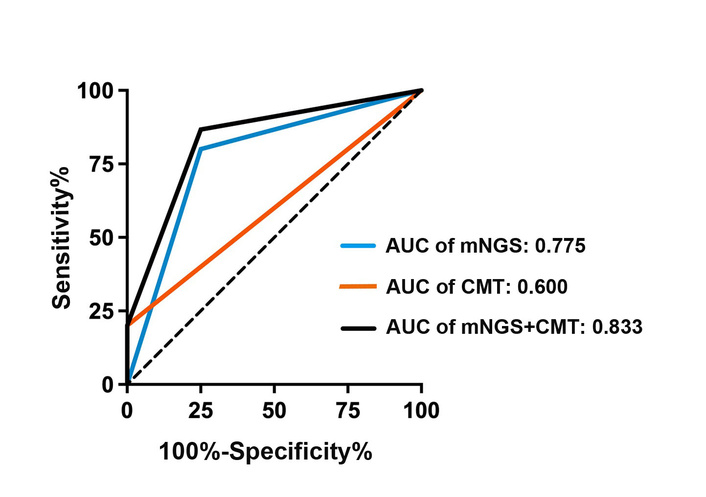
Comparison of the diagnostic value between mNGS and CMTs. AUC: area under the receiver operating characteristic curve; CMT: conventional microbiological test; mNGS: metagenomic next-generation sequencing.
For the 4 HIV patients without coinfections, FCD combined with mNGS and CMT results facilitated therapeutic adjustments, specifically the discontinuation of unnecessary antibiotic treatment. Among the remaining 15 coinfected HIV patients, 10 patients underwent treatment adjustments on the basis of mNGS results, of which 8 patients achieved effective treatment, 1 patient was lost to follow-up, and 1 patient experienced treatment failure. In addition, 2 patients underwent treatment adjustments guided by the combined results of mNGS and CMT, both of whom were successfully treated. Furthermore, one patient was treated on the basis of only CMT results with effective outcomes, whereas two other patients were effectively treated on the basis of empirical therapy. A Sankey diagram is shown in Figure 4.
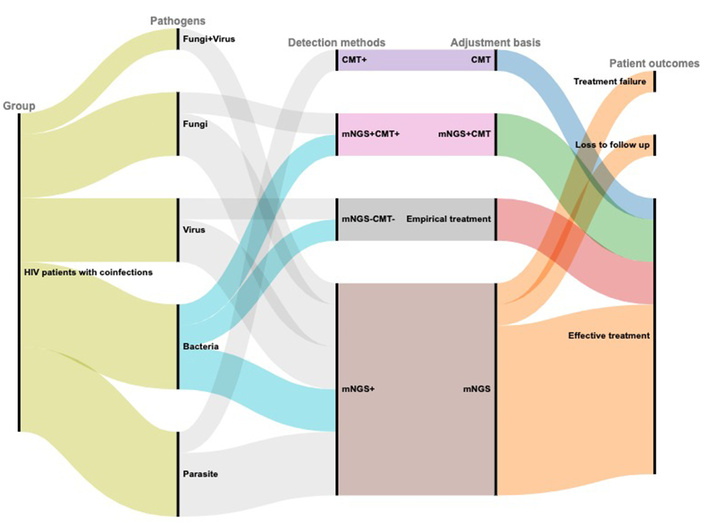
Sankey diagram demonstrating the treatment adjustments and outcomes based on mNGS and CMT in HIV patients with coinfections. This figure was generated via the RAWGraphs website https://rawgraphs.io. CMT: conventional microbiological test; HIV: human immunodeficiency virus; mNGS: metagenomic next-generation sequencing.
Owing to the immunodeficiency associated with HIV infection, patients with HIV are more susceptible to a wide range of infections. HIV/AIDS not only facilitates the occurrence of diseases caused by opportunistic pathogens that seldom infect healthy individuals but also substantially exacerbates the clinical manifestations of other pathogens. Common coinfections include tuberculosis, fungal infections, and P. jirovecii pneumonia. For example, tuberculosis is more likely to cause active disease in individuals with HIV [4, 9], whereas coinfection with HBV more rapidly leads to liver damage, significantly increasing the risk of mortality [10, 11]. The viral envelope glycoproteins of HIV indirectly promote the occurrence and progression of these coinfections by modulating viral replication efficiency and facilitating immune evasion [12].
These coinfections substantially increase morbidity and mortality among HIV-infected patients. Therefore, it is not surprising that coinfections in HIV/AIDS patients should receive more attention from the global health community. Although CMTs based primarily on culture remain the gold standard for identifying pathogenic microorganisms, the mycological culture process is cumbersome, time-consuming, and has a low positive detection rate. For example, the culture of certain fungi may take several weeks, and many pathogens cannot be cultured at all. In this study, among seven patients ultimately diagnosed with fungal infections, only two yielded positive culture results. Although the GM or G test results were positive in these cases, the results suggest the possibility of fungal infection, but cannot identify specific pathogens. This diagnostic uncertainty may lead to empirical antifungal therapy, which carries a degree of therapeutic uncertainty.
mNGS offers a rapid and comprehensive pathogen detection method for infectious diseases. The adoption of mNGS technologies in clinical diagnostics is increasing, and several studies have reported the application of mNGS in central neurological infections of HIV patients, including tuberculosis meningitis and neurosyphilis [13–15], as well as in sepsis and pneumocystis [6, 16]. However, research comprehensively evaluating the suitability of mNGS across diverse types of coinfections in HIV-infected patients remains limited. In this study, we retrospectively analyzed the diagnostic efficacy of the mNGS method on the basis of different types of samples to evaluate its diagnostic value for coinfections in HIV-positive patients. These results indicate that mNGS has broader pathogen coverage than CMTs do, suggesting that mNGS has greater diagnostic sensitivity and accuracy than CMTs do, underscoring its potential clinical utility in identifying coinfections in HIV-infected individuals.
Our study was limited to a single-center design with a relatively small sample size, which may have introduced potential selection bias. Although mNGS has been accepted and applied in HIV-infected patients, there are currently no standardized guidelines for interpreting test reports in this population. In cases in which mNGS detects multiple potential pathogenic microorganisms, low-abundance viruses (such as circovirus, Epstein-Barr virus, and cytomegalovirus) and commonly colonizing bacteria (such as Staphylococcus epidermidis) are often subjectively classified as nonpathogenic. However, the interpretation criteria in our study were based on previously established evidence from similar sequencing platforms and sample populations. In practice, a more comprehensive approach should be adopted, taking into account the unique susceptibility of HIV-infected patients to opportunistic infections, to enable more accurate clinical judgments. Finally, while our study highlights the positive impact of mNGS on clinical decision-making, the economic burden of routine mNGS implementation in clinical practice must be carefully evaluated. The high cost of mNGS may limit its accessibility, particularly in resource-limited settings where the HIV prevalence is highest. Future studies are needed to address these limitations.
In addition to mNGS, several novel pathogen nucleic acid detection methods have been developed. For example, the target-navigated CBT-Cys “Stapling” technique, coupled with the CRISPR/Cas12a amplification technique, integrates enzyme-free ligation with CRISPR/Cas12a-mediated cleavage amplification. This approach uses rolling circle amplification (RCA) to generate abundant target amplicons, which subsequently trigger CRISPR-mediated signal amplification, resulting in significantly increased detection sensitivity. This dual amplification strategy is particularly suitable for the sensitive detection of low-abundance pathogen nucleic acids, thereby facilitating early diagnosis and infection monitoring [17]. Therefore, with ongoing technological advancements, detection methods for coinfections in HIV patients are expected to become increasingly sophisticated and effective in the future.
ART: antiretroviral therapy
AUC: area under the receiver operating characteristic curve
BALF: bronchoalveolar lavage fluid
CMTs: conventional microbiological tests
CSF: cerebrospinal fluid
FCD: final clinical diagnosis
FNs: false negatives
G: (1,3)-β-D-glucan
GM: galactomannan
HIV: human immunodeficiency virus
mNGS: metagenomic next-generation sequencing
MTB: Mycobacterium tuberculosis
P. jirovecii: Pneumocystis jirovecii
qPCR: quantitative polymerase chain reaction
ROC: receiver operating characteristic
We are thankful to all the participants and their families.
YMY, DZ, and JD: Writing—original draft, Conceptualization, Methodology, Project administration, Supervision. YG: Data curation, Visualization. YW: Formal analysis. MYL: Methodology. JYG: Investigation, Writing—review & editing. HTS and XFC: Software. YCX and QWY: Conceptualization, Methodology, Project administration, Supervision, Funding acquisition. All authors read and approved the submitted version.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This study was approved by the Ethics Committee of Peking Union Medical College Hospital (Approval Number: I-24PJ0589) and conducted in accordance with the 2013 Declaration of Helsinki.
All participants provided written informed consent.
Consent to publication is not needed, as the data used in this study are fully anonymized and contain no identifiable information.
All data are available from the corresponding author on reasonable request.
This work was supported by the National Key R&D Program of China [2022YFC2305301], CAMS Innovation Fund for Medical Sciences [2025-I2M-KJ-001], National Science and Technology Major Project [2024ZD0532804], National Natural Science Foundation of China [82272380], National Key Research and Development Program of China [2021YFC2301002], National High Level Hospital Clinical Research Funding [2022-PUMCH-C-060, 2022-PUMCH-B-028], Beijing Natural Science Foundation [5232026], the Fundamental Research Funds for the Central Universities, Peking Union Medical College [3332024203], the Peking Union Medical College Hospital Talent Cultivation Program (Category D) [UHB12045]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1153
Download: 15
Times Cited: 0
Yadessa Tegene Woldie ... Mark Spigt
Violetta Vlasova ... Konstantin Shmagel
Zhimin Huang, Xiaohui Wang
Werner Krause
Yang Zhou ... Hongzhou Lu
