Affiliation:
Department of Biotechnology, Institute of Applied Sciences & Humanities, GLA University, Mathura 281406, Uttar Pradesh, India
Email: sarmistha_pharmacol@yahoo.com; sarmistha.saha@gla.ac.in
ORCID: https://orcid.org/0000-0001-5324-1957
Explor Med. 2025;6:1001362 DOI: https://doi.org/10.37349/emed.2025.1001362
Received: June 01, 2025 Accepted: July 31, 2025 Published: September 26, 2025
Academic Editor: Apostolos Zaravinos, European University Cyprus, Cyprus
The article belongs to the special issue Lipid Peroxidation and Cancer
An increasingly popular therapeutic approach for the treatment of cancer is the modification of signaling pathways mediated by oxidative stress. Epigenetic dysregulation serves as a key characteristic of human cancer, as almost half of all cancer cases involve mutations in epigenetic regulators like microRNAs (miRNAs). These small non-coding RNAs play a crucial role by generating functional RNA molecules that range from 18 to 25 nucleotides. miRNAs are essential for regulating gene expression at the mRNA level, but they have also been demonstrated in recent studies to influence the growth and development of cancer. miRNAs play a significant role in the generation of reactive oxygen species (ROS) and in various processes influenced by ROS. Therefore, exploring the relationship between ROS and miRNAs is becoming increasingly crucial, as it holds the potential to advance the development of effective cancer therapies and prevention strategies. This article aims to provide a comprehensive overview of the key characteristics and functional roles of miRNAs that are linked to oxidative stress in different cancers, paving the way for future research and therapeutic innovations. However, a lot of concerns and uncertainties regarding ROS-miRNAs and antioxidant defense systems still need to be resolved despite a great deal of research in this field.
Oxidative stress (OS) represents a critical imbalance between antioxidant factors and reactive oxygen species (ROS) within cells. This phenomenon is pivotal in driving essential physiological responses, utilizing noncoding RNAs (ncRNAs), transcription factors (TFs), and complex signal transmission pathways [1]. ROS, generated as byproducts of cellular oxidative metabolism, include powerful molecules like superoxide anion (O2–), hydroxyl radical (OH–), hydrogen peroxide (H2O2), nitric oxide (NO), and singlet oxygen (1O2). These reactive species are indispensable for a range of vital processes, including signal transduction, cellular apoptosis, chemoresistance, cell differentiation, and ultimately, cell death [2, 3]. Excessive ROS generation has recently been found in a number of malignancies, and it has been strongly linked to carcinogenesis. The fundamental process by which ROS regulation contributes to the development of cancer is yet unknown, though.
MicroRNAs (miRNAs) are powerful small non-coding RNAs that play a crucial role in regulating gene expression by binding directly to the 3’-untranslated region (3’-UTR) of target mRNAs, effectively preventing their translation. miRNAs are defined as short non-coding RNAs of 18–23 nucleotides that target the mRNA to adversely inhibit gene expression [4]. RNA polymerase II and III play a crucial role in transcribing miRNA molecules into immature primary miRNAs, referred to as pri-miRNAs. The development of mature miRNA occurs in the nucleus, where pri-miRNA is effectively processed through two essential steps involving the Dicer and Drosha complexes. Dicer, an endoribonuclease with a helicase domain and RNase activity, and Drosha, a class 2 ribonuclease III enzyme, work together to ensure the proper maturation of miRNA. This processing is vital for the functionality and regulation of gene expression [5]. The mature miRNA functions as a functional miRNA in the cytoplasm when it combines with the RNA-induced silencing complex (RISC).
miRNAs are strongly linked to tumor growth, metastasis, and cancer progression, according to earlier research [6, 7]. These results suggest that dysregulated miRNA expression is a characteristic of cancer. Determining the nature of the relationship between ROS and miRNAs is crucial because it has been linked to the development of cancer. Remarkably, OS controls the expression of some miRNAs known as ROS-miRs or redoximiRs, which alter target gene expression in response to ROS [8, 9]. For instance, a study by Mesenguer et al. [10] revealed that in MELAS cells, the OS/NFB signaling pathway triggers the production of miR-9/9*, simultaneously suppressing its target genes, GTPBP3, MTO1, and TRMU. Additionally, previous research demonstrated that miR-21 inhibits the antioxidant response in human umbilical vein endothelial cells (HUVECs), playing a pivotal role in regulating ROS homeostasis [11]. These findings strongly suggest that ROS can act both as downstream effectors and upstream regulators of miRNAs.
According to earlier research, ROS can either increase or decrease the production of miRNA and influence target genes, which in turn affects downstream biological function [12]. Cross-talk between miRNAs and redox signaling components has been demonstrated more and more [13, 14]. There are known redox sensors, which include kinases (like Akt and IKK) and TFs [including p53, NFB, c-Myc, and nuclear factor erythroid 2 related factor 2 (Nrf2)] that initiate cellular redox signaling.
It was recently found that RNA polymerase II/III can transcribe miRNAs as pri-miRNAs, which are longer primary transcripts. The two-step processing of pri-miRNA produces the mature form of miRNA, which is then linked to the effector RISC. The miRNA processing pathway is mediated by two important genes, Dicer and Drosha. According to a study, aging-related OS in cerebromicrovascular endothelial cells (CMVECs) decreased the expression of Dicer [15]. Notably, human microvascular endothelial cells (HMECs) produced lower ROS when Dicer was knocked down [16]. Following OS, DGCR8/heme oxygenase-1 (HMOX1) regulation reduces pre-miRNA and miRNA expression in myoblasts [17]. The heme-binding domain of DGCR8 is essential for pri-miRNA recognition for DROSHA’s processing of miRNA, and heme is necessary for DGCR8 function.
miR-5096 plays a significant role in promoting cell death and inhibiting the proliferation and invasion of breast cancer cells. Research has shown that its overexpression in these cells leads to increased iron levels, ROS, hydroxyl radicals, lipid peroxides, and glutathione (GSH). This is accomplished by inhibiting the function of SLC7A11, which triggers ferroptosis in breast cancer cells [18]. The key regulator of the ferroptosis process is glutathione peroxidase 4 (GPX4), a selenoprotein that operates through a two-step catalytic mechanism. In the first step, the selenocysteine residue at the active site undergoes self-oxidation while reducing lipid peroxides (such as PE-AA-OOH and PE-AdA-OOH) into non-toxic phospholipid alcohols (PE-AA-OH and PE-AdA-OH). In the second step, GSH is oxidized to GSSG, followed by the reduction of the oxidized selenocysteine residues with two molecules of GSH to restore their functionality [19]. Furthermore, miR-15a-3p has been identified as a direct inhibitor of GPX4 by binding to its 3’-UTR, thereby enhancing the ferroptosis process in colorectal cancer. This interaction leads to elevated levels of intracellular ROS, Fe2+, and malondialdehyde (MDA) [20]. Notably, in cisplatin-resistant lung adenocarcinoma cells, there is a significant reduction in the expression of miR-324-3p. However, overexpressing miR-324-3p could potentially reverse this resistance by directly targeting and suppressing GPX4 expression [21].
The overexpression of miR-155 suppresses Foxo3a, which lowers the levels of key antioxidant enzymes like catalase (CAT) and superoxide dismutase (SOD2), boosting the production of ROS and growing pancreatic cells (Table 1, Figure 1) [22]. Additionally, the miR-1287-5p/GPX4 axis has emerged as a critical factor in determining the susceptibility of osteosarcoma cells to cisplatin, effectively regulating both ferroptosis and cell proliferation [23]. Other significant axes include the miR-15a/GPX4 in prostate cancer (Table 1) [24], the circIL4R/miR-541-3p/GPX4 in hepatocellular carcinoma [25], the circKIF4A/miR-1231/GPX4 in papillary thyroid cancer [26], and the circDTL/miR-1287-5p/GPX4 in non-small cell lung cancer (NSCLC) [27]. Each of these axes contributes to the regulation of ferroptosis in its respective tumors, highlighting the intricate mechanisms that could be targeted for therapeutic intervention. GPX3 is an important antioxidant enzyme present in plasma, and it is regulated by the suppression of miR-921 [28]. Additionally, research indicates that miR-330-3p can facilitate cancer cell metastasis by inhibiting the production of the potent antioxidant enzyme human manganese superoxide dismutase (hSOD2b) (Table 1) [29]. Certain miRNAs, like miR-509-5p, have been identified as targeting SOD2, demonstrating their potential to exert tumor-suppressive effects that could help reduce breast cancer cell invasion and metastasis [30].
miRNAs linked to oxidative stress and possible cancer-related processes.
| miRNA | Target | Type of signaling | Ref. |
|---|---|---|---|
| miR-34a | SIRT1 | Oxidant | [100] |
| miR-33a | SIRT6 | Antioxidant | [101] |
| miR-29b | SIRT1 | Antioxidant | [102] |
| miR-128 | SIRT1 | Oxidant | [103] |
| miR-155 | Nrf2 | Oxidant | [104] |
| miR-7 | Keap1 | Antioxidant | [105] |
| miR-141 | Keap1 | Antioxidant | [106] |
| miR-30 | p53 | Antioxidant | [107] |
| miR-34a | NOX2 | Oxidant | [100] |
| miR-921 | GPX3 | Oxidant | [108] |
| miR-1915-3p | Bcl-2 | Oxidant | [63, 109] |
| miR-15 | Bcl-2 | Oxidant | [31, 110] |
| miR-16 | Bcl-2 | Oxidant | [110] |
| miR-509-5p | SOD2 | Antioxidant | [111] |
| miR-330-3p | hSOD2b | Oxidant | [112] |
| miR-143/miR-145 | SOD1 | Oxidant | [113] |
| miR-551b | Catalase | Antioxidant | [114] |
| miR-6785-5p and miR-642a-3p | FOXO4 | Antioxidant | [89] |
| miR-27b | NF-κB | Oxidant | [56] |
SIRT1: Sirtuin 1; Nrf2: nuclear factor erythroid 2 related factor 2; Keap1: Kelch-like ECH-associated protein 1; GPX3: glutathione peroxidase 3; SOD2: superoxide dismutase 2.
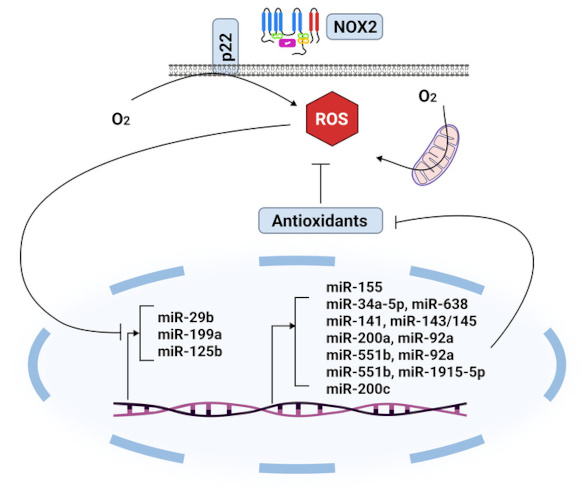
ROS-inhibited miRNAs and miRNAs that block antioxidant enzymes. ROS: reactive oxygen species.
Various malignancies are influenced by miR-15a-3p, highlighting its potential as a therapeutic target. Research by Liu et al. [31] indicates that miR-15a-3p is involved in the ferroptosis process in colorectal cancer. By directly binding to the 3’-UTR of GPX4, miR-15a-3p reduces GPX4 activity, which consequently elevates the levels of intracellular ROS, Fe2+, and MDA. This suggests that manipulating miR-15a-3p could offer new strategies to enhance ferroptosis in cancer treatment. It has been found that colorectal cancer has lower levels of miR-539, while colon cancer displays higher expression of tumor necrosis factor (TNF)-α induced protein 8 (TNFAIP8/TIP8). This differential expression suggests that TIP8 may play a crucial role in promoting angiogenesis, migration, and proliferation in colorectal cancer. Moreover, the study delves into the mechanisms by which miR-539 contributes to cancer cell regulation. It appears that miR-539 activates the SAPK/JNK pathway, which promotes ferroptosis and inhibits the growth of colorectal cancer cells. This action occurs through the indirect down-regulation of GPX4 and the modulation of TIP8 expression. These insights provide valuable directions for potential therapeutic approaches in targeting colorectal cancer [32]. Another study found that miR-433 regulates glutamyl-cysteine ligase (GCL), a heterodimeric enzyme that limits the rate of GSH biosynthesis [33].
Nrf2 is a key member of the basic leucine zipper (bZIP) TFs, specifically belonging to the Cap’n’Collar (CNC) family [34]. Its activity is intricately linked to Kelch-like ECH-associated protein 1 (Keap1), which functions as a suppressor of Nrf2 by promoting its proteasomal degradation [35]. Nrf2 plays a multifaceted role in inflammation, radioresistance, protein degradation, and antioxidant metabolism [36]. Notably, it possesses the capacity to both directly and indirectly regulate miRNAs [36, 37]. Research by Singh et al. [38] highlighted how Nrf2 reprograms the glucose metabolism of cancer cells by inhibiting the production of miR-1 and miR-206.
Furthermore, it has been identified that Nrf2 directly interacts with the genes miR-29 and miR-125b (Figure 2) [39, 40]. The up-regulation of miR-125b by Nrf2 is significant, as it leads to the suppression of the aryl hydrocarbon receptor repressor, thereby providing a protective effect for cancer cells against certain harmful drugs [40]. On the other hand, several miRNAs, including miR-28, miR-34a, miR-93, and miR-200a, have been shown to regulate the Nrf2 gene (Figure 2) [41–44]. For instance, miR-28 has been demonstrated to interact with the 3’-UTR of Nrf2, inhibiting its expression in breast cancer cells [41]. Similarly, the overexpression of miR-34a was found to decrease both Nrf2 and its target gene expression, with miR-34a playing a crucial role in the liver’s Nrf2-dependent antioxidant system [42]. It is known that apoptosis and cell death are increased when miR-155 is inhibited by targeting the TF Nrf2 (Figure 2) [45]. Peroxiredoxin-like 2A (PRXL2A), an antioxidant that shields cells from OS, has similarly been demonstrated to be regulated by miR-125b. By preventing PRXL2A from being up-regulated, down-regulation of miR-125b protects the tumor cells from OS. It has been demonstrated that miR-144 directly influences cellular resistance to OS by altering Nrf2 expression. Thus, Nrf2 protein expression is markedly reduced by miR-144 overexpression (Figure 1). Additionally, miR-144 can lower cellular levels of GSH and inhibit gene expression triggered by antioxidant response elements (AREs) [46].
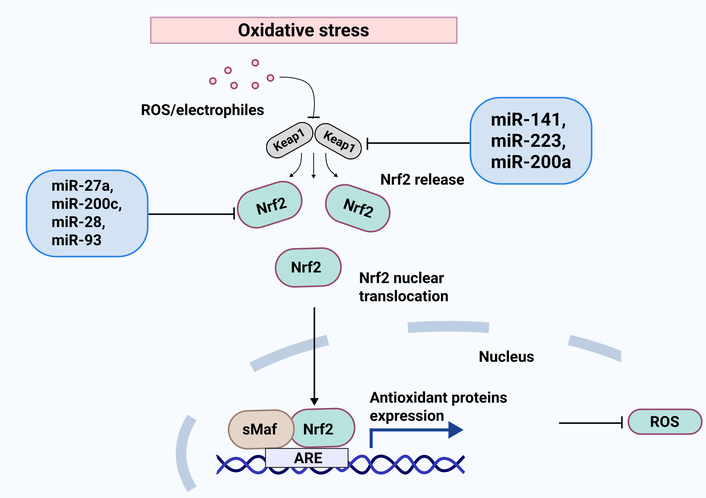
Keap1-Nrf2 signaling in effective miRNAs and oxidative stress responses. Keap1 negatively regulates Nrf2, making it a target for ubiquitin ligase. After ROS separates Keap1 from Nrf2, the Nrf2 cloud moves to the nucleus to begin transcriptional activity in the promoter of the antioxidant gene. Furthermore, the direct targeting of miRNAs regulates both Nrf2 and Keap1, which results in the suppression or induction of the antioxidant defense mechanism. Keap1: Kelch-like ECH-associated protein 1; Nrf2: nuclear factor erythroid 2 related factor 2; ROS: reactive oxygen species.
Angiogenesis, the formation of new blood vessels from existing ones, plays a crucial role in various vital processes, such as organ regeneration, tissue repair, cancer development, and metastasis [47]. Recent research highlights the significance of the MRN (MRE11a/RAD50/NBN) complex in endothelial cells, which is regulated by the expression of miR-494 in response to genotoxic stress [48]. This regulation is important as it can inhibit both DNA repair and angiogenesis, shedding light on potential therapeutic targets. Moreover, ROS contributes positively by enhancing the expression of vascular endothelial growth factor (VEGF) and activating the MAPK pathway [49]. Notably, previous studies have revealed that oxidized phospholipids can interact with VEGFR2, thereby initiating angiogenesis through the Src signaling pathway, presenting another area for exploration in therapeutic strategies.
Additionally, the involvement of Sirtuin 1 (SIRT1) and the ATM/p38 pathway in ROS-mediated angiogenesis offers further avenues for investigation. The first miRNA identified as down-regulating SIRT1 in relation to cellular aging is miR-34a [50]. Additionally, other miRNAs, including miR-141, miR-181, and miR-199, have also been found to regulate SIRT1 levels across various cells and tissues [51]. Notably, in hypoxic conditions, a reduction in miR-199 expression can promote the up-regulation of SIRT1, which may contribute to decreased apoptosis. By modulating SIRT1, miR-29b has also been demonstrated to provide protection against OS and H2O2 toxicity (Figure 1) [52]. Furthermore, by triggering apoptosis, miR-128 made colon cancer cells more susceptible to the cytotoxicity caused by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) [53].
Another transcriptional component that OS affects via the miRNA regulatory mechanism is NF-κB [54]. NF-κB is a redox-sensitive TF that induces apoptosis, as evidenced by its activation by increased ROS [55]. Additionally, recent research has demonstrated that up-regulation of miR-27b reduces OS conditions and inhibits the activation of NF-κB brought on by lipopolysaccharide [56].
Numerous investigations into the expression of miRNAs in colorectal cancers have discovered that miR-143 and miR-145 may be regulators of carcinogenesis [57]. In this context, it has also been established that certain miRNA influences OS factors to carry out their biological functions in malignancies. For instance, it was discovered that miR-210, which has an HIF-1α-binding site in its promoter, is significantly up-regulated in hypoxic environments and increases the development of breast, pancreatic, and colon cancers [58]. Recent research has shown that overexpression of miR-143 may increase cell death and inhibit the growth of colon tumors in vivo [59]. Similarly, up-regulation of miR-145 has been shown to decrease colon cancer cell growth and proliferation and modify apoptosis [60, 61]. In the pathophysiology of hepatocellular carcinoma, miRNAs were also identified as important actors that regulate iron homeostasis [62]. Four OS-responsive miRNAs were identified in this context, and they were influenced by a p53-dependent mechanism (miR-1915-3p and miR-34a-5p) as well as a p53-independent one (miR-638 and miR-150-3p) [63].
Wilson et al. [64] showcase the potential of miR-103 in inhibiting major exonucleases in endothelial cells, which may suppress both developmental and pathological angiogenesis effectively. Furthermore, research by Yang and colleagues [65] indicates that overexpressing miR-328-3p can diminish cell growth and enhance the radiosensitivity of osteosarcoma cells by targeting H2AX, both in vitro and in vivo. Lastly, recent findings by Marampon et al. [66] suggest that the Nrf2/antioxidant enzymes/H2AX/miRNAs (including miR-22, miR-34a, miR-126, miR-146a, miR-210, and miR-375) axis holds promise as an innovative approach for radiosensitization in the clinical management of rhabdomyosarcoma.
Metabolic changes, such as those in glycolysis, fatty acid oxidation, and oxidative phosphorylation, are indicative of the growth of tumors [67]. La Sala et al. [68] have shown that miR-21 is up-regulated by glucose treatment and inhibits ROS homeostatic genes such as NRF2, SOD2, and KRIT1. According to earlier research, miR-33a/b is an intronic miRNA that is found near the genes for sterol regulatory element binding protein (SREBP) 1 and 2 [69]. These two miRNAs control the production of high-density lipoproteins (HDL) by cotranscription with their host gene.
There is growing evidence that miRNAs regulate cancer stem cells (CSCs) and are linked to the generation of ROS during the development of tumors and cancer. Let-7a, miR-21, miR-34a, miR-200, and miR-210 are among the miRNAs that may be implicated in regulating ROS generation in CSCs [70–74]. According to a different study, miR-21 inhibits the expression of SOD2, SOD3, and sprouty homolog 2 (SPRY-2) while increasing ROS generation through the MAPK pathway [75]. Furthermore, several investigations have demonstrated that miR-34a inhibits epithelial-mesenchymal transition (EMT) producers and CSC-related genes like CD44, which in turn reduces cell invasion, metastasis, and self-renewal ability [76]. Furthermore, normal human and mouse mammary stem/progenitor cells, as well as human breast CSCs, showed down-regulation of all five members of the miR-200 family [77]. When comparing MCF-7 spheroid cells and CD44+/CD24 MCF-7 cells to MCF-7 parental cells, miR-210 expression is higher in the former [78]. By inhibiting E-cadherin both in vitro and in vivo, overexpression of miR-210 promotes motility, invasion, proliferation, and self-renewal ability.
p53 serves as a crucial regulator in how cells respond to OS. It not only facilitates the repair of damaged DNA but also helps to reduce levels of ROS. At the same time, p53 can also elevate ROS production, which may lead to cellular senescence or apoptosis, highlighting its dual role in cell health [79]. Several miRNAs have been identified as significant players in cellular senescence, exhibiting different expression patterns in senescent cells. These include miR-21, miR-22, miR-29, miR-34a, miR-106b, miR-125b, miR-126, miR-146a, the miR-17-92 cluster, the miR-200 family, and miR-210 [80–86]. Additionally, miR-217 has been shown to inhibit angiogenesis by down-regulating the target gene SIRT1 in endothelial cells, resulting in an early senescence-like phenotype [87]. Furthermore, research conducted by Liu et al. [88] demonstrated that suppressing miR-92a enhances cell proliferation while simultaneously reducing ROS levels and caspase-3 activity, through the modulation of the Nrf2-KEAP1/ARE signaling pathway. Furthermore, it has been demonstrated that resistant gastric cancer cells become more chemo-sensitive when miR-6785-5p and miR-642a-3p are down-regulated and FOXO4 expression is subsequently increased [89].
In their research on chronic cholestatic liver injury, Yang and colleagues [90] made notable findings regarding the interplay between bile duct ligation, lithocholic acid treatment, and the c-Myc/miR-27/prohibitin 1 axis. They found that this axis was enhanced, which subsequently led to the suppression of Nrf2 expression and ARE binding. As a result, there was a reduction in GSH synthesis and a decrease in antioxidant capacity [90]. The miR-382-5p/SLC7A11 axis represents a promising target for lidocaine therapy, which has demonstrated the ability to induce ferroptosis in ovarian and breast cancer cells and effectively inhibit their growth in vitro. Furthermore, animal studies have provided encouraging insights, showing that mice with transplanted ovarian cancer tumors treated with lidocaine exhibited an up-regulation of miR-382-5p and a down-regulation of SLC7A11. Notably, the tumor volume in these mice was significantly reduced compared to the control group, suggesting that lidocaine could be a valuable addition to cancer treatment strategies [91]. However, before it is used in clinical practice, a number of issues must be taken into account. Lidocaine causes numerous side effects, including cardiovascular side effects, such as bradycardia, atrioventricular block, hypotension, muscle tremor, convulsions, respiratory depression, and paresthesia, as well as central nervous system side effects. Thus, more clinical testing is required to determine the effectiveness and side effects of lidocaine.
Propofol is an anesthetic that is widely used in clinical settings and offers promising therapeutic benefits in cancer treatment. Research has demonstrated that it can enhance the effects of erastin and significantly inhibit the migration, invasion, and proliferation of gastric cancer cells. By specifically targeting the miR-125b-5p/STAT3 axis, propofol can effectively induce ferroptosis, a form of regulated cell death, in these cancer cells. Additionally, studies have shown that propofol can play a key role in managing tumor volume in mice with transplanted gastric cancer, highlighting its potential as a valuable adjunct in cancer therapy [92]. According to recent research, ketamine, another anesthetic, can induce liver cancer cells to undergo ferroptosis by down-regulating the lncRNA PVT1. As a ceRNA, downregulation of lncRNA PVT1 enhanced miR-214-3p’s binding to GPX4 while decreasing its adsorption [93].
Metformin, a widely used hypoglycemic medication, has shown promising potential in cancer research. It specifically targets the miR-324-3p/GPX4 axis, which plays a crucial role in reducing the proliferation of breast cancer cells. In experiments conducted on mice with transplanted breast cancer tumors, metformin treatment led to an increase in the expression of miR-324-3p and a decrease in GPX4 levels. Notably, the tumors in these treated mice were significantly smaller compared to those in the control group, highlighting metformin’s potential as a therapeutic agent in breast cancer treatment [94].
Several natural compounds have shown promise in targeting cancer, alongside various synthetic chemical medications. One particularly noteworthy compound is Icariside II (ICS II), a flavonoid recognized for its anti-tumor properties. Research indicates that ICS II can effectively induce ferroptosis in renal cell carcinoma cells, the most common malignant tumor of the kidney, by targeting the p53-independent miR-324-3p/GPX4 axis. ICS II has no discernible impact on the viability of normal cells, but it can stop renal cell carcinoma cells from proliferating, invading, and migrating [95]. Curcumenol is an active compound found in Wenyujin with anti-tumor properties. The mechanism targeted by curcumenol is the lncRNA H19/miR-19b-3p/FTH1 pathway [96].
Previous research has demonstrated that anti-PD-1 antibodies have the potential to reactivate tumor-infiltrating CD8+ T cells within the tumor microenvironment (TME). This reactivation leads to the production of interferon-γ (IFN-γ), which can play a crucial role in promoting ferroptosis in tumor cells. Based on this concept, Guo et al. [97] developed miR-21-3p-loaded gold nanoparticles (miR-21-3p-AuNp) and administered them to mice with melanoma transplants. Following this, they administered anti-PD-1 as a form of immunotherapy. The findings were promising, as the combination treatment resulted in a more significant delay in tumor growth compared to treatment with either miR-21-3p-AuNp or anti-PD-1 alone. Moreover, there was a notable up-regulation of miR-21-3p levels and a corresponding down-regulation of TXNRD1 levels in the tumor tissue, suggesting a synergistic effect that could enhance therapeutic outcomes.
In vitro studies conducted by Luo et al. [98] have revealed the promising role of the miR-101-3p/TBLR1 axis in lung cancer treatment by effectively suppressing cell growth and promoting both ferroptosis and apoptosis in lung cancer cells. Their innovative approach involved fluorescent labeling experiments that demonstrated the successful accumulation of specially designed nanodrugs, including combinations of miR-101-3p and nanocarriers in the tumor tissues of mice with lung cancer. When administered via tail vein injections, these nanodrugs led to noteworthy changes in the TME, including increased expression of miR-101-3p alongside a decrease in TBLR1 expression. Furthermore, the study observed elevated levels of ROS and lipid ROS, accompanied by a reduction in GSH content. The findings also highlighted significant alterations in various markers: a decrease in GPX4 and PTGS2 (ferroptosis markers), a decline in Bcl-2 (an anti-apoptotic marker), and an increase in cleaved caspase-3 (an apoptosis marker). Most impressively, the treated mice showed a significantly smaller tumor volume compared to the control group, indicating the potential of these nanodrugs as an effective therapeutic strategy against lung cancer [98]. A study involving healthy individuals indicated that a flavonoid supplement containing quercitrin, rutin, and hesperidin can effectively reduce ROS and oxidative stress [99].
All things considered, a great deal of research has been done to clarify the molecular processes behind the ROS/miRNA axis and its function in carcinogenesis. In fact, ROS and miRNAs share traits with regard to carcinogenesis. Through transcriptional, posttranscriptional, and epigenetic control, ROS, as upstream regulators, alter the expression of miRNAs. However, miRNAs interfere with the generation of ROS (downstream mediator) and play a role in ROS-mediated processes. ROS and miRNAs can work together or against one another to control the spread of cancer. Many aspects of their interplay, meanwhile, are yet unknown and require more research. The capacity to target numerous genes within established pathways and the conservation of miRNA across multiple species with known sequences are two benefits of employing miRNA-target therapy. Interestingly, there are a number of miRNA-based treatments under development. For instance, the first miRNA-targeted treatment for HCV in clinical trials is anti-miR-122 modified with locked nucleic acid (LNA). Many aspects of their interplay, meanwhile, are yet unknown and require more research. The functional roles of miRNA in cellular adaptation to ROS vary depending on the tissue and cell type, as this article discusses.
ROS-dependent miRNAs have been revealed to be targetable by several chemically synthesized medicines and natural products for tumor therapy; nonetheless, this class of medications has many common shortcomings. First, it is unknown how natural items and chemically synthesized medications control miRNAs or related molecules. Second, there are a lot of targets that chemically manufactured medications and natural products can target, which leads to a lot of side effects and poor specificity. Thus, future research is focused on gene medicines with excellent specificity. At this time, preliminary research has been conducted on gene therapies for miRNAs, and the results have been confirmed in animals. Finding therapeutic targets, enhancing drug sensitivity, and comprehending the function of miRNA in cancers are all greatly impacted by this.
ROS plays a crucial role as an upstream regulator that significantly influences the expression of miRNAs through transcriptional, post-transcriptional, and epigenetic mechanisms. In a dynamic interplay, miRNAs also impact ROS-related processes and can modulate ROS synthesis as downstream mediators. This complex relationship between ROS and miRNAs can either promote or inhibit cancer progression, highlighting the intricate balance that exists within cellular pathways. However, much of their interaction remains poorly understood, underscoring the urgent need for further research. The consequences of this interplay are profound, as they are determined by the specific context of downstream molecules and various signaling pathways, which ultimately shape cellular phenotypes. Given ROS’s dual roles in both the initiation and progression of cancer, existing therapeutic strategies face considerable limitations. Moreover, the functional roles of miRNAs in responding to ROS are not uniform; they vary by tissue and cell type, emphasizing the need for a deeper understanding of these mechanisms. The connection between miRNA regulation and ROS-mediated activity opens up exciting new avenues for the development of innovative anticancer therapies.
3’-UTR: 3’-untranslated region
AREs: antioxidant response elements
CSCs: cancer stem cells
GPX4: glutathione peroxidase 4
GSH: glutathione
ICS II: Icariside II
Keap1: Kelch-like ECH-associated protein 1
MDA: malondialdehyde
miRNAs: microRNAs
Nrf2: nuclear factor erythroid 2 related factor 2
OS: oxidative stress
PRXL2A: peroxiredoxin-like 2A
RISC: RNA-induced silencing complex
ROS: reactive oxygen species
SIRT1: Sirtuin 1
SOD: superoxide dismutase
TFs: transcription factors
TME: tumor microenvironment
VEGF: vascular endothelial growth factor
SS: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. The author read and approved the submitted version.
The author declares that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1824
Download: 21
Times Cited: 0
Santiago Gelerstein-Claro ... Ramón Rodrigo
Anju Singh ... Shrikant Kukreti
