Affiliation:
Institute of Ecology and Genetics of Microorganisms, Perm Federal Research Center, Ural Branch of the Russian Academy of Sciences, 614081 Perm, Russia
Email: violetbaudelaire73@gmail.com
ORCID: https://orcid.org/0000-0002-1656-7277
Affiliation:
Institute of Ecology and Genetics of Microorganisms, Perm Federal Research Center, Ural Branch of the Russian Academy of Sciences, 614081 Perm, Russia
ORCID: https://orcid.org/0000-0002-4342-5362
Affiliation:
Institute of Ecology and Genetics of Microorganisms, Perm Federal Research Center, Ural Branch of the Russian Academy of Sciences, 614081 Perm, Russia
ORCID: https://orcid.org/0000-0001-9840-7578
Affiliation:
Institute of Ecology and Genetics of Microorganisms, Perm Federal Research Center, Ural Branch of the Russian Academy of Sciences, 614081 Perm, Russia
ORCID: https://orcid.org/0000-0002-2763-3620
Affiliation:
Institute of Ecology and Genetics of Microorganisms, Perm Federal Research Center, Ural Branch of the Russian Academy of Sciences, 614081 Perm, Russia
ORCID: https://orcid.org/0000-0001-6355-6178
Explor Med. 2025;6:1001323 DOI: https://doi.org/10.37349/emed.2025.1001323
Received: March 24, 2025 Accepted: May 09, 2025 Published: May 21, 2025
Academic Editor: Lee M. Wetzler, Boston University School of Medicine, USA
The article belongs to the special issue Global Perspectives on the Clinical Diagnosis, Treatment, and Functional Cure of HIV Infection in the Post-ART Era
Aim: This study investigated the effects of successful hepatitis C virus (HCV) treatment with direct-acting antivirals (DAAs) on CD4+ T cell recovery in HIV/HCV coinfected immunological non-responders (INRs) to antiretroviral therapy (ART). The study assessed changes in CD4+ and CD8+ T cell counts, immune activation, inflammation, T cell exhaustion, and the size of the naïve CD4+ T cell pool following DAA therapy to determine whether HCV suppression enhances immune restoration in coinfected INRs.
Methods: Three groups were analyzed: DAA-treated INRs (n = 9), untreated HIV/HCV coinfected INRs (n = 10), and healthy controls (n = 10). Plasma cytokine levels and viral loads were quantified using multiplex immunoassay and real-time PCR. Peripheral blood mononuclear cells were analyzed via flow cytometry to evaluate T cell subsets, activation (HLA-DR+CD38+), and exhaustion markers (PD-1, TIGIT).
Results: Both INR groups showed significantly lower CD4+ T cell counts and elevated CD4+ T cell proliferation compared to controls, with no significant difference between DAA-treated and untreated patients. Deficits in naïve CD4+ T cells were observed in both INR groups but reached statistical significance only in untreated individuals. Activated CD4+ and CD8+ T cells and proinflammatory cytokines of IFN and IL-10 families remained elevated in INRs after DAA treatment. DAAs reduced PD-1 and TIGIT expression on CD4+ T cells, suggesting attenuated exhaustion, but did not alter exhausted T cell frequencies in CD4+ or CD8+ T cells.
Conclusions: HCV coinfection in people living with HIV (PLWH) not only increases the risk of immunological non-response to ART but also has lasting impacts on the immune system. Even after successful HCV clearance with DAAs, INRs experience persistent immune dysregulation, including low CD4+ T cell counts, deficits in the naïve CD4+ T cell compartment, and ongoing inflammation. This indicates that HCV eradication alone is insufficient to reverse the long-term immune damage resulting from coinfection.
Hepatitis C virus (HCV) coinfection is common among people living with HIV (PLWH), affecting millions worldwide [1]. This is largely due to the overlap in transmission routes, particularly intravenous drug use [2]. Current estimates indicate that approximately 39 million individuals are PLWH [3], and 4 to 5 million of them are also chronically infected with HCV [4]. The prevalence among PLWH is particularly high in Eastern Europe, reaching up to 80% in some countries [5]. The presence of HIV complicates the pathogenesis of HCV infection, leading to accelerated liver disease progression, including rapid fibrosis and cirrhosis [6]. Furthermore, HIV/HCV coinfected subjects face an increased risk of developing hepatocellular carcinoma at an earlier age compared to those with HCV alone. In contrast, the impact of HCV on the pathogenesis of HIV infection is less well understood and is an area of ongoing investigation.
One of the most significant complications associated with HCV in PLWH is an increased risk of immunological non-response to antiretroviral therapy (ART) [7, 8]. Specifically, it occurs 3.5 times more often in PLWH with HCV coinfection compared to those without HCV [9]. In these immunological non-responders (INRs), HIV suppression does not coincide with an increase in CD4+ T lymphocyte counts, putting them at higher risk of morbidity and mortality [10–12]. The adverse effects of hepatitis C on CD4+ T cell regeneration in PLWH are believed to be linked to the high levels of immune activation and inflammation caused by chronic HCV infection [13–16]. This persistent inflammation contributes to CD4+ T cell exhaustion, as evidenced by the elevated frequency of cells expressing inhibitory receptors [17–19]. Another factor associated with poor CD4+ T cell restoration is the profound deficiency in the naïve CD4+ T cell compartment observed in HCV-coinfected INRs [20].
The development of direct-acting antivirals (DAAs) has enabled sustained HCV suppression in HIV/HCV coinfected INRs. This raises the possibility that successful treatment with DAAs may normalize the indexes of immune activation, inflammation, T cell exhaustion, and the size of the naïve T cell pool, consequently improving CD4+ T lymphocyte regeneration. In the current study, we assessed these parameters in DAA-treated HIV/HCV coinfected INRs to elucidate the effects of successful HCV therapy on CD4+ T cell recovery.
The study included HIV/HCV coinfected patients who had received ART for more than two years and had an undetectable viral load (< 50 copies/mL). INRs were defined as patients with the number of peripheral CD4+ T lymphocytes less than 350/μL. The study group comprised 9 HIV/HCV coinfected INRs who had successfully completed a DAA regimen at least 12 months prior to the start of the study. The control groups consisted of 10 INRs who did not receive DAAs, and 10 healthy volunteers without HIV and HCV infections.
Blood samples were collected from the cubital vein into Vacutainer tubes containing ethylenediaminetetraacetic acid (EDTA). CD4+ T lymphocyte counts were assessed using a BD Simultest™ IMK-Lymphocyte kit (Cat #340182, BD Biosciences, USA) and a CytoFLEX S flow cytometer (Beckman Coulter, USA). Blood plasma was separated by centrifugation (3,000 rpm for 10 min), and cytokine levels were measured using a Bio-Plex Pro™ Human Inflammation Panel 1, 37-Plex kit (Cat #171AL001M, Bio-Rad, USA) on a MAGPIX analyzer (Luminex Corporation, USA). HIV and HCV viral loads were determined by real-time polymerase chain reaction (PCR) using commercial kits: AmpliSense HIVMonitor-FRT (ILS, Russia) and RT-Hepatogen-C Quantitative (DNA-Technology, Russia), respectively. DAA therapy was considered successful if the HCV viral load was reduced to less than 200 copies/mL, which was the sensitivity limit of the test system.
Peripheral blood mononuclear cells (PBMCs) were isolated by density centrifugation using Diacoll (1.077 g/mL, Diaem, Russia). The isolated cells were then stored in liquid nitrogen in a cryopreservation medium containing 90% fetal bovine serum (FBS, Biowest, France) and 10% dimethyl sulfoxide (DMSO; AppliChem, Germany). On the day of the study, the cryopreserved cells were thawed at 37°C, washed in 10 mL of complete culture medium [RPMI-1640, supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin (Sigma, USA)], and then washed in 10 mL of Dulbecco’s phosphate buffered saline (DPBS; Gibco, USA).
PBMCs were analyzed using the CytoFLEX S flow cytometer. Viable cells were identified by the absence of staining with the Zombie UV Fixable Viability Kit (BioLegend, USA). The following antibodies were used: 1. Anti-CD3-BV605, anti-CD4-PE, and anti-CD8-BV510 (BioLegend, USA) to identify CD4+ and CD8+ T lymphocytes. 2. Anti-CCR7-PE/Cy7 (BioLegend, USA) and anti-CD45RO-APC-eFluor780 (Invitrogen, USA) to classify CD4+ and CD8+ T cells into naïve (CCR7+CD45R0–), central memory (TCM, CCR7+CD45R0+), effector memory (TEM, CCR7–CD45R0+), and effector memory cells re-expressing CD45RA (TEMRA, CCR7–CD45R0–). 3. Anti-TIGIT-AF488 and anti-PD-1-PB (BioLegend, USA) to assess the expression of the exhaustion markers. 4. Anti-CD38-PE/Fire700 (BioLegend, USA) and anti-HLA-DR-APC-R700 (BD Biosciences, USA) to identify activated cells.
Statistical analysis and data visualization were performed using the GraphPad Prism version 8.0.1 (GraphPad Prism Software Inc., San Diego, CA, USA). Quantitative data in the text and tables are presented as medians and 25–75 percentiles. To compare two groups of quantitative data, we used the Mann-Whitney U-test. For more than two groups, we used ANOVA with Tukey’s corrections.
All groups were comparable in terms of age (Table 1). Both genders were equally represented in the HCV+DAA– INR group and the healthy control group. However, the DAA-treated INR group consisted solely of men. HIV viral load did not differ among the groups of INRs. Notably, the DAA-treated INRs had been infected with HIV and treated with ART for a longer duration compared to the other HIV infected group.
Clinical characteristics
| Parameter | HIV+HCV+DAA– INRs | HIV+HCV–DAA+ INRs | Healthy controls |
|---|---|---|---|
| Number of enrollments | 10 | 9 | 10 |
| Age (years) | 41 (40–42) | 43 (35–47) | 41 (40–44) |
| Males (%) | 50pHCV–DAA+ < 0.05 | 100pHC < 0.05 | 50 |
| HIV-infection duration (years) | 8.0 (6.5–10.0)pHCV–DAA+ < 0.05 | 15.0 (10.0–20.5) | –– |
| ART duration (years) | 3.0 (2.0–4.7)pHCV–DAA+ < 0.05 | 6.0 (5.0–11.0) | –– |
| HIV viral load (copies/mL) | < 50※ | < 50※ | –– |
| Time elapsed post-DAA therapy (months) | –– | 21.5 (20.0–27.3) | –– |
The data are presented as medians and interquartile ranges. ※: the test-system sensitivity limit; ––: no data; ART: antiretroviral therapy; DAA: direct-acting antivirals; INRs: immunological non-responders. Significance of differences between groups was established based on the ANOVA test with Tukey’s corrections
The CD4+ T cell counts were comparable between DAA-treated (313; 157–352) and HCV-coinfected INRs (202; 183–297; Figure 1A). However, both patient groups had significantly lower CD4+ T cell counts compared to healthy control subjects [707 (646–880); p < 0.001]. A similar pattern was observed when comparing the CD4+/CD8+ T cell ratio between INRs and healthy individuals (p < 0.001; Figure 1B). In contrast, the CD8+ T cell counts were similar across all groups studied (p > 0.05; Figure 1C).
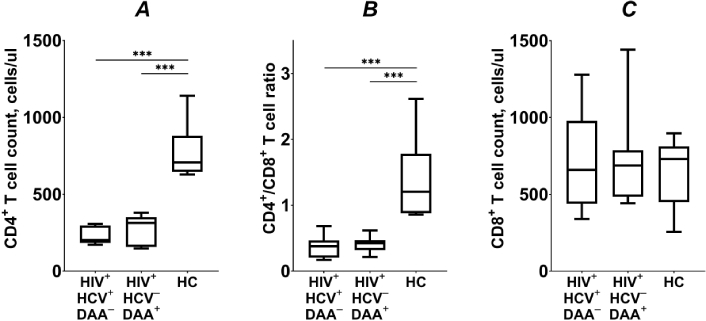
T lymphocyte counts (A, C) and CD4+/CD8+ T cell ratio (B) in HIV/HCV coinfected (HIV+HCV+DAA–) and DAA-treated (HIV+HCV–DAA+) immunological non-responders and healthy control group (HC). Medians (horizontal lines within rectangles), interquartile ranges (rectangles), and 10–90% intervals (vertical lines) are shown. ***: p < 0.001; ANOVA test with Tukey’s corrections
In PLWH, the CD4+ T cell pool is regenerated through homeostatic proliferation [21, 22]. Consequently, the frequency of proliferating (Ki-67+) CD4+ T lymphocytes was significantly higher in both groups of INRs compared to healthy control subjects (p < 0.01; Figure 2A).
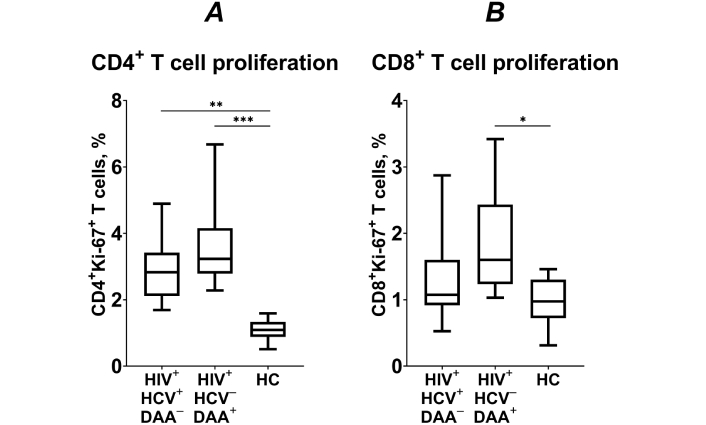
The frequency of proliferating (Ki-67+) CD4+ (A) and CD8+ T cells (B) HIV/HCV coinfected (HIV+HCV+DAA–) and DAA-treated (HIV+HCV–DAA+) immunological non-responders and healthy control group (HC). Medians (horizontal lines within rectangles), interquartile ranges (rectangles), and 10–90% intervals (vertical lines) are shown. *: p < 0.05, **: p < 0.01, ***: p < 0.001; ANOVA test with Tukey’s corrections
The frequency of Ki-67+ CD4+ T lymphocytes negatively correlated with the blood CD4+ T cell counts across all three study groups (R = –0.742, p < 0.0001). This suggests that the high level of CD4+ T lymphocyte proliferation observed in the DAA-treated group may be associated with the overall deficiency in CD4+ T cell counts.
The proportion of proliferating CD8+ T cells was similar between HCV coinfected INRs and healthy volunteers (p > 0.05; Figure 2B). However, in patients who underwent DAA treatment, the proportion of proliferating CD8+ T cells was significantly higher compared to healthy controls (p < 0.05; Figure 2). Hence, DAA treatment appears to enhance CD8+ T cell proliferation, potentially reflecting an improved immune response following successful HCV suppression.
We analyzed the frequency of CD4+ T cells at different stages of differentiation and found no significant differences in the frequencies of TCM, TEM, and TEMRA between the three groups. However, we did find a significant deficiency in naïve CD4+ T cells in the group of HCV coinfected INRs compared to healthy control subjects (p < 0.05; Figure 3). Patients who underwent DAA treatment also exhibited lower frequencies of naïve CD4+ T cells compared to healthy volunteers, but these differences were not statistically significant (p > 0.05). The frequency of naïve, TCM, TEM, and TEMRA CD8+ T cells did not differ significantly between HCV coinfected INRs, DAA-treated INRs, and healthy individuals (p > 0.05).
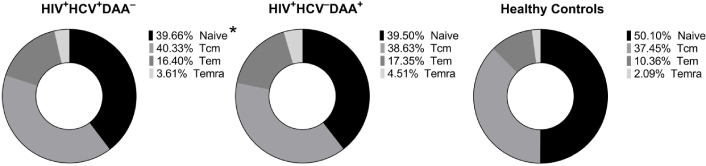
The frequency of naïve, central memory (TCM), effector memory (TEM), and terminally differentiated effector memory (TEMRA) CD4+ T cells in HIV/HCV coinfected (HIV+HCV+DAA–) and DAA-treated (HIV+HCV–DAA+) immunological non-responders and healthy control group (HC). *: p < 0.05 (HIV+HCV+DAA– vs. HC); ANOVA test with Tukey’s corrections
The proportion of activated (CD38+HLA-DR+) CD4+ T lymphocytes was significantly higher in both groups of INRs compared to healthy individuals (p < 0.01; Figure 4A). Similarly, the frequency of activated CD8+ T cells was also significantly higher in INRs (p < 0.05; Figure 4B). These findings indicate that the level of immune activation remains elevated in HCV coinfected INRs even after DAA treatment.
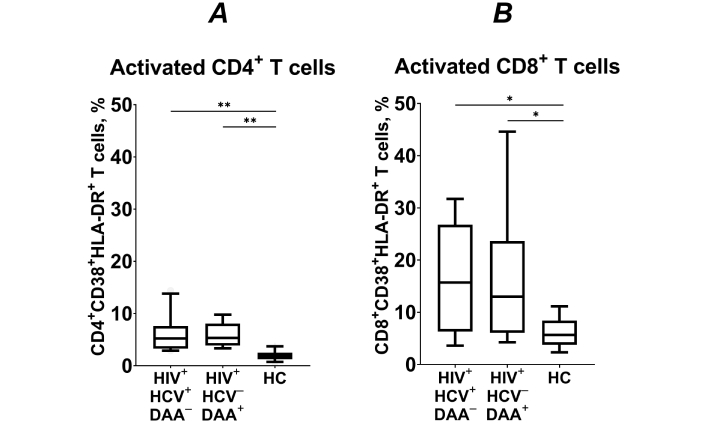
The frequency of activated (CD38+HLA-DR+) CD4+ (A) and CD8+ T cells (B) in HIV/HCV coinfected (HIV+HCV+DAA–) and DAA-treated (HIV+HCV–DAA+) immunological non-responders and healthy control group (HC). Medians (horizontal lines within rectangles), interquartile ranges (rectangles), and 10–90% intervals (vertical lines) are shown. *: p < 0.05, **: p < 0.01; ANOVA test with Tukey’s corrections
To assess systemic inflammation, we measured proinflammatory cytokine levels in the blood plasma of participants from three groups. Interferon family members (IFN-α, IFN-β, and IFN-γ) were significantly elevated in the blood plasma of HCV-coinfected INRs (p < 0.05, Figure 5). In INRs who underwent DAA treatment, IFN-α and IFN-γ levels remained elevated and were higher than in healthy controls. In contrast, IFN-β levels significantly decreased (p < 0.05) post-treatment, but still exceeded those of healthy individuals (p < 0.05).
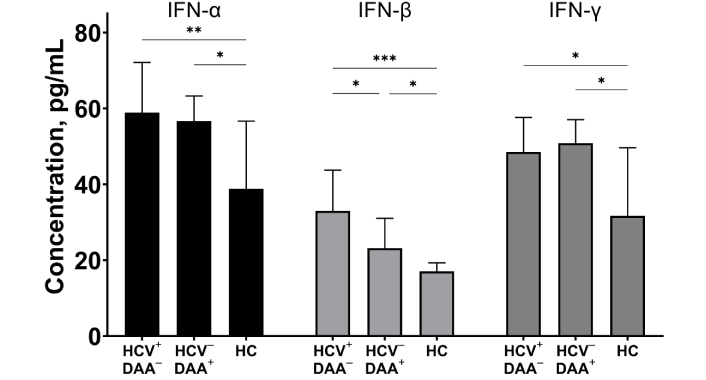
The concentrations of IFN-α, IFN-β, and IFN-γ in blood plasma of HIV/HCV coinfected (HIV+HCV+DAA–) and DAA-treated (HIV+HCV–DAA+) immunological non-responders and healthy donors (HC). Medians (bars) and interquartile ranges (vertical lines) are shown. *: p < 0.05, **: p < 0.01, ***: p < 0.001; ANOVA test with Tukey’s corrections
Plasma levels of cytokines from the IL-10 family were elevated in INRs. Specifically, we observed significantly higher levels of IL-20, IL-28A, and IL-29 in both HCV-coinfected and DAA-treated patients compared to controls (p < 0.05, Figure 6). Although there was a trend toward decreased IL-28A levels in DAA-treated INRs, this change did not reach statistical significance.
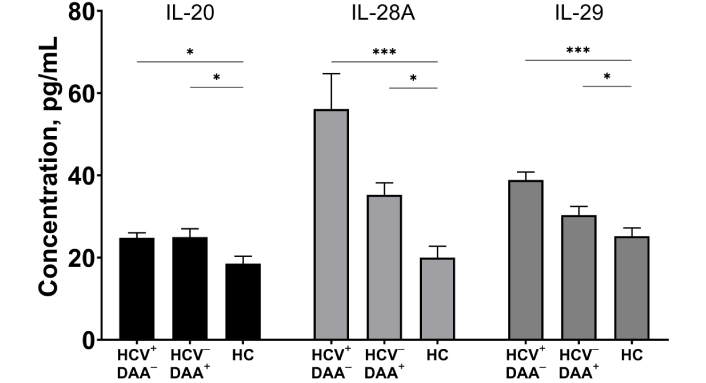
The concentrations of IL-20, IL-28A, and IL-29 in blood plasma of HIV/HCV coinfected (HIV+HCV+DAA–) and DAA-treated (HIV+HCV–DAA+) immunological non-responders and healthy donors (HC). Medians (bars) and interquartile ranges (vertical lines) are shown. *: p < 0.05, **: p < 0.01, ***: p < 0.001; ANOVA test with Tukey’s corrections
We analyzed the expression of the inhibitory receptors programmed cell death protein 1 (PD-1) and T cell immunoreceptor with immunoglobulin and ITIM domain (TIGIT), which are commonly used to evaluate T cell exhaustion in PLWH [23–25]. We found significantly increased frequencies of PD-1+ (p < 0.01) and TIGIT+ CD4+ T lymphocytes in both groups of INRs compared to healthy individuals (p < 0.05; Figure 7A, C).
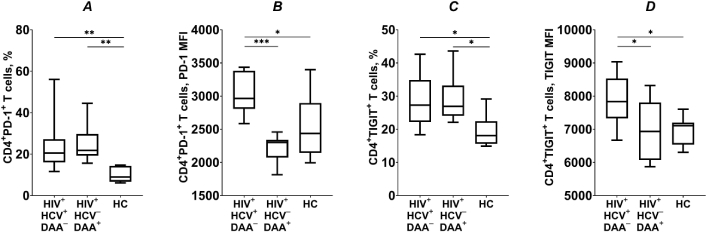
The frequency of CD4+ T cells expressing inhibitory receptors PD-1 and TIGIT (A and C), and their expression levels (B and D), in HIV/HCV coinfected (HIV+HCV+DAA–) and DAA-treated (HIV+HCV–DAA+) immunological non-responders and healthy control group (HC). Medians (horizontal lines within rectangles), interquartile ranges (rectangles), and 10–90% intervals (vertical lines) are shown. *: p < 0.05, **: p < 0.01, ***: p < 0.001; ANOVA test with Tukey’s corrections; PD-1: programmed cell death protein 1; TIGIT: T cell immunoreceptor with immunoglobulin and ITIM domain
However, in DAA-treated patients, the expression levels of the inhibitory receptors PD-1 (p < 0.001) and TIGIT (p < 0.05) on the surface of CD4+ T lymphocytes were significantly lower than those observed in HCV coinfected INRs without DAA treatment. These levels were comparable to those of healthy volunteers (p > 0.05; Figure 7B, D). Our findings suggest that in HCV coinfected INRs, DAA treatment may effectively reduce the exhaustion of CD4+ T lymphocytes, potentially restoring their functionality.
In HCV coinfected INRs, the proportion of CD8+ PD-1+ T lymphocytes was significantly higher compared to the healthy controls (p < 0.05; Figure 8A). After the DAA-treatment, the frequency of CD8+ PD-1+ T cells remained elevated in INRs, but was not significantly different from HCV-coinfected patients or healthy individuals (p > 0.05). Expression levels of PD-1 were comparable across all groups studied (p > 0.05; Figure 8B). The frequency of CD8+ TIGIT+ T lymphocytes, as well as TIGIT expression levels, was similar across all three groups (p > 0.05; Figure 8C, D). It is important to note that TIGIT expression in CD8+ T cells is strongly correlated with differentiation status and is predominantly observed on cells exhibiting a memory phenotype [26]. Therefore, TIGIT may not accurately reflect the state of exhaustion in these cells.
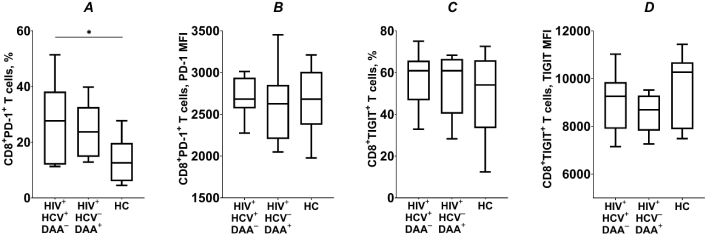
The frequency of CD8+ T cells expressing inhibitory receptors PD-1 and TIGIT (A and C), and their expression levels (B and D), in HIV/HCV coinfected (HIV+HCV+DAA–) and DAA-treated (HIV+HCV–DAA+) immunological non-responders and healthy control group (HC). Medians (horizontal lines within rectangles), interquartile ranges (rectangles), and 10–90% intervals (vertical lines) are shown. *: p < 0.05; ANOVA test with Tukey’s corrections
In PLWH, hepatitis C coinfection increases the risk of immunological non-response to ART and rapid progression to AIDS [15]. However, new DAA therapies for hepatitis C have shown high success in achieving sustained virological response in HIV/HCV coinfected patients [27]. This has led to a reduction of liver-related complications and improved overall health outcomes [28]. While DAAs are highly effective at eradicating HCV, their impact on the immune system of coinfected INRs is still an area of active investigation.
Our research shows that DAA treatment does not significantly improve CD4+ or CD8+ T cell counts in INRs. Other studies [28–30] also confirm that in PLWH, there are no substantial changes in these populations or the CD4+/CD8+ ratio after DAA therapy. The persistent deficits in CD4+ T cell counts and CD4+/CD8+ ratios observed in both the HCV-coinfected and DAA-treated INR groups, compared to healthy controls, suggest that HCV clearance alone is insufficient to reverse the long-term immunological damage caused by years of coinfection.
We observed that the deficits in the naïve CD4+ T cell compartment in HCV-coinfected INRs persist after successful DAA therapy. This persistence may be attributed to the significant impairment of thymic function associated with HCV coinfection. Studies have demonstrated that chronic HCV infection leads to reduced numbers of naive CD4+ T cells and compromised thymic output [31, 32]. In the context of HIV infection, it has been noted that HCV-coinfected patients have lower numbers of recent thymic emigrants compared to those with HIV alone [20]. Furthermore, the numbers of recent thymic emigrants are inversely correlated with levels of liver enzymes indicative of hepatic damage [20]. Notably, our findings suggest that thymic damage caused by chronic HCV infection may be irreversible, as the frequency of naïve CD4+ T cells does not increase following DAA therapy. Naïve CD4+ T cells play a crucial role in generating diverse antigen-specific immune responses and replenishing the memory T cell pool [33]. Consequently, the inability to replenish the naïve CD4+ T cell pool, even after successful HCV treatment, may impede effective immune reconstitution, leaving these patients vulnerable to opportunistic infections and other complications.
While naive T cells are necessary to replenish the diverse pool of T lymphocytes, in HIV infection, the main burden of regeneration lies with memory T cells [34]. During lymphopenia, they actively proliferate to increase their numbers [21, 22]. In the current study, we observed a negative correlation between the number of peripheral CD4+ T cells and their proliferation. This suggests that in both groups of INRs, homeostatic mechanisms are actively engaged in efforts to restore the CD4+ T cell pool. However, even after successful HCV eradication, the CD4+ T lymphocytes’ proliferation is insufficient to fully restore the immune system. In the meantime, the heightened proliferative activity of CD8+ T cells observed in the DAA-treated INRs suggests that successful HCV clearance can enhance the immune response mediated by the CD8+ T cell compartment. This may reflect an improved ability to mount effective antigen-specific responses following HCV eradication.
Previous research has shown that HCV-coinfected PLWH exhibit an increased proportion of activated CD4+ and CD8+ T lymphocytes, which is generally viewed as an unfavorable sign [35]. In HIV infection, the primary source of immune activation and inflammation is typically the damage to the gut epithelial barrier, which allows microbial products to translocate into systemic circulation [36]. HCV coinfection appears to impair hepatic clearance of microbial products, as indicated by increased plasma levels of lipopolysaccharide, which contributes to heightened immune activation in coinfected PLWH [20]. Most studies have reported a decline in immune activation markers following DAA treatment in HIV/HCV coinfected patients responding well to ART [37–40]. PLWH who respond effectively to ART also experience decreased systemic inflammatory markers, such as sCD163 and sCD14, after HCV eradication [27, 29]. However, our findings indicate that even after successful DAA therapy, INRs continue to display persistently elevated activation levels of CD4+ and CD8+ T cell populations, compared to healthy controls. Furthermore, in DAA-treated INRs, we observed persistently elevated plasma levels of type I and II interferons (IFN-α, IFN-β, IFN-γ) as well as IL-10 family cytokines (IL-20, IL-28A, IL-29). This suggests that long-term damage from chronic HCV infection in INRs may be irreversible, and that hepatic clearance of bacterial products in these patients does not improve post-DAA treatment. This is particularly concerning, as chronic inflammation can have detrimental effects on immune function and T cell homeostasis. Prolonged exposure to high levels of cytokines like IFN-α, IFN-γ, and IL-10 family members has been linked to T cell dysfunction, exhaustion, and impaired regenerative capacity [41, 42]. Moreover, chronic type I interferon signaling has been identified as a key driver of HIV disease progression [43–46].
The regulation of T cell activity is crucial in the context of inflammation and heightened activation levels. Inhibitory receptors like PD-1 and TIGIT play a vital role in this process [47, 48]. However, prolonged and excessive expression of these receptors can lead to functional exhaustion of T cells, characterized by impaired effector functions and reduced proliferative capacity [19, 49, 50]. Our analysis revealed no significant differences in the frequencies of PD-1 and TIGIT-expressing CD4+ T cells between the DAA-treated and HCV coinfected INR groups. Interestingly, we observed that the expression levels of these inhibitory receptors were significantly lower in the DAA-treated INR group, resembling those of healthy subjects. This suggests that successful HCV clearance through DAA treatment can help reduce CD4+ T cell exhaustion in INRs. The present finding is crucial, as restoring the functional capacity of CD4+ T cells is essential for improving immune reconstitution in these patients.
It should be noted that this study has several limitations. The small sample size of 10 participants per group may limit the generalizability of the findings. Additionally, an imbalance in sex distribution, with one group of INRs predominantly consisting of men, could introduce confounding effects due to sex differences in immune and metabolic responses. Furthermore, although all patients were tested negative for hepatitis B virus (HBV), many may have a history of cytomegalovirus (CMV) infection, which could also influence immune parameters and potentially affect the results. Future studies involving larger and more diverse cohorts are warranted to validate and extend these results.
The HCV coinfection in PLWH not only increases the risk of immunological non-response to ART, but also has lasting impacts on the immune system of INRs. Importantly, the dysregulated immune profile persists in INRs even after HCV clearance, suggesting that the accumulated immune damage from years of coinfection may not be fully reversible. Although DAA treatment effectively eradicates HCV, it does not significantly improve CD4+ counts in INRs. The persistent deficits in the naïve CD4+ T cell compartment, along with ongoing immune activation and inflammation after DAA therapy, suggest that HCV clearance alone is insufficient to reverse long-term immune damage in these patients. Our findings suggest that successful HCV eradication may reduce CD4+ T cell exhaustion in INRs, as evidenced by decreased expression of inhibitory receptors on these cells. However, further investigation is needed to confirm this hypothesis. Overall, addressing immunological non-response to ART in HCV coinfected patients may require a more comprehensive approach. This could include interventions targeting the persistent inflammatory response and strategies aimed at regenerating the naïve CD4+ T cell compartment. Additional research is necessary to identify the optimal combination of treatments to restore immune homeostasis in INRs.
ART: antiretroviral therapy
DAAs: direct-acting antivirals
FBS: fetal bovine serum
HCV: hepatitis C virus
HIV: human immunodeficiency virus
IL: interleukin
INRs: immunological non-responders
PD-1: programmed cell death receptor 1
PLWH: people living with HIV
TIGIT: T cell immunoreceptor with immunoglobulin and ITIM domain
VV: Conceptualization, Investigation, Visualization, Writing—original draft. ES and LK: Conceptualization, Investigation, Writing—review & editing. NS: Project administration, Resources, Investigation. KS: Conceptualization, Validation, Writing—review & editing, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The work described has been carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. The work plan (No. 17-54-30006) was approved by the ethics committee of the Perm Regional Center for the Prevention and Control of AIDS and Infectious Diseases (committee registration number IRB00008964).
Informed consent to participate in the study was obtained from all participants.
Not applicable.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
This work was carried out within the framework of the State assignment #124021900006-5. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1898
Download: 17
Times Cited: 0
Yadessa Tegene Woldie ... Mark Spigt
Zhimin Huang, Xiaohui Wang
Werner Krause
Yun-Meng Yan ... Qi-Wen Yang
Yang Zhou ... Hongzhou Lu
