Affiliation:
1Molecular Biology Research Center & Center for Medical Genetics, School of Life Sciences, Central South University, Changsha 410078, Hunan, China
2National Clinical Research Center for Infectious Diseases, Shenzhen Clinical Medical Research Center for Tuberculosis, Shenzhen Third People’s Hospital, Shenzhen 518112, Guangdong, China
†These authors are co-first authors who have made equal contributions to this work.
ORCID: https://orcid.org/0000-0003-1785-5315
Affiliation:
2National Clinical Research Center for Infectious Diseases, Shenzhen Clinical Medical Research Center for Tuberculosis, Shenzhen Third People’s Hospital, Shenzhen 518112, Guangdong, China
†These authors are co-first authors who have made equal contributions to this work.
ORCID: https://orcid.org/0000-0002-5134-1230
Affiliation:
2National Clinical Research Center for Infectious Diseases, Shenzhen Clinical Medical Research Center for Tuberculosis, Shenzhen Third People’s Hospital, Shenzhen 518112, Guangdong, China
†These authors are co-first authors who have made equal contributions to this work.
ORCID: https://orcid.org/0000-0002-8053-2319
Affiliation:
2National Clinical Research Center for Infectious Diseases, Shenzhen Clinical Medical Research Center for Tuberculosis, Shenzhen Third People’s Hospital, Shenzhen 518112, Guangdong, China
†These authors are co-first authors who have made equal contributions to this work.
ORCID: https://orcid.org/0009-0002-9436-3154
Affiliation:
2National Clinical Research Center for Infectious Diseases, Shenzhen Clinical Medical Research Center for Tuberculosis, Shenzhen Third People’s Hospital, Shenzhen 518112, Guangdong, China
ORCID: https://orcid.org/0009-0000-8547-4042
Affiliation:
2National Clinical Research Center for Infectious Diseases, Shenzhen Clinical Medical Research Center for Tuberculosis, Shenzhen Third People’s Hospital, Shenzhen 518112, Guangdong, China
ORCID: https://orcid.org/0009-0008-0471-0217
Affiliation:
2National Clinical Research Center for Infectious Diseases, Shenzhen Clinical Medical Research Center for Tuberculosis, Shenzhen Third People’s Hospital, Shenzhen 518112, Guangdong, China
ORCID: https://orcid.org/0000-0002-7717-7440
Affiliation:
2National Clinical Research Center for Infectious Diseases, Shenzhen Clinical Medical Research Center for Tuberculosis, Shenzhen Third People’s Hospital, Shenzhen 518112, Guangdong, China
ORCID: https://orcid.org/0009-0003-9661-7135
Affiliation:
2National Clinical Research Center for Infectious Diseases, Shenzhen Clinical Medical Research Center for Tuberculosis, Shenzhen Third People’s Hospital, Shenzhen 518112, Guangdong, China
ORCID: https://orcid.org/0009-0001-8072-4628
Affiliation:
2National Clinical Research Center for Infectious Diseases, Shenzhen Clinical Medical Research Center for Tuberculosis, Shenzhen Third People’s Hospital, Shenzhen 518112, Guangdong, China
Email: pengfeizhao@aliyun.com
ORCID: https://orcid.org/0000-0003-2034-7696
Affiliation:
2National Clinical Research Center for Infectious Diseases, Shenzhen Clinical Medical Research Center for Tuberculosis, Shenzhen Third People’s Hospital, Shenzhen 518112, Guangdong, China
3Pharmaceutical Department, Dongguan Songshan Lake Central Hospital, Dongguan 523320, Guangdong, China
Email: mingbinzheng@126.com
ORCID: https://orcid.org/0000-0003-0463-7968
Affiliation:
2National Clinical Research Center for Infectious Diseases, Shenzhen Clinical Medical Research Center for Tuberculosis, Shenzhen Third People’s Hospital, Shenzhen 518112, Guangdong, China
Email: luhongzhou@fudan.edu.cn
ORCID: https://orcid.org/0000-0003-3590-4790
Explor Med. 2025;6:1001373 DOI: https://doi.org/10.37349/emed.2025.1001373
Received: July 28, 2025 Accepted: October 14, 2025 Published: November 27, 2025
Academic Editor: Lee M. Wetzler, Boston University School of Medicine, USA
The article belongs to the special issue Global Perspectives on the Clinical Diagnosis, Treatment, and Functional Cure of HIV Infection in the Post-ART Era
Despite antiretroviral therapy (ART) effectively suppressing viral replication and reducing transmission risk, human immunodeficiency virus (HIV) infection still sustains cycles of chronic inflammation, immune dysfunction, and dysbiosis of microbiota, driving barrier disruption, microbial translocation, and systemic inflammation. These pathological states accelerate cluster of differentiation 4+ (CD4+) T cell depletion, contribute to viral persistence, and exacerbate the risk of death caused by complications. Current microbiome interventions, such as prebiotics and fecal microbiota transplantations (FMTs), exhibit limited efficacy in regulating HIV infection-associated chronic inflammation, immune dysfunction, and dysbiosis of microbiota due to transient colonization and poor pathogen specificity. Bacteriophages (phages), which are viruses that precisely target bacteria, represent a promising alternative to ameliorate these intervention deficiencies and to optimize microbiome modulation, especially in HIV patients. Their precise host range and genetic tractability enable targeted modulation of pathogenic, commensal, and pathobiontic microbiota, which in turn enhances immunity against imbalanced microbiome-associated diseases. In this review, we explored phage therapy’s potential to disrupt HIV-associated pathologies affecting the host microbiome. We elucidated the mechanisms by which phage therapy targeted dysbiotic bacteria in HIV and reviewed the supporting preclinical and early clinical evidence for its role in preventing acquisition, enhancing viral clearance, restoring immunity, and managing comorbidities. Finally, we analyzed the challenges in translating phage therapy into clinical practice, which mainly include phage selection, regulatory frameworks, and delivery systems, and evaluated potential solutions to address these challenges. Collectively, our review emphasized how phage therapy can bring a paradigm shift in HIV management, where integrating microbiome-immune crosstalk with virology and synthetic biology may enable a functional cure within the next decade.
Human immunodeficiency virus/Acquired Immunodeficiency Syndrome (HIV/AIDS) remains a major challenge for global public health. According to the World Health Organization (WHO) and the Joint United Nations Programme on HIV/AIDS (UNAIDS), there were an estimated 40.8 million people living with HIV (PLWH) at the end of 2024 [1]. Although interventions such as antiretroviral therapy (ART), HIV pre-exposure prophylaxis (PrEP), and harm reduction programs have successfully reduced HIV transmission rates, PLWH continue to experience chronic inflammation and immune dysfunction. Chronic inflammation and immune deficiency in PLWH promote dysbiosis of the gut and lung microbiota, thereby initiating a vicious cycle of barrier dysfunction, microbial translocation, and systemic inflammation. This cycle exacerbates cluster of differentiation 4+ (CD4+) T cell exhaustion, enhances viral persistence, and disrupts commensal microbial communities [2]. These pathological processes contribute to the maintenance of latent HIV reservoirs and increase the burden of clinical comorbidities. Despite advances in treatment, HIV transmission continues to remain a substantial global health challenge.
Microbiota intervention measures for PLWH mainly include prebiotics [3], fecal microbiota transplantation (FMT) [4], and short-chain fatty acid (SCFA) administration [5]. However, these measures have limited efficacy, poor durability, and poor pathogen targeting. Considering these limitations, we propose shifting research attention to bacteriophage (phage) therapy, an emerging and highly targeted antimicrobial approach [6]. By enabling the precise recognition and lysis of specific bacteria, phage therapy offers a powerful tool for reshaping dysbiotic microbial communities. We have confirmed this in clinical studies of phage therapy for multidrug-resistant infections, which demonstrate that precise host recognition and lysis by phages can selectively remodel dysbiotic microbial communities [7]. It holds considerable promise as a novel strategy for ameliorating chronic inflammation and immune dysfunction in PLWH. Multiple evidence show that phage therapy can regulate the balance of the microbiome (reducing Prevotella, Streptococcus, etc., and enhancing the abundance of Bacteroides and Lactobacillus) and its metabolites [8, 9], which are key regulators of HIV-related pathology [10]. Persistent immune deficiency and chronic inflammation of PLWH lead to the imbalance of intestinal flora, forming a vicious cycle of “microbiota imbalance-intestinal/lung barrier destruction and pathogen translocation”. This cycle accelerates CD4+ T cell exhaustion, promotes viral replication, chronic inflammation, and becomes a key driver of ART-related drug-resistant infections and complications. HIV-related chronic inflammation severely damages the commensal flora [11], impairs mucosal immunity, and increases the metabolic burden in the liver and kidneys [12, 13]. Notably, phage therapy repairs the mucosal barrier, avoids microbial translocation, and prevents HIV invasion [14]. It is interesting to note that SCFAs and bile acids (BAs) can significantly affect the pathogenesis of HIV by regulating immune cell functions, such as T helper 17 cell (Th17) [15], regulatory T cell (Treg) differentiation, pro-inflammatory/anti-inflammatory balance [16], and dendritic cells [17] (Figure 1). As phages have been shown to regulate the balance of the microbiome and its lipid metabolism, including SCFAs and BAs, phage therapy is now possible to protect the integrity of the intestinal barrier, which helps in regulating immune homeostasis and suppressing chronic inflammation.
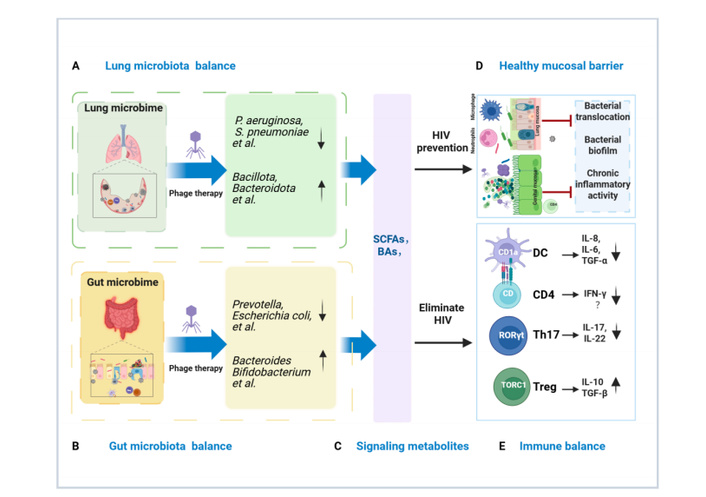
Phage therapy alleviates mucosal barrier damage, microbial translocation, and chronic inflammation in the gut and respiratory tract of HIV infected individuals. (A) Lung microbiota balance: Lung dysbiosis features pathogenic biofilm and damage. Lytic phages selectively eliminate these bacteria to restore a healthy microbiota. (B) Gut microbiota balance: In HIV, gut dysbiosis with pathobiont overgrowth damages the epithelium. Lytic phages precisely eliminate these bacteria to restore a healthy microbiota. (C) Metabolite signaling: Depletion of pathobionts restores microbiota-derived immunomodulators (SCFAs and BAs), recovering epithelial tight junctions in gut/lung mucosae and activating regulatory pathways (RORγt+ T cells, TORC1) that suppress inflammation. (D) Gut/Lung mucosal restoration: Reconstituted barriers reduce microbial translocation, disrupt biofilms, and resolve chronic inflammation. (E) Gut/Lung immune balance: Changes to these metabolites consequently increase Treg cell responses and decrease Th17 cell responses. This shift elevates levels of the immunoregulatory factors IL-10 and TGF-β while reducing levels of the proinflammatory factors IL-17 and IL-22. Combined with weakened DC responses, this cascade inhibits levels of the proinflammatory factors IL-6, IL-8, and TGF-α, which are beneficial for reducing inflammation and promoting HIV clearance. HIV: human immunodeficiency virus; SCFAs: short-chain fatty acids; BAs: bile acids; DC: dendritic cell; CD4: cluster of differentiation 4; Th17: T helper 17 cell; Treg: regulatory T cell; IL: interleukin; INF-γ: interferon-gamma; TGF-β: transforming growth factor-beta. Created in BioRender. He, Z. (2025) https://BioRender.com/gv2gwnv.
This review focuses on the therapeutic potential of phages in HIV management, with an emphasis on two primary areas: first, the targeting of pathogenic components within the HIV-associated microbiota, and second, the clinical applications of phage therapy. These applications are explored across four key domains: (i) the prevention of HIV infection, (ii) the inhibition of viral replication, (iii) the treatment of opportunistic infections, and (iv) the management of HIV-related complications. Finally, we synthesize the prevailing challenges and outline future trajectories for the field. We posit that, over the next decade, a systems-level approach integrating phage therapy with microbiome-immune crosstalk, virology, and synthetic biology holds significant promise for transforming HIV prevention, treatment, and strategies for achieving a functional cure.
The microbiome, including bacteria, archaea, viruses, fungi, and protists, features a virome rich in phages that influence host physiology. HIV infection disrupts mucosal barriers and immune homeostasis, affecting the microbiome and virome. Modulating the viral community alters immune function, thereby mitigating HIV pathogenesis [18]. Stabilizing the virome or altering phage populations offers a promising strategy to restore the mucosal ecosystem, complementing ART.
Phages undergo lytic or lysogenic life cycles. In the lytic cycle, obligately lytic phages adsorb to host receptors, inject their genome, and hijack bacterial machinery to replicate and assemble progeny virions. Subsequent expression of holin and endolysin proteins disrupts the cytoplasmic membrane and degrades peptidoglycan, resulting in lysis and release of infectious progeny [19]. In contrast, temperate phages can integrate into the host genome or persist episomally. Environmental stressors may trigger prophage excision and re-entry into the lytic cycle. Due to concerns regarding virulence gene transfer, transduction, and horizontal gene transfer, therapeutic phages must be carefully selected to exclude lysogenic, virulence, or transduction-related genes [20]. Rigorous genomic screening is essential to ensure safety and prevent unintended outcomes in clinical applications [21] (Figure 2).
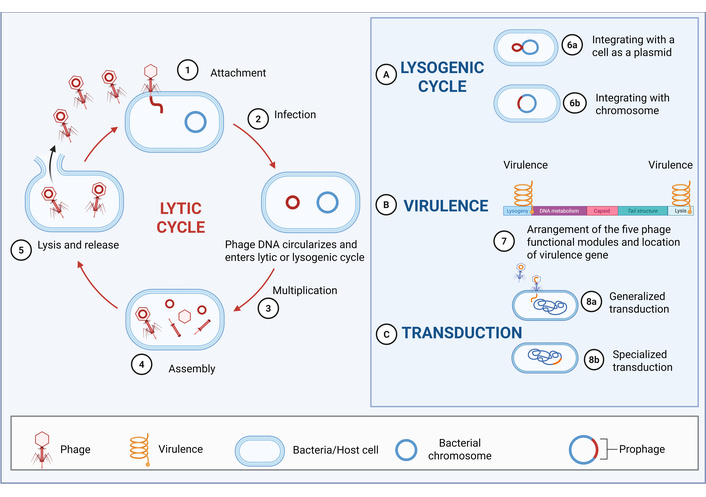
Phage life cycle and safety. This diagram illustrates the different life cycles of phages (lytic cycle, lysogenic cycle), as well as the mechanisms of phages in virulence and transduction (including generalized transduction and specialized transduction). The left side depicts the lytic cycle, where phages sequentially undergo attachment, infection, multiplication, assembly, and lysis and release. The right part (A) shows the lysogenic cycle, in which phages can integrate with a cell as a plasmid (6a) or integrate with the chromosome (6b). (B) Presents the arrangement of the five phage functional modules and the location of virulence genes, reflecting the influence of phages on virulence. (C) Shows the transduction process, including generalized transduction (8a) and specialized transduction (8b). Created in BioRender. He, Z. (2025) https://BioRender.com/ez4qqfn.
During phage-bacteria interactions, bacteria deploy a multilayered defense network comprising surface modifications [22], restriction-modification systems, and CRISPR-Cas machinery to specifically block phage infection. Phage’s co-evolution with bacteria drives microbiome diversity, influences community structure, regulates metabolism and immunity, and ultimately shapes human health and ecosystem dynamics [23, 24]. Therefore, by exploiting phage-bacterium coevolutionary dynamics, phage therapy precisely remodels microbiome architecture to reestablish ecological balance.
The microbiome’s influence on HIV pathogenesis is well-established. If the microbiome is disturbed in PLWH, it will lead to HIV-related pathology [25]. Phage therapy corrects HIV-associated dysbiosis by selectively remodeling gut and lung microbiota composition and function. In PLWH, enriched bacteria exacerbate disease progression through metabolite-mediated dysregulation of host immunity and barrier function: Prevotella enrichment leads to a decrease in SCFAs [26] and activation of myeloid dendritic cells (mDCs), thereby inducing immune abnormalities. Pseudomonas and Escherichia coli secrete barrier-disrupting mucolytics (e.g., indole derivatives) that directly promote HIV replication [27]; Desulfovibrio releases pro-inflammatory hydrogen sulfide [28]; Acinetobacter-derived lipopolysaccharide (LPS) and α-ketoglutarate stimulate interleukin (IL)-8-dependent neutrophil recruitment [29]; and Ruminococcus generates barrier-impairing metabolites [30, 31]. These changes initiate a vicious cycle. The initial breach of the epithelial barrier permits microbial translocation into systemic circulation, triggering a state of chronic immune activation. This persistent activation exhausts the CD4+ T cell compartment and creates a favorable environment for robust viral replication. These processes operate synergistically to drive the progression of HIV pathogenesis.
At the same time, emerging evidence identifies microbe-derived metabolites (particularly abundant colonic SCFAs and BAs) as critical mediators of innate and adaptive immunity [32]. These microbially produced/modified compounds mechanistically link dysbiosis to chronic inflammation and immune dysfunction in PLWH [33], positioning microbial metabolites as central effectors in disease progression. Furthermore, compositional fluctuations within the microbiota—particularly dynamic shifts in translocated microorganisms [34]—induce metabolic reprogramming of immune cells characterized by upregulated glycolysis/hypoxia-related genes [35] and downregulated mitochondrial function genes [36, 37]. This drives early elevation of proinflammatory cytokines, including IL-8 and interferon-gamma-inducible protein 10 (IP-10), activates Th17 immune responses, and exacerbates inflammation while paradoxically promoting mucosal repair. The subsequent attenuation of this response constitutes a key biological switch enabling T-cell homeostasis restoration [15].
Administration of phage cocktail in HIV restored eubiosis through (i) enrichment of SCFA-producing taxa (e.g., Bacillota, Bacteroidota) and concomitant SCFAs elevation, (ii) immune reconstitution and intestinal/lung barrier integrity via increased SCFAs and BAs, and decreased proinflammatory cytokines. Therefore, phage-mediated ecological restoration serves as a novel therapeutic strategy for metabolic disorders in HIV management. By reestablishing eubiosis, phage therapy directly targets the ecological dysfunction that underlies many HIV-associated comorbidities. These effects reduce susceptibility to metabolic diseases, cardiovascular issues, and opportunistic infections. Therefore, the clinical value of phage therapy lies in its capacity to translate microbiome modulation into tangible improvements in overall health.
PLWH exhibit elevated incidence of microbiome-associated comorbidities (including dyslipidemia, cardiovascular disorders, metabolic diseases, and chronic inflammation) compared to HIV-negative individuals. As dominant constituents of the microbiome, phages critically shape the structural and functional evolution of microbial communities, with particular significance in HIV pathogenesis. Consequently, phage therapy, through precise regulation of the microbiome and systemic immune balance, exhibits significant translational potential in HIV prevention, management of HIV-associated comorbidities, and control of opportunistic infections (Figure 3). The following sections describe these aspects in detail:
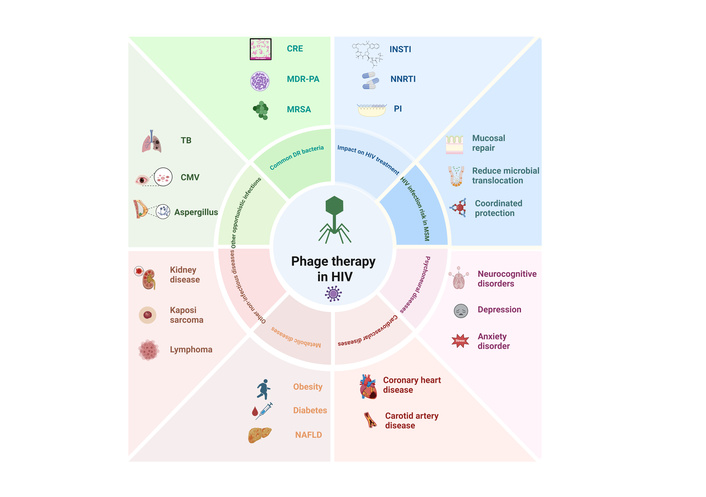
Schematic diagram of the application of phage therapy in HIV prevention, HIV-related opportunistic infections, and complications. In PLWH, phage therapy prevents HIV infection by repairing the mucosa, bacterial translocation, and immune coordination protection. At the same time, it enhances the efficacy of ART drugs, including INSTIs, NNRTIs, and PIs, to eliminate HIV and reduce drug toxicity and side effects. Phage therapy can eliminate pathogens by targeting specific bacterial flora in common drug-resistant bacterial infections, including vancomycin-resistant Staphylococcus aureus, CRE, and MDR-PA. In addition, phage therapy may also be applied to uncommon drug-resistant bacterial infections, such as mycobacterial infections, CMV infections, and fungal infections, which target specific bacteria, regulate microbial communities, repair mucosal barriers, and reduce chronic inflammation. Phage therapy may also alleviate HIV-related complications, neuropsychiatric diseases (neurological diseases, anxiety disorders, and depression), cardiovascular diseases (coronary heart disease, atherosclerosis), metabolic diseases (diabetes, obesity, and fatty liver) and other diseases (kidney disease, lymphoma, and Kaposiʼs sarcoma), by repairing the mucosal barrier, regulating the microbiota and systemic active or auxiliary immune levels, and improving clinical outcomes. HIV: human immunodeficiency virus; PLWH: people living with HIV; CRE: Carbapenem-resistant Enterobacteriaceae, MDR-PA: multidrug-resistant Pseudomonas aeruginosa, MRSA: methicillin-resistant Staphylococcus aureus, INSTI: integrase strand transfer inhibitor, NNRTI: Non-Nucleoside Reverse Transcriptase Inhibitor, PI: Protease Inhibitor, TB: tuberculosis, CMV: cytomegalovirus, NAFLD: non-alcoholic fatty liver disease. Created in BioRender. He, Z. (2025) https://BioRender.com/zrd067i.
The U.S. Centers for Disease Control and Prevention (CDC) endorses a combination of highly effective HIV prevention strategies. The most effective include treatment as prevention (TasP), where ART suppresses viral load in people with HIV, and PrEP for those at risk (highest). Other key strategies include consistent condom use and, for some populations, voluntary male medical circumcision (lowest). Despite this hierarchy, all approaches face limitations. This prevention gap primarily reflects the dynamics of rectal mucosal transmission, which accounts for 66% of new infections among men who have sex with men (MSM), a population in which mucosal integrity is easily compromised during viral entry [38, 39]. Other challenges include suboptimal PrEP adherence (67% in Africa) and microbiome-dependent drug metabolism, which results in reduced efficacy of tenofovir gel in bacterial vaginosis due to drug degradation [40].
Thus, despite systemic viral suppression, microbiome disturbances increase the risk of HIV infection [41] and are an independent predictor of HIV risk. Phage therapy is a precise microbiome regulation method that selectively targets pathogens while protecting commensal flora to restore ecological balance, repair mucosal barriers, avoid microbial translocation, synergize with and enhance the efficacy of blocking drugs, and coordinate the bodyʼs immunity to reduce HIV infection risk. In one approach, encapsulating broad-spectrum lytic phages in mucoadhesive nanoparticles [e.g., chitosan or poly(lactic-co-glycolic acid)] for vaginal or rectal delivery prolongs mucosal residence and enhances epithelial penetration, thereby enabling selective depletion of pathobionts such as Prevotella spp. while preserving beneficial commensals [42, 43]. By mitigating dysbiosis and reinforcing mucosal barrier integrity, lytic phage therapy via mucoadhesive nanoparticles may reduce HIV susceptibility and offer a novel microbiome-based approach to PrEP. This strategy overcomes key therapeutic limitations in disease prevention. Microbiome analysis showed that regardless of HIV infection status, the intestinal flora of MSM showed a trend of enrichment of Prevotella and loss of Bacteroidetes. Therefore, in this population, phages can specifically eliminate Prevotella, protect the abundance of intestinal Bacteroidetes and Erysipelothrixaceae (including Cartinibacterium tricaudatum and Holdermanella dimorphicum), promote T cell activation and homing receptor expression [38, 44], maintain microbiome balance, and improve body immunity. HIV-infected women also showed a similar pattern of dysbiosis, characterized by reduced dominance of Lactobacillus and increased flora diversity, which in turn increased the risk of sexually transmitted infections and impaired bioavailability of local antiviral drugs [45]. For example, phages can suppress the abundance of anaerobic vaginal pathogens such as Gardnerella, thereby reducing the impact of these microorganisms on the effective tissue concentration of tenofovir [40], coordinating blocking drugs to prevent HIV infection. In addition, for translocated microorganisms that cause early inflammatory cascades and disrupt mucosal repair [15], phage therapy can repair the mucosal barrier, reduce translocated microorganisms, and quench the risk of HIV infection. Through this targeted lytic effect, phage intervention can suppress the production of specific microbial metabolites, restore intestinal homeostasis, and improve disease outcomes [12].
In addition, engineered phage therapy has shown multifaceted potential in combating viral infection and restoring mucosal homeostasis, for example, T7 phage surface display binds HIV fusion inhibitors (e.g., Griffith peptide or C34 peptide). These constructs were formulated into mucoadhesive gels, which blocked viral entry and achieved in vitro neutralization (IC50 = 0.8 nM). In another study, phage-mediated targeted lysis of pathogenic bacteria (such as Prevotella and Pseudomonas) has been shown to repair the integrity of the intestinal barrier through a dual mechanism: restoring commensal bacteria that produce SCFAs and enhancing the expression of epithelial tight junction proteins [46]. This cascade reaction can reduce microbial translocation, plasma soluble cluster of differentiation 14 (sCD14) and LPS levels, thereby alleviating systemic inflammatory responses [47]. In addition, phages can achieve synergistic post-exposure protection by regulating microbial dysbiosis to drive host immune metabolic reprogramming, thereby inhibiting early mucosal HIV amplification [15] (Figure 3).
Phages dramatically shape microbial community composition. Alterations in the intestinal virome following HIV infection, including a reduced abundance of Caudovirales, have been linked to increased immune activation [48]. Phage therapy aimed at reestablishing virome-bacteria homeostasis may contribute to the reduction of chronic systemic inflammation, thus diminishing the immune stimulation that facilitates HIV replication and viral persistence [49]. For example, the Lak phages infect bacteria of the genus Prevotella and regulate the balance of the microbiota [50]. Dysbiosis is the core pathological feature of HIV infection, characterized by an increase in Prevotella, a decrease in Bacteroides and Faecalibacterium, and an abnormal expansion of Proteobacteria [51] (Figure 1). Emerging evidence suggests that phage therapy, by ameliorating gut dysbiosis, may represent a novel strategy to complement current HIV eradication efforts [52]. Phage therapy ameliorates dysbiosis, thereby improving ART efficacy and alleviating chronic inflammation through the dual metabolic-immune pathways. Studies have shown that phages can target specific microbiota to improve the efficacy of anti-HIV drug treatment. For example, an increased abundance of Bifidobacterium was observed in individuals receiving integrase strand transfer inhibitor (INSTI) treatment [53]. The targeted reduction of Bifidobacterium abundance by specific phages altered lipid metabolism in patients on INSTI regimens. This microbial shift may indirectly inhibit weight gain and modulate drug distribution [53]. Furthermore, phages can regulate the gut microbiota to interfere with microbial-dependent drug metabolism. This intervention can increase bacterial β-glucuronidase activity [54], which disrupts BA metabolism and subsequently impairs the hepatic metabolism of ART drugs like tenofovir disoproxil fumarate (TDF). The resulting increase in plasma drug concentrations may enhance the efficacy of HIV inhibition [55]. Second, phages have been shown to target microbiota, regulate the probiotic flora, repair the immune barrier function, and reduce inflammation. Studies have found that a decrease in the abundance of Bacteroidetes leads to insufficient production of SCFAs, such as butyrate, weakens intestinal epithelial tight junctions, and promotes the translocation of Gram-negative bacterial LPS (such as from Klebsiella) into circulation. Further studies have found that SCFAs deficiency inhibits CD4+ naive T cell proliferation, leading to delayed immune reconstitution after ART (the risk of impaired CD4+ T cell recovery increased 2.3 times for every 0.5 unit decrease in the Shannon index of the microbiota). Phage targeting specific bacteria, such as Prevotella, can directly affect colonic mDCs, modulate CD40 expression, and inhibit mucosal T cell activation. Prevotella abundance is positively correlated with sCD14 levels, a marker of microbial translocation.
Increasing evidence has further revealed the intricate cross-kingdom interactions between viruses, bacteria, and host immunity that together promote disease progression [56, 57]. The human virome, including phages, is considered a key regulator of microbiome ecology and disease pathogenesis, playing an important role in type 1 and type 2 diabetes (T2D) and inflammatory bowel disease [57]. In HIV-infected individuals, severe immunodeficiency (CD4+ T cells < 200/μL) is associated with elevated levels of Anelloviridae, Adenoviridae, and Papillomaviridae viruses, which in turn predict poor immune recovery on ART. At the same time, phage communities in HIV infection show highly individualized evolutionary trajectories, and the β-diversity of phage communities is closely related to ART drug exposure [58]. The virome, including phages, drives systemic inflammatory factors, such as IL-6 and D-dimer, aggravating disease progression [59]. In addition, changes in the diversity and composition of translocated microbial species can indirectly regulate virome dynamics and CD4+ T cell reconstitution by affecting Th17 cell differentiation and mucosal barrier repair [15]. However, research results are conflicting, with some studies reporting that intestinal phage communities do not undergo significant changes after HIV infection, ART, or CD4+ T cell depletion [48], highlighting the need for further investigation of these complex interactions.
Phage therapy can directly target mycobacteria and eliminate them, leading to improved clinical outcomes [60]. On the other hand, the microbiome, including phages, of HIV-infected patients with Mycobacterium tuberculosis (MTB) shows unique changes that vary with tuberculosis (TB) status, HIV infection, and ART. Specifically, analysis of the microbiome of different TB sites showed reduced beta diversity in patients with bacteriologically confirmed TB compared with non-TB (nTB) controls [61–63]. Although there are some inconsistencies in the results of studies on the TB-specific gut microbiome, key changes generally involve changes in SCFAs-producing species (e.g., Lachnospiraceae, Ruminococcus, Blautia, Dorea, and Faecalibacterium), inflammation-associated species (e.g., Prevotella), and potentially beneficial genera (e.g., Bifidobacterium) [64]. This further demonstrates that TB-specific dysbiosis is associated with the metabolism of key SCFAs. HIV infection status and ART significantly affect these microbiome characteristics and are associated with treatment efficacy [65]. HIV-positive patients with MTB show enrichment of Mycobacterium/Bifidobacterium, while ART treatment reduces both genera. Lung involvement is associated with enrichment of Mycobacteria and depletion of Streptococci [62]. Phage therapy, as an adjunct to antibiotics for the treatment of resistant mycobacterial infections, can reduce antibiotic doses and improve their efficacy. Thus, phage therapy offers all these therapeutic benefits with a good safety profile and minimal toxicity [60]. Mycobacterium abscessus is a nontuberculous mycobacterium (NTM) with a high degree of intrinsic and acquired antibiotic resistance, which severely limits treatment options and has a poor prognosis [66]. However, phage therapy cleared a persistent M. abscessus infection in a cystic fibrosis patient, allowing for a successful lung transplant [66]. Further studies have confirmed that phage therapy has a significant efficacy (> 50%) against mycobacterial infections [60]. Overall, these advances provide unprecedented treatment options for serious, previously untreatable mycobacterial infections and lay the foundation for future clinical trials.
Since CMV infection is associated with specific changes in the composition of the intestinal flora, including a decrease in the Firmicutes to Bacteroidetes abundance ratio [67], phage therapy targets specific intestinal flora and regulates the balance of the microbial community, which may improve intestinal flora disorders that lead to CMV replication, intestinal mucosal barrier damage, and elevated levels of pro-inflammatory cytokines in the colon, ultimately improving the progression of colitis. Additionally, phage therapy promotes intestinal flora symbiosis [68], increases SCFAs production, and improves the relationship between Treg/Th17 imbalance caused by CMV [69]. Phage therapy has also been reported to break the vicious cycle between CMV and intestinal flora, which may be initiated through type I interferon signaling [70, 71]. The interaction between CMV infection and lung flora has been less studied, and the application of phage therapy to understand this phenomenon deserves further study.
Invasive fungal disease (IFD) is a common opportunistic infection and a significant cause of hospitalization and mortality in HIV-infected patients [72]. Crucially, the diversity of lung microbiota composition appears to predict survival in patients with invasive pulmonary aspergillosis (IPA) [73], suggesting that the microbiome plays an important role in fungal infections. When it comes to the microbiome, the public often focuses on bacteria and viruses, but they often ignore the fact that the “fungal community” that accounts for a certain proportion may also play a key role [74]. During invasive fungal lung infections, phage therapy targets bacterial species in the lungs, improving bacterial richness and alpha diversity, as well as the ratio of Prevotella to Veillonella; this may improve the prognosis of IPA patients [73]. Furthermore, phage therapy mitigates invasive fungal colonization by targeting facilitating bacteria such as Pseudomonas aeruginosa, Burkholderia cepacia, and Haemophilus influenzae, thereby limiting their proliferation and reducing bacterial burden on the bronchial mucosa in cystic fibrosis patients [75]. Therefore, phages that target microbiota modulation have become a promising new approach for antifungal therapy [76]. Strategies such as phage therapy and probiotics may alter the composition of the intestinal microbiota by directly modulating immune cells or releasing health-promoting metabolites that affect systemic immunity [77].
Dysbiosis in PLWH promotes chronic inflammation, which underpins the development of serious comorbidities such as cardiovascular disease, obesity, T2D, and kidney disease. By specifically targeting the bacterial components of this dysbiosis, phage therapy offers a novel approach to managing inflammation and preventing these HIV-related complications.
Microbiome dysbiosis is mechanistically linked to cardiovascular pathogenesis, including heart failure, atherosclerosis, and hypertension, with mechanisms involving inflammation, metabolic reprogramming, and microbial-derived metabolites [78, 79]. In PLWH, phages targeting the elimination of pro-inflammatory microbiota (e.g., Fusobacterium nucleatum) can increase SCFAs production, and reduce the accumulation of atherogenic metabolites [e.g., imidazole propionate (ImP)], thereby jointly inhibiting the progression of vascular lesions [3, 79]. In addition to the targeted elimination of pro-inflammatory pathogens [80], phage therapy can also reduce LPS translocation, inhibit Toll-like receptor-mediated inflammation, reduce IL-6/IL-8/IL-17 levels [81–83], and slow the progression of carotid plaques, showing therapeutic potential.
Microbiome dysbiosis underlies the development of metabolic diseases in PLWH [84]. HIV disrupts SCFAs-mediated glucose regulation through mucosal damage, translocation of microbiota in mucosa, chronic inflammation, and tryptophan catabolism via the kynurenine pathway [85, 86], and phage-specific targeting can mitigate the risk of T2D. In addition, ART improves survival, but it leads to insulin resistance, dyslipidemia, and fat redistribution. These are precursors to metabolic syndrome, T2D, and cardiovascular disease [87]. Emerging evidence supports phage-mediated precise elimination of Prevotella to restore butyrate synthesis and improve insulin sensitivity [88], thereby modulating homeostasis model assessment of insulin resistance (HOMA-IR) and adiponectin in PLWH and improving diabetes outcomes [89]. Therefore, phage therapy strategies targeting the microbiome are an important component of long-term HIV management [88].
Obesity is a significant comorbidity in PLWH, and rapid weight gain is associated with a higher risk of diabetes and metabolic sequelae compared with the general population [90]. Due to obesity- and HIV-induced dysbiosis, gut microbiota-derived SCFAs and BAs—key regulators of inflammation and metabolism, are reduced [91, 92]. Targeted phage therapy for dysbiosis could mitigate the direct effects of HIV proteins and ART on adipocyte biology, genetic susceptibility, microbial translocation, adaptive immune dysregulation, tissue inflammation, and accelerated fibrosis [93]. In PLWH, enrichment of Ruminococcus and Streptococcus, taxa linked to MASLD, compromises intestinal tight junctions, facilitating translocation of bacteria and LPS to the liver, where LPS-driven activation of hepatic stellate cells worsens steatosis and may promote fibrosis [94, 95]. Phage-mediated reduction of Gram-negative bacterial load may ultimately improve liver enzyme profiles and reduce lipid deposition [94, 95]. Given the current lack of effective obesity interventions for PLWH, phage-targeted bacterial regulation of the microbiota has become a key therapeutic strategy in the metabolic diseases [90] and is expected to open up new therapeutic avenues for obesity management in this population.
Neurocognitive function declines more rapidly in PLWH, frequently leading to HIV-associated neurocognitive disorder. For example, the severity of distal neuropathic pain (DNP) in this population is associated with reduced intestinal α-diversity and increased abundance of Blautia/Clostridium [96]. HIV-induced gut microbiota dysbiosis results in significantly lower levels of circulating butyrate and valerate than in HIV-seronegative people, disrupts the mucosal endothelial barrier, and leads to persistent microbial translocation and systemic inflammation [97, 98]. Therefore, phages target specific microbiota, affect key SCFA metabolites, repair intestinal epithelial integrity, and regulate immune levels [99], ultimately changing this pro-inflammatory environment and disrupting gut-brain axis signal transduction, which may mediate the pathogenesis of HIV-associated neurocognitive disorder. In addition, phages mediate intestinal microbiota balance, which has a parallel mechanism with mood regulation [100]. Notably, alterations in the gut microbiota of individuals with HIV, including a decline in Bacteroides and a rise in aerotolerant bacteria, have been associated with adverse emotional outcomes [101]. Conversely, in HIV-HCV co-infected individuals, depression is linked to elevated Bacteroides abundance and disrupted BA metabolism [102]. Although phage regulation of neuroactive microbial pathways and serotonin metabolism represents a promising therapeutic approach, its mechanistic evidence is still preliminary and warrants further study.
Kidney disease, including HIV-associated nephropathy (HIVAN), is a serious complication of HIV infection, and gut dysbiosis is associated with this pathogenesis. ART can alleviate direct renal damage caused by HIV to varying degrees, but HIV-affected or ART-induced gut dysbiosis persists for a long time and may accelerate renal failure through inflammatory cascades and the production of uremic toxins [103]. The microbiota of patients with chronic kidney disease (CKD) shows a significant expansion of uremic toxin precursor-producing bacteria [104]. Phages intervene in the regulation of microbial flora and metabolites, reduce chronic inflammation and repair mucosal barriers, avoid microbial translocation (such as Enterobacteriaceae) [105], and affect Kupffer cells through portal circulation, reduce systemic inflammation and glomerular damage, and reduce renal fibrosis formation [95].
In addition to renal pathology, HIV also induces skin flora imbalance (such as a decrease in skin bacilli) and barrier dysfunction, and there is a bidirectional relationship between the two [106]. Local phage therapy may restore ecological balance and improve diseases such as atopic dermatitis [106]. Microbiome composition also affects vaccine response. The enrichment of Bifidobacterium/Faecalibacterium in PLWH is associated with higher titers of coronavirus disease 2019 (COVID-19) vaccine-induced IgGs [107], suggesting that application of phages to control microbial species can optimize vaccine immunogenicity. The elevated risk of HIV-associated cancers—including Kaposi sarcoma, lymphoma, and anal cancer—is further promoted by gut dysbiosis. This occurs through several oncogenic pathways: chronic inflammation, local and systemic immunosuppression, direct DNA damage, and the production of pro-tumorigenic microbial metabolites [108]. Notably, anal precancerous lesions in HIV-infected individuals are associated with enrichment of Prevotella [109]. Thus, local phage therapy could be a strategy to target carcinogenic microbiota. Elucidating the tripartite interactions between HIV, the microbiota, and extraintestinal pathologies remains critical for developing targeted interventions to restore microbiota balance and improve clinical outcomes.
The application of phage therapy to modulate the HIV-associated microbiome is fraught with complexity, primarily due to the intricate triad of microbiome-immune-viral pathogenesis interactions. A major obstacle is the considerable inter-individual variation in microbial composition, influenced by genetics, lifestyle, and environment, which confounds the establishment of universal definitions for “health” and “dysbiosis”. This variability, compounded by significant heterogeneity in clinical study designs, challenges the reproducibility and broader applicability of research outcomes [110]. This can be addressed by establishing cross-scale phage therapy research platforms that perform dynamic predictive modeling through multi-omics data fusion (metagenomics/metabolomics/immunoassays). Poorly defined interactions between HIV, sexual behavior, dietary habits, and microbial ecology further obscure causal relationships, which can be mitigated by machine learning-driven barrier integrity assessment (via SCFA ratios such as propionate/butyrate) and CD4+ T cell-stratified precision pharmacokinetics (for sustained-release formulations with counts < 200/μL) to enhance mechanistic elucidation. Mechanistically, the host-phage-microbiome [111] is perturbed by HIV-mediated alterations in quorum sensing [112] and antiretroviral drugs (ARVs), which result in a unique and complex microenvironment. For this unique and complex microenvironment, synergistic combination strategies can be adopted to improve efficacy. Critical knowledge gaps persist due to technical limitations in analyzing high-dimensional omics data, particularly in proteomics and metabolomics. These gaps may be addressed through staged ecological reprogramming—a sequential process involving pathogen-specific phage deployment, probiotic or FMT-mediated introduction of beneficial colonies, and the use of engineered phages to promote a stable microbial ecosystem.
These complexities introduce research uncertainties into the design of phage therapy in the context of HIV. First, the broad spectrum of infecting pathogens (ranging from ESKAPE pathogens to rare fungi like Talaromyces marneffei) can be targeted precisely using the staged ecological reprogramming strategy. Secondly, ART (e.g., the rilpivirine/dolutegravir regimen) exerts varying effects on the host microbiome, and personalized treatment hinders the study of regular patterns—this can be addressed by real-time monitoring methods to spatially track phage localization and bacterial clearance dynamics. In addition, bacterial-phage competition increases the evolutionary escape rate of bacteria in immunosuppressed hosts by 2-3-fold, elevating drug resistance risk, which can be prevented through resistance gene monitoring.
Despite clear therapeutic potential, phage delivery confronts multiple translational challenges, including formulation and storage instability, scalable GMP compliant production and purification, limited mucosal penetration and rapid host immune neutralization, emergent bacterial resistance and risk of endotoxin mediated inflammation or horizontal gene transfer, unclear pharmacokinetics and dosing regimens, and unresolved regulatory and clinical evidence requirements that must be addressed to enable safe and effective clinical deployment. A central challenge in therapeutic phage delivery is their unique pharmacokinetic-pharmacodynamic (PK/PD) behavior, stemming from the capacity of phages to replicate at the infection site. This results in nonlinear and context-dependent kinetic profiles that integrate pathogen load, mucosal colonization time, and spatial structure into treatment exposure [113, 114]. Clinical translation is constrained by phage immunogenicity that provokes neutralizing antibodies and cellular responses, by rapid bacterial evolution of phage resistance, and by the practical barriers to producing scalable, individualized phage cocktails. Addressing these challenges necessitates a dual strategy: first, the multidisciplinary quantification of critical phage metrics (e.g., mucosal residence time, in situ replication, clearance) to build predictive PK/PD models; and second, the development of pragmatic delivery solutions, including immune-evasive encapsulation, phage cocktails, integrated diagnostic libraries, and harmonized production standards. Thus, reconciling the complexity of HIV-related diseases with therapeutic goals demands multidimensional innovation to transform phage therapy into viable clinical translation.
In summary, phage therapy introduces a novel therapeutic paradigm for HIV management, enabling the precision targeting of HIV-associated pathobionts (e.g., Prevotella spp.), repair of the mucosal barrier, and modulation of the immune-metabolic axis—notably via the restoration of SCFA and BA homeostasis. Collectively, these mechanisms pivot the treatment strategy from passive viral suppression to the active ecological control of the microbiome-metabolism-immunity triad. Consequently, microbiome engineering is positioned as a strategic intervention to mitigate chronic inflammation, eradicate latent viral reservoirs, prevent opportunistic infections and non-AIDS comorbidities, and advance the field toward a functional cure for HIV.
AIDS: Acquired Immunodeficiency Syndrome
ART: antiretroviral therapy
BAs: bile acids
CD4+: cluster of differentiation 4+
CMV: cytomegalovirus
FMTs: fecal microbiota transplantations
HIV: human immunodeficiency virus
IL: interleukin
INSTI: integrase strand transfer inhibitor
IPA: invasive pulmonary aspergillosis
LPS: lipopolysaccharide
mDCs: myeloid dendritic cells
MSM: men who have sex with men
MTB: Mycobacterium tuberculosis
phage: bacteriophage
PK/PD: pharmacokinetic-pharmacodynamic
PLWH: people living with human immunodeficiency virus
PrEP: pre-exposure prophylaxis
sCD14: soluble cluster of differentiation 14
SCFA: short-chain fatty acid
T2D: type 2 diabetes
TB: tuberculosis
Th17: T helper 17 cell
Treg: regulatory T cell
HL, MZ, and PZ: Conceptualization, Supervision, Writing—review & editing. YZ, PX, DL, and ZH: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. ZL, XD, GD, LZ, and YC: Writing—review & editing. All authors read and approved the submitted version.
Hongzhou Lu, who is the Editorial Board Member and Guest Editor of Exploration of Medicine, had no involvement in the decision-making or the review process of this manuscript. The other authors declare no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This study was supported by the Shenzhen Clinical Medical Research Center for Major Emerging Infectious Diseases [lcyssq2022082309120007], the Shenzhen Strategic Emerging Industries Special Fund Project [F-2022-Z99-502266], the Shenzhen High Level Hospital Construction Internal Supporting Funds (Infectious Diseases Department) Project [XKJS-CRGRK-009], and the Shenzhen “Three Project” Medical Team Project [SZSM202311033]. The funder has no role in research design, data collection and analysis, publication decisions, or manuscript preparation.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1416
Download: 24
Times Cited: 0
Yadessa Tegene Woldie ... Mark Spigt
Violetta Vlasova ... Konstantin Shmagel
Zhimin Huang, Xiaohui Wang
Werner Krause
