Affiliation:
1Nanobiotech lab, Department of Zoology, Kirori Mal College, University of Delhi, Delhi 110007, India
ORCID: https://orcid.org/0000-0003-2789-9979
Affiliation:
1Nanobiotech lab, Department of Zoology, Kirori Mal College, University of Delhi, Delhi 110007, India
ORCID: https://orcid.org/0000-0003-2707-7545
Affiliation:
1Nanobiotech lab, Department of Zoology, Kirori Mal College, University of Delhi, Delhi 110007, India
2Fellow, Delhi School of Public Health, Institution of Eminence, University of Delhi, Delhi 110007, India
Email: akverma@kmc.du.ac.in
ORCID: https://orcid.org/0000-0001-6341-5930
Explor Med. 2022;3:393–413 DOI: https://doi.org/10.37349/emed.2022.00102
Received: April 29, 2022 Accepted: July 25, 2022 Published: August 31, 2022
Academic Editor: Esma R. Isenovic, University of Belgrade, Serbia
The article belongs to the special issue Reactive Oxygen Species (ROS) in Pathophysiological Conditions
Osteoporosis is a metabolic bone disorder that affects both sexes and is the most common cause of fractures. Osteoporosis therapies primarily inhibit osteoclast activity, and are seldom designed to trigger new bone growth thereby frequently causing severe systemic adverse effects. Physiologically, the intracellular redox state depends on the ratio of pro-oxidants, oxidizing agents (reactive oxygen species, ROS) and antioxidants. ROS is the key contributor to oxidative stress in osteoporosis as changes in redox state are responsible for dynamic bone remodeling and bone regeneration. Imbalances in ROS generation vs. antioxidant systems play a pivotal role in pathogenesis of osteoporosis, stimulating osteoblasts and osteocytes towards osteoclastogenesis. ROS prevents mineralization and osteogenesis, causing increased turnover of bone loss. Alternatively, antioxidants either directly or indirectly, contribute to activation of osteoblasts leading to differentiation and mineralization, thereby reducing osteoclastogenesis. Owing to the unpredictability of immune responsiveness and reported adverse effects, despite promising outcomes from drugs against oxidative stress, treatment in clinics targeting osteoclast has been limited. Nanotechnology-mediated interventions have gained remarkable superiority over other treatment modalities in regenerative medicine. Nanotherapeutic approaches exploit the antioxidant properties of nanoparticles for targeted drug delivery to trigger bone repair, by enhancing their osteogenic and anti-osteoclastogenic potentials to influence the biocompatibility, mechanical properties and osteoinductivity. Therefore, exploiting nanotherapeutics for maintaining the differentiation and proliferation of osteoblasts and osteoclasts is quintessential.
Osteoporosis occurs due to imbalance in bone modelling, characterized by declination of bone mass and microarchitecture of bone tissues that lead to higher risk of bone fragility resulting in bone fracture [1]. Globally, it is one of the common disorders affecting both males and females. Risk factors causing osteoporosis include genetic variations, previous history of fractures, excessive sports activity, low calcium intake, other nutrient deficiencies, excessive lipid intake and low antioxidant states of the body [2]. With increased life expectancy of global population, occurrence of osteoporosis in old aged people is inevitable. Statistically, it is calculated 10.3% of population who are above 50 years of age are suffering from osteoporosis in the USA alone and will rise up to 19% and 32% by the end of 2030 [3]. Osteoporotic patients suffer pain and poor quality of life that affects their social life tremendously and also causes economic burden on them. Early prevention, diagnosis, therapy and proper management of the degenerative disorder are extremely important.
A critical imbalance modulating activity of osteoblasts and osteoclasts is responsible for both bone formation and bone resorption. Negative balance between these two specialized cells—osteoblastic and osteoclastic regulation leads to osteoporosis [4]. Negative balances are caused by factors including oxygen supply, improper nutrients like calcium and vitamin D deficiency, cytokines, endocrines, growth factors and hormonal changes. Free radicals and mitochondrial DNA deletion are factors for reactive oxygen species (ROS) induced osteoporosis that mainly occurs in males. This leads to ineffective oxidative phosphorylation and poor electron transport chain followed by increased production of oxygen-free radicals. Recent studies have reported oxidative stress as a probable perpetrator leading to the evident uncoupling of osteoblast and osteoclast functions in osteoporosis [5]. The differentiation of osteoblasts and osteoclasts is considered to be very crucial in the pathogenesis of osteoporosis [6].
ROS is responsible for many other diseases like cancer and neurodegenerative disease. Many chaperones and other molecules responsible for restoring homeostasis equilibrium, become inefficient due to chronic oxidative stress in the cell. But hormetic dose, a biphasic dose which responds with stimulation at low dose and inhibition at high dose, represents a new therapeutic approach for neuroprotection [7]. This hormetic dose mediates endogenous antioxidant pathways like nuclear factor erythroid 2-related factor 2 (Nrf2) and sirtuin (SIRT) and helps in the prevention of neuronal related disease. SIRT, the heat shock proteins, lipoxin A4, and Nrf2-dependant enzymes are the family members of vitagenes [8]. Other gases like nitric oxide (NO), carbon monoxide (CO), and hydrogen sulphide (H2S) are also responsible for hormetic-based neuroprotection [9]. NO in the physiological condition and appropriate amounts is neuroprotective, but can be neurotoxic when the levels are increased in the brain cells. It also plays a very important role in the central nervous system by regulating sleep-wake cycle, synaptic plasticity and hormonal secretion [10].
Osteoblasts are derived from osteoprogenitors originating from bone marrow. Remodeling of bone implicates dynamic complex interactions among cells and multiple molecular moieties that include hormones, cytokines and growth factors. Physiologically, osteoclasts remove damaged and old tissues of bone that can be consequently substituted by new bone cells and tissues formed by osteoblasts. Osteocytes transduce signals required to sustain the mechanical load. Literature suggests the osteocytes regulate the bone remodeling process as well as the viability and functionality of bone, maintaining normal levels of mineralization and constantly restoring the microdamage and the microfractures. Healthy bone is tightly regulated and maintained in order to prevent significant alterations in bone mass or mechanical strength after each remodeling cycle. It has been reported that signaling pathways and transcription factors are responsible for osteoblast differentiation. It is yet unclear how reducing their differentiation in osteoporosis would be of significance [11]. Oxidative stress has a detrimental inhibitory effect on osteoblast proliferation as reported by Mody and colleagues [12]. They demonstrated on bone-marrow stromal cell line (M2-10B4) and on pre-osteoblastic mouse cell line (MC3T3-E1) by alkaline phosphatase assay which is a marker of early differentiation. Further, ROS also inhibits the mineralization in these mentioned cell lines. Other studies on rabbit bone marrow stromal cells (BMSCs) and calvarial osteoblasts cell line were induced with low dose of hydrogen peroxide (H2O2, 0.1 mmol/L) and high dose (1 mmol/L), and BMSCs showed low expression of differentiation markers but calvarial cells exhibited cell death [13]. MC3T3-E1 cells when exposed to a high dose of free radicles cause cell death by necrosis [14]. Liu and groups [15] used metallothionein, an ROS scavenger that scavenges hydroxyl and superoxide radicals, and demonstrated the protective nature of metallothionein as it downregulated nuclear factor kappa B (NF-κB) signaling pathway activated by H2O2 in BMSCs as H2O2 is responsible for inhibiting the osteoblastic differentiation.
Osteoclasts play a major role in destroying the calcified bone tissue by a complex cascade mechanism. ROS has a direct impact on the regulation of osteoclastogenesis. Osteoclasts are rich in mitochondria. Mitochondria and ROS play an important role in osteoclastic differentiation and activation. Mitochondrial biogenesis synergizes osteoclastic differentiation and bone metabolism [16]. Superoxide generated by mitochondria during oxidative stress directly causes bone degradation [11]. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is the prime source of ROS production by osteoclast [17]. In organ culture of a mouse calvarial, provided xanthine and xanthine oxidase generated superoxide which is responsible for increased bone resorption. Local injection of these agents into calvarial bone of mouse in vivo triggered increasing osteoclastic bone resorption. H2O2 induced calvarial bones stimulate increased number of osteoclast formation with increased activity of matured osteoclast. In osteoclast of rats, H2O2 showed the resorption pit generation [18]. NF-κB signaling pathway is mainly associated with oxidative stress and osteoclastogenesis. NF-κB further influences the bone microenvironment by modulating the cytokine tumor necrosis factor-α (TNF-α), which is invariantly produced in osteoclastogenesis that is activated by mitogens and other cytokines in osteoblast in response to ROS [11].
ROS generation disrupts the fibronectins present in the extracellular matrices of the bone. Fibronectin polymerization provides the substratum to the osteoblasts and facilitates various cellular activities like proliferation, adhesion, migration, differentiation and also cell shape. As the metabolic turnover is comparatively slow for fibronectin when compared to other cellular components, several nonenzymatic modifications are triggered including generation of free radicals during the aging process. Excess ROS partially degrades and modifies the fibronectin molecules and hence are unable to form bone nodules [19]. Here, the various ROS activities on bone cells are summarized in Table 1.
Effects of ROS on different bone cells and materials
| Bone agents | Regulatory molecules | Activity of ROS | Reference |
|---|---|---|---|
| Osteoblast | Hydroxyl and superoxide radicals | Inhibition of differentiation and mineralization; induction of necrosis | [11] |
| Osteoclast | Superoxide, H2O2 and NADPH oxidase | Enhanced generation of ROS regulates osteoclast differentiation and facilitates resorption of bone tissuesGeneration of resorption pit | [17] |
| Osteocytes | NO, H2O2 | Induction of apoptotic cell death | [20, 21] |
| Fibrinonectin | Oxygen-free radicals | Partial degradation and modification of ECM molecules; inhibition of bone nodule formation | [19] |
| Pro-inflammatory cytokines | IL-1, IL-6, TNF-α | Increases the expression of osteoclast markers | [11] |
IL-1: interleukin-1; ECM: extracellular matrix
The physiological intracellular redox levels depend on the proportion of the pro-oxidants, ROS and the antioxidants [22]. Oxidative stress mainly refers to an overabundance of free radicals that include ROS and reactive nitrogen species (RNS). ROS is highly reactive and manifests as numerous chemical species, including free radicals and nonradical species like hydroxyl radical (•OH–), superoxide anion radical (•O2–), and H2O2. O2– is considered to be a primary ROS that is responsible for the generation of secondary ROS which aggressively interacts with other moieties causing deleterious effects. ROS is ubiquitous and generated even during normal metabolic activities leading to activation of several enzymes such as superoxide dismutase— cytoplasmic enzyme, NADPH oxidase—membrane enzyme along with other mitochondrial oxidases [23, 24]. Regulation of ROS, especially H2O2 may be responsible for the transmission of cell signaling that requires coordination of several vital processes like proliferation, differentiation, apoptosis, repair processes and inflammation [25, 26]. Thiols are the natural antioxidants in the animal systems that include glutathione (GSH, γ-glutamyl-cysteinyl-glycine). Nonthiol polyphenols which are largely found in numerous plants, vitamins (vitamin C, alfa-tocopherol and vitamin A), and some enzymes like catalase, can easily eliminate ROS as well as enzymes for GSH that use as substrate (GSH reductase, GSH peroxidase, etc.) [27]. In the cells, the concentrations of GSH in the range of 2–10 mmol/L are primarily responsible for cellular redox environment [28] and may be present in the biologically active reduced -thiol form. GSH normally gets oxidised to oxidised GSH (GSSG) and consequently reduced to GSH/GSSG level that is often responsible for the metabolic stress. Hence, GSH/GSSG ratio can be used as an indicator of cellular redox state [29]. Homeostasis of cellular redox is maintained by de novo GSH synthesis, reduction of GSSG, and uptake of exogenous GSH. GSH is also involved in various signaling pathways which regulate the transcriptional activity and translation by reactions of glutathionylation [30]. There are several other thiol antioxidants that may originate from the reduction of lipoic acid (LA), dihydrolipoic acid (DHLA) that includes thioredoxin, glutaredoxin, and cysteine (Cys). Some of the in vivo studies suggested that Cys and DHLA are capable of scavenging ROS and RNS directly and also via activation of other antioxidants like vitamin C, vitamin E and GSH [31–33]. Many bone diseases are associated with ROS induced oxidative stress. Oxidative stress during postmenopausal osteoporosis occurs because of estrogen deficiency, followed by the activated levels of NADPH oxidase and/or downregulated synthesis of antioxidant enzymes as well as GSH levels [34–37]. ROS generation in the secondary osteoporosis occurs in inflammatory bowel disease (IBD) primarily due to the decreased GSH levels and defensive antioxidant activities [20]. Prolonged treatment with steroidal anti-inflammatory drugs in osteoporosis causes oxidative stress, mainly because of the activation of enzymes which helps in generating ROS [38, 39].
The alteration in the redox state is connected with bone remodelling, which permits bone to regenerate continuously [40–42]. Bones are dynamic tissues that keep on renewing themselves by coordination of these bone cells: osteoclasts, osteoblasts and osteocytes [43, 44] and bone remodelling can occur by interactions of these cells with various molecular agents which include hormones, cytokines and growth factors. It is a physiological time taking process, approximately six months, in which osteoclasts help to eliminate damaged or old bone tissues and subsequently substituted with new tissues synthesis by osteoblasts. Function of osteocytes on the other hand helps in the transmission of signals essential to sustain mechanical loads. Healthy bones are highly sustained and tightly regulated with no alterations in the mechanical strength or bone mass during the occurrence of remodelling [45]. ROS induced oxidative stress increases with estrogen deficiency and/or aging in postmenopausal women. Nrf2 signaling pathway is emerging as an important factor in the regulation of bone metabolism [46]. It causes altered remodelling of bone and adversely affects bone homeostasis that leads to skeletal fragility. Possible decrease in the antioxidants in osteoporotic women is associated with the Nrf2 signaling pathway. Deficiency of Nrf2 indicates enhanced intracellular levels of ROS and imperfects generation of various antioxidant enzymes including glutathione in both precursors of osteoclast and the osteoblast progenitor cells [47, 48]. Nrf2 crosstalks with other transcription factors to integrate and increase the efficacy of adaptive metabolic strategies that cause acquired elasticity. The ubiquity of hormetic dose responses is based on the adaptive mechanism of Nrf2. Recently, clinical studies suggested that ROS-antioxidant systems may have a key role in the pathogenesis of bone loss [49]. ROS can easily activate the differentiation of osteoclasts from preosteoclasts which is followed by enhanced bone resorption [50, 51] (Figure 1). Plethora of literature suggests that human bone marrow mononuclear cells when incubated with H2O2 show enhanced osteoclasts count and their activity increases significantly along with tartrate resistant acid phosphatase (TRAP) levels [46], thereby favouring osteoclastogenesis. On the other hand, osteoblasts and osteocytes that are localized in the bone matrix undergo apoptosis [20] (Figure 1). Apart from this, ROS also activates various molecular signaling pathways like mitogen-activated protein kinase (MAPK), c-Jun N terminal kinase (JNK), extracellular signal-regulated kinase 1/2 (ERK1/2), and p38 MAPK for growth, proliferation, differentiation and arrest of apoptosis by osteoblast or osteocytes [9, 52–54]. Increased levels of ROS may further block the osteoblast activities and their differentiation followed by low intensity of mineralization and minimal osteogenesis [55, 56]. However, antioxidants have a paradoxical effect, as they help in differentiation of osteoblasts followed by formation of bone [31, 57–59], maintain osteocytes required for osteogenesis, while simultaneously they minimise the osteoclast activities and their differentiation. Osteoclast and osteoblast activities are regulated by various factors which are produced by osteoblasts themselves and also by osteocytes for the bone remodelling such as receptor activator of NF-κB ligand (RANKL) and osteoprotegerin (OPG). These two are sensitive to the increased levels of oxidative status causing upregulation of RANKL and downregulation of OPG via activation of ERK1/2, pathways like JNK and other transcriptional factors [20]. RANKL in response activates the osteoclasts by interacting with the RANK receptors present in the preosteoclasts and promotes osteoclastogenesis, while OPG which is the soluble receptor produced by wingless (Wnt)/β-catenin signaling pathway activation actually competes for the RANK receptor and blocks the RANKL, subsequently inhibiting the osteoclast activity [60–64]. Oxidative stress blocks the osteoblasts activation, thus the OPG production and related activity of RANKL will prevail under these limited conditions, which enhances the induction of differentiation and activation of osteoclasts significantly. Thereafter, increases in the RANKL/OPG ratio are also an indicator of bone resorption activity [35, 65, 66], as their regulation is the deciding factor of osteoclastogenesis and osteobalstogenesis. Upregulation of RANKL/OPG ratio increases bone resorption activity as it enhances bone remodelling turnover and decreases the rate of bone formation activity which are ultimately responsible for several skeletal metabolic disorders like osteoporosis [20]. The expression of OPG and RANKL is regulated by different cytokines and hormones [67]. Limited data are available on the molecular mechanisms of osteocytes. Osteocytes constitute 90% of the total bone cell population and are embedded in the bone matrices. Morphologically, these cells are mechano-sensory cells [68] similar to neurons, having a central cell body with dendritic extensions that help in communication with other cells, blood capillaries and nerve endings. It has been reported that the mature osteocytes undergo apoptosis when there is microdamage or other hormonal signals like oestrogen deficiency, and occasionally due to oxidative stress too [63, 66, 69]. Osteocytes further produce sclerostin protein and Dickkopf-1 Wnt signaling pathway inhibitor 1 (DKK1) which further block the synthesis of OPG. Sclerostin is released by Wnt/β-catenin signaling pathway [70, 71] that leads to increased RANKL/OPG levels which promote osteoclast activity, osteoblast apoptosis and bone degradation. This results in an inadequate O2 intake, altered hormones, improper nutrients and other factors required to sustain the viability, metabolic alterations, osteocyte apoptosis and oxidative stress initiated by remodelling process and bone resorption [58, 61, 63, 72, 73]. Excessive ROS generation triggered by increased apoptosis of osteocytes causes further imbalance in the bone remodelling process that may result in altered and weakened bone formation, analogous to changes observed during aging, treatment with glucocorticoid, osteoporosis and in a number of other skeletal disorders related to oxidative stress [74–76]. Alternatively, osteocytes produce high levels of OPG and this contributes to the differentiation of osteoblasts and the mineralization process [39].
Innumerable reports regarding in vivo and in vitro studies confirm the contribution of thiol and nonthiol moieties as antioxidants. They act via triggering the osteoblast differentiation, mineralization, thereby reducing the osteoclast activity. These antioxidants are not only direct scavengers of ROS but also maintain significant levels of GSH for the conjugation with GSH reductase. Thus, they can eliminate GSSG and can sustain the standard GSH/GSSG levels thereby balancing the intracellular redox state [32, 57, 59]. Consequences of antioxidants on bone metabolism have been reported, suggesting the low levels of antioxidants presented in plasma of aged or osteoporotic animals [77]. TNF-α is very sensitive to minute levels of antioxidants [78] with the signaling pathways accelerating the bone cell. Administration of supplementary antioxidants like LA, vitamin C, vitamin E, and N-acetylcysteine (NAC) shows positive effectiveness in overcoming osteoporosis [20]. LA showed positive effects in maintaining the integrity of bone structures of inflammatory and ovariectomy mediated osteoporosis [79]. Vitamin E helps in promoting healing efficacy in osteoporotic fractures that have developed in ovariectomized (OVX) rats. The parameters include increased bone mineral density that further induces bone regeneration [80]. Ascorbic acid and NAC—a Cys analogue showed negligible bone loss in OVX mice. Glutathione inhibitor, l-buthionine-(S, R)-sulphoximine causes extensive bone loss [81]. Therefore, NAC at different doses showed protective role against oxidative stress in osteoblasts, and also stimulated differentiation of mice calvarial cells [82]. NAC basically prevents the osteoblastic apoptosis that occurs due to GSH induced oxidative stress [83], inhibits osteoclastogenesis and further prevents pathways like NF-κB which are responsible for activation of osteoclast [65]. The role of GSH plays a critical role in differentiation of both types of bone cells and also in progressive bone related diseases like arthritis and osteoporosis [20]. Some studies in sarcoma osteogenic-2 (Saos-2) cells—human osteoblast cells, report that GSH and NAC stimulate osteoblast differentiation, as validated by the alkaline phosphatase (ALP) activity along with the increased expression of osteogenic markers like osteocalcin (OCN) and runt-related transcription factor 2 (Runx-2), important biomarkers that are overexpressed by the mature osteoblasts [57]. LA blocks the TNF-α signaling pathway, which in return inhibits the expression of JNK and NF-κB responsible for apoptosis of bone marrow stromal cells of human cell line [84]. LA also blocks the RANK-RANKL interaction in human BMSC (human BMSC) and controls osteoclastogenesis. Literature survey suggests that the increased levels of GSH/GSSG play a very crucial role in differentiation and mineralization of osteoblast [57], which concludes that the importance of GSH redox state is present in the phenotypic expression of osteoblast and osteoclast [81]. GSH and NAC also contribute by downregulating the RANKL/OPG level reported in SaOS-2 cells, while the upregulated calcium levels lead to osteoblast mineralization [57]. Several studies further support the role of antioxidant on osteocytes like apoptosis of osteocytes can be prevented by GSH, LA and NAC, decreasing the RANKL/OPG ratio and sclerostin levels stimulated by ROS [33]. This is supported by the study conducted on murine long bone osteocyte Y4 (MLO-Y4) cell line (murine osteoblast cell) sharing many features of mature osteocytes helpful for perusing experiments on osteocyte viability and cell death mechanism related to bone disease [67, 72]. Oxidative stress has been reported on osteocyte cell line via starvation, which mimics the microdamage that occurs in osteocytes [20]. With the administration of antioxidants, cells undergo apoptosis and increase the OPG expression by JNK signaling and expression of sclerostin and RANKL levels are regulated by JNK and ERK1/2 pathways [33]. Furthermore, studies have demonstrated that the catalase enzyme has antioxidant properties that can abolish the expression of TRAP, a biomarker of osteoclast cells induced by H2O2 treatment in primary culture of human bone marrow cells [46].
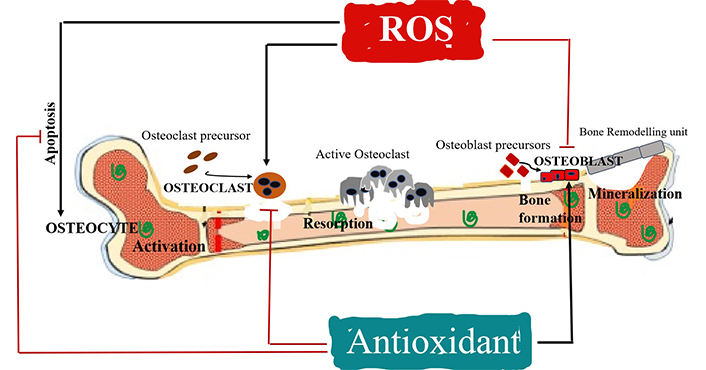
Effects of ROS and antioxidants on the activity of osteoclasts, osteoblasts and osteocytes in bone remodelling. ROS activates osteoclast differentiation and osteocyte apoptosis (+), while inhibits osteoblast activity (–) inducing bone resorption; antioxidants activate osteoblast differentiation (+) and inhibit osteoclast activity and osteocyte apoptosis (–) inducing bone formation
Current medication for osteoporosis is primarily based on modulating the osteoclast activity, initially by suppressing its activity that causes restoration of existing bone mass, but rarely focuses on new bone growth. Owing to their versatility and remarkable intrinsic features, nanotherapeutics have gained noteworthy recognition. Nanoassisted technologies have emerged as efficient delivery vehicles to enhance the bioavailability of drugs, calcium supplements etc. especially for the bone disorders (Figure 2). Some mineral-based therapies have been demonstrated to accomplish new bone formation by downregulating the osteoclast activity. The nano-hydroxyapatite (nano-HA) crystal further has the potential to combat osteoporosis as reported in in vivo study suggesting its role in initiating bone formation to the point of healing [85].
Bone is an important site for the successful implantation to heal bone disorder. Various types of bioactive coating materials are used including titanium and ceramic to enhance the bone integration and reduce the infiltration of inflammatory cells at the site of bone implanted. However, observed clinical evidences demonstrated that the implantation of titanium nanomaterials (TiIs) in a diabetic patient resulted in overproduction of ROS at the bone interface. The coated TiIs with Tantalum (TaTi) significantly improved the diabetes induced impaired osteogenesis via TiIs by inhibiting ROS-mediated p38 MAPK pathway in osteoblast cells [86]. But use of these coating materials is limited owing to their toxicity and cytotoxicity even at low doses [87]. Calcium based nanomaterials include HA, calcium phosphate and bisphosphonates, which may be considered to be implanted as a nanomaterial-based scaffold that mediates stem cells differentiation into bone lineage cells [88]. Therefore, these centres have promoted both the mineralization and differentiation of the stem cells.
The design used for bone tissue engineering scaffold required a biocompatible, biodegradable, and hydrophilic platform for the desired treatment. Materials that have the potential for drug delivery system (DDS) against osteoporosis and bone tissue engineering include chitosan, silica, poly (lactic-co-glycolic acid) (PLGA), poly (ethylene glycol) and liposomes. Nanoparticles can be loaded into these implants independently or in combination to be suitable to treat and target the bone disorder [89]. Nanoparticles enable targeting, drug protection and improved biodistribution and are enlisted in Table 2. The review further addressed the conceptual framework of the role of nanoparticles in bone remodelling and regeneration by regulating ROS level. In the past decades, nanoparticles have gained considerable awareness as a DDS to enhance the therapeutic efficacy of drugs, and lessen their adverse toxicity. After the drug has been delivered and distributed systemically via the blood circulation, it will finally be absorbed by the body. The distribution is generally unequal because it undergoes rapid degradation in the body and very sparsely infiltrated into the bone tissue. Due to poor vascularization and existence of blood brain barrier, drug penetration into the bone is prevented: when compared to other organs (spleen, liver, or kidney). Therefore, drugs are usually administered in high doses or repeatedly, which results in systematic toxicity. Hence, it would be safer and more effective to deliver drugs in a sustained manner at bone targeted site. The nanoparticles deliver the drug at the target site, and release the therapeutic factors which encourage either bone growth or inhibit bone resorption (Figure 2). In this way, DDS amends the drug doses, safeguards it from degradation and reduces the off-target effects. For bone disorders, nanoparticles coupled with bone targeting agents to form the key factor in targeted DDS [90]. Studies reported that, chitosan nanoparticles loaded with bone-morphogenetic protein 2 (BMP-2) as a growth factor were used to target and promote bone formation and angiogenesis in osteoporotic rabbit model [91]. Moreover, nanoparticles provide newer possibilities in modulating the ROS levels that are essential for maintaining the homeostasis in bone microenvironment. In order to direct the nanoparticles to bone, it is important to attach the moieties that specifically bind with strong affinity to the mineralized matrix-HA.
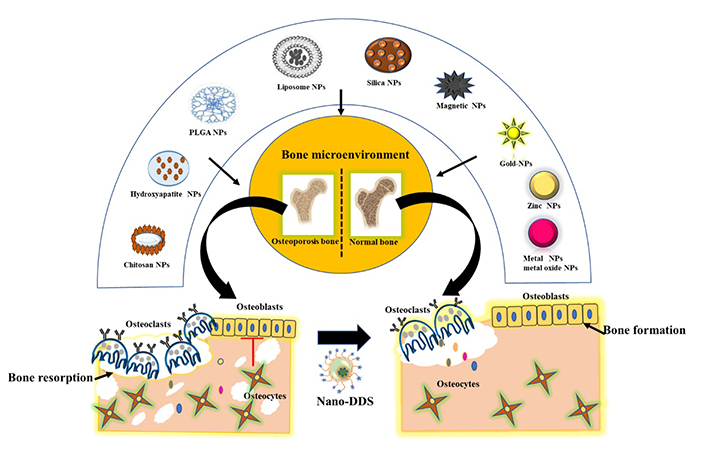
Schematic illustration of various forms of organic and inorganic nanoparticles for targeted bone delivery to combat osteoporosis by restoring the normal functioning and homeostasis of bone cells. Hence, this nano-drug delivery system mediates osteoblastogenesis and inhibits osteoclastogenesis. NPs: nanoparticles
Tetracycline and bisphosphonates have been reported to have strong binding affinity with calcium present on HA that facilitates bone targeting with these moieties having dual functions, enhancing the antioxidant activity required for inhibiting the osteoclastogenesis and and subsequently promoting the bone formation. Regardless of the strong binding affinity of tetracycline with calcium, staining of teeth to yellow colour in children has revealed its potential toxicity. Therefore, use of this antibiotic was discontinued in paediatric regimens. Song et al. [85] have reported the targeting mechanisms for tetracycline and its derivative to HA surface and resolved the issues of the nonspecific binding to bone in an in vitro model, but it has not been validated in any biological model as yet [92]. Unlike tetracycline, bisphosphonates have emerged as attractive targeting molecules in recent years, as they are widely used due to their strong affinity to bind with HA and can be exploited in the treatment of osteoporosis and bone osteolytic disease. That gold nanoparticles coated with bisphosphonates (alendronate) to target the osteoclast to reduce bone resorption activity which leads to bone formation by facilitating the weakening of ROS by RANKL and remarkably enhances the glutathione peroxidase-1 (Gpx-1) has been reported [93]. Recently, Sun et al. [94] fabricated pyrophosphate (PPi) functionalized biomineral-binding liposomes loaded with icariin (PPi-TEG-Chol) to combat osteoporosis in a rat osteopenia model. Herein, PPi acts as the targeting moiety that anchors to the HA surface and mediates the sustained release of icariin. This is responsible for the suppressed levels of tartrate-resistant acid phosphatase 5b (TRACP 5b) in serum, which is a sensitive marker of bone resorption. This study concluded that enhanced osteoblast activity and inhibition of osteoclast function, along with induced cancellous bone mineralization are essential for successful therapy with no adverse effects [94].
Oligopeptides are the eight repeating sequences of amino acids that specifically bind to bone resorption surface and bone formation surface. It has been reported that aspartate (Asp8) specifically attaches to osteoclast cells and the sequence of (AspSerSer)6 targets the osteoblast cells. Currently, series of DDS have been designed to specifically target bone microenvironment such as surface functionalization of PLGA nanoparticles with poly-aspartic acid peptide (poly-Asp) demonstrated the strong binding affinity to HA, which is validated through bone resorption surfaces to target osteoclasts [95]. Liang et al. [96] synthesized osteoblast specific CH6 aptamer functionalized lipid nanoparticles (LNPs), encapsulated osteogenic pleckstrin homology domain-containing family O member 1 (Plekho1) small interfering RNA (siRNA) (CH6-LNPs-siRNA) that mediated bone formation, increased bone mass and improved bone microarchitecture in both osteopenia and healthy rodents [96]. Fu et al. [97] synthesized aspartic-acid functionalized PLGA- polyethylene glycol (PEG) di-block polymers to target bone HA to deliver drugs both in vitro and in vivo in rat and zebra models [97].
Bone targeting moieties
| Bone targeting agents | NPs | Outcomes | Reference |
|---|---|---|---|
| Tetracycline | PLGA (SIM-loaded TC-PLGA) | SIM-loaded TC-PLGA NPs exhibit enhanced bone targeting efficiency as confirmed by in vitro and in vivo OVX rats | [98] |
| Bisphosphonate | Lipid bilayer NPs (HA-coated zoledronic acid functionalized LNPs) | HZL in the LNP exhibited a strong affinity towards HA, further enhancing its efficacy in the treatment of OP | [99] |
| Pyrophosphates | Liposomes NPs (PPi functionalized biomineral binding liposomes NPs loaded icariin) | PPis act as targeting moieties that anchor to HA surface and mediate the sustained release of icariin that is responsible for the enhancement of osteoblast activity and inhibits the osteoclast function, as well as induces cancellous bone mineralization | [94] |
| Aspartate (Asp8) (Oligopeptides) | PLGA NPs | Poly-Asp-PLGA NPs have strong binding affinity to HA, which is validated through bone resorption surfaces to target osteoclasts | [95] |
NPs: nanoparticles; TC: tetracycline; SIM: simvastatin; HZL: hydroxyapatite-coated zoledronic acid functionalized lipid bilayer NPs; OP: osteoporosis
Commissioning of new bone growth is a key factor in bone remodelling and forms the next generation treatment of osteoporosis. The nanoparticles are categorically divided into two types as hard and soft nanoparticles. The soft nanoparticles include liposomes, dendrimers, micelles and polymeric nanoparticles while the hard nanoparticles synthesized involve both inorganic and metallic nanoparticles such as silica, gold, quantum and carbon dots (Table 2). Herein, the role of nanoparticles in delivering the drugs that suppress the osteoclast activity is highlighted along with the nanoparticles used in modulating ROS levels in bone microenvironment that further leads to the inhibition of bone resorption but simultaneously induces bone formation (Figures 2 and 3).
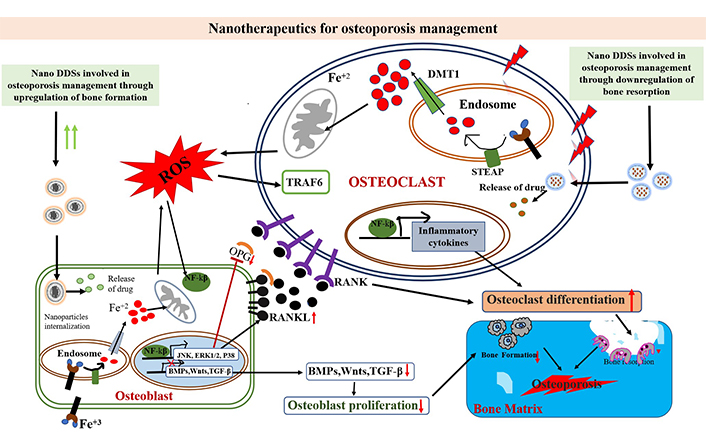
Effects of nano DDS against the action of oxidative stress at the molecular cellular level. Oxidative stress triggers mitochondria to produce excessive ROS, that further activates the NF-κB pathway in cells. The inflammatory factors are induced by NF-κB that promotes osteoclast differentiation; the activated BMPs, Wnts, and TGF-β signaling pathways can promote osteoblast proliferation. But NF-κB can inhibit these signaling pathways. The activated JNK, P38, and MAPK signaling pathways by NF-κB in osteoblasts further promote RANKL expression and inhibit OPG expression, leading to osteoclast differentiation, eventually leading to osteoporosis. DMT1: divalent metal transporter 1; STEAP: six-transmembrane epithelial antigen of prostate; TRAF6: tumor necrosis factor receptor associated factor 6; TGF-β: transforming growth factor β
Liposomes are small spherical vesicles consisting of two or more lipid bilayers and cholesterols. A rationale design of the nano-carrier system involves the permeation of the drug in the bilayer or the inner core to facilitate a targeted, sustained drug release system with reduced cytotoxicity. This formed an efficient drug delivery vehicle for clinical application based on pharmacological and pharmacokinetic efficacy [100]. Currently, Xiao [101] synthesized adhesive hydrogel liposome composites [gelatin methacryloyl-dopamine-melatonin (GelMA-DOPA-MT)] for controlled release of melatonin at the local site. The MT was responsible for the differentiation of osteoblast and bone formation was promoted by modulating the oxidative stress. This study demonstrated that MT inhibited apoptosis caused by H2 O2 – that triggered generation of oxidative stress, promoted osteogenesis in MC3T3-E1 cells (preosteoblast cells) and in rat model developed by ovariectomy. This contributed to the increased bone mass and enhanced osteogenic potential in osteoporosis [101]. LNP loaded with BMP-9 induces differentiation of MSCs to osteoblastic lineage cells that can effectively combat osteoporosis [102].
In the golden age of pharmaceutical nanocarriers, PLGA nanoparticles have been an attractive option for the sustained drug release, targeted delivery to specific organ and tissues due to their versatile nature that offers ease of delivery of multiple cargos (proteins, peptides and genes). In a study, PLGA nanoparticles loaded p47phox siRNA reduced the ROS/oxidative stress induced by damage of chondrocyte in osteoarthritis (OA). In articular chondrocytes, the levels of ROS production are very low and regulated by NADPH when compared to an OA patient. This study showed inhibition of the ROS levels p47phoxsi-nanoparticles and decreased cartilage damage in OA animal model. Moreover, PLGA nanoparticles were synthesized as nanodecoys for scavenging RANKL and TNF-α from the preosteoclast membrane, which inhibited postmenopausal osteoporosis and regulated bone integrity. These biomimetic nanodecoys when given to OVX mice systematically escaped capture by macrophages and effectively downregulated the RANKL and TNF-α to inhibit osteoclastogenesis and promote osteoblastogenesis [103].
Chitosan nanoparticles are made from biopolymer with excellent biodegradable, biocompatible and physicochemical properties making them efficient delivery systems as they can be administered via various routes of administration for drug, protein and gene delivery. Li et al. [104] engineered chitosan microsphere composites consisting of nano-HA loaded resveratrol (n-HA/resveratrol/chitosan). The microsphere composites exhibited anti-inflammatory properties as evidenced by the lower expression of pro-inflammatory cytokines TNF-α, IL-1β and inducible NO synthase (iNOS) in RAW 264.7 cells. This study signifies enhanced osteogenic differentiation by upregulation of Runx-2, ALP, collagen type 1 (Col-1) and OCN, and promotes mineralization in differentiation medium. When implanted in an osteoporotic rat model, it resulted in entochondrostosis and bone regeneration [105]. Literature suggested that hyper activation of osteoclasts’ activity was mediated via the enhanced ROS generation as discussed above. Another report suggests that chitosan derived nitrogen-doped carbon dots (N-CDs) were synthesized to scavenge ROS. N-CDs successfully inhibited RANKL-induced ROS generation and showed impairment of NF-κB and MAPK pathways, which enabled treatment of osteolytic lesions in mice model [105].
Currently, Shi et al. [106]. have prepared the biogenic silver nanoparticles using chitosan/agar hydrogel. These nanocomposites elevated the levels of bone mineral specific markers, maintained the calcium homeostasis, mediated collagen formation and scavenged ROS in treating osteoporotic rat models [106].
Risedronate functionalized chitosan nanoparticles (RISCN) were used to treat osteoporosis. The outcome of this study showed that RISCN treatment indicated remarkable enhancement in bone mineral density and healed trabecular microarchitecture in rat osteoporosis model [107]. Moreover, our lab has designed layer by layer deposition of siRNA and polyethyleneimine (PEI) on chitosan coated gold nanoparticles (CS-AuNPs) for the silencing of Sclerostin (Sost) gene/sclerostin expression. The in vitro model (mouse embryonic fibroblast and MC3T3-E1) revealed that siRNA delivery vehicle successfully resulted in a threefold decrease in expression of Sost gene/sclerostin that inhibits the osteoblast differentiation and enhanced osteoblast formation. Sost gene encoded protein sclerostin that inhibits the bone formation and promotes osteoclast activity. Therefore, this established the potential utility of CS-AuNPs for biomolecules delivery to promote bone formation, this being a potential alternative to treat osteoporosis as shown in Figure 4.
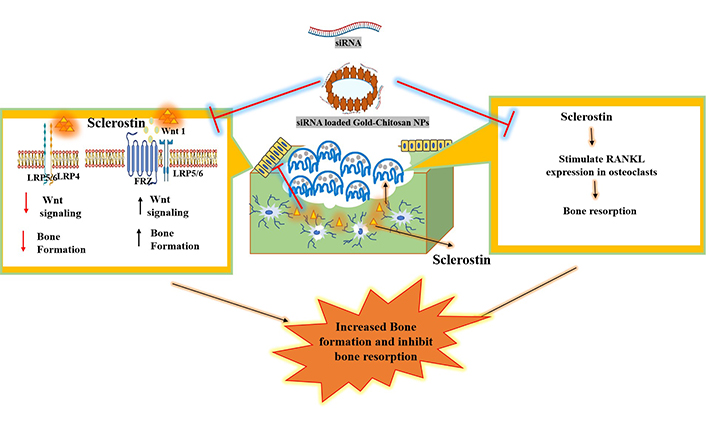
Proposed schematic representation of siRNA loaded CS-AuNPs that augments the specific delivery of Sost-siRNA to silence the expression of sclerostin protein encoded by Sost gene. This secretory protein secreted from osteocytes that promote bone resorption and inhibit bone formation. The overexpression of sclerostin protein inhibit the Wnt signalling pathway by suppressing the conformational changes occurred due to FRZ transmembrane receptor and initiate the osteoclastogenesis. Delivery of Sost-siRNA not only silences the expression but also is responsible for upregulation of bone formation (unpublished data). LRP5/6: low-density lipoprotein receptor-related protein 5/6; FRZ: Frizzled
HA nanoparticles have extensively been used in bioengineering applications due to the unique biocompatibility as well as osteo-conductive properties and nanomaterial characteristics mimicking the bone matrix. Marycz et al. [108] constructed nano-HA (nHAp) doped iron oxide (IO) nanoparticles with loaded microRNA-21 (miR-21) and miR-124. Under the effect of magnetic field, nanocomposite increased the differentiation of precursor cells, activated the osteogenic markers [Runx-2, Osteopontin (OPN), Collagen-1 (Coll-1)] and downregulated the expression of carbonic anhydrase II (CaII) and cathepsin K (Ctsk) and the metabolism and differentiation of preosteoclast cells. In addition, it also demonstrated the immunomodulatory properties exhibiting suppressed pro-inflammatory cytokines such as TNF-α, iNOS or IL-1β, in lipopolysaccharide (LPS)-stimulated RAW 264.7 cells, thereby maintaining the bone homeostasis. Another research evaluated the potency of various types of nano-HA [chitosan/HA nanocomposites (nCh/HA) and silver/HA nanoparticles (nAg/HA)] used to treat osteoporotic bone disorder. These nanocomposites significantly inhibited the expression of Sost, bone ALP (BALP) and bone sialoprotein (BSP) and subsequently downregulated the expression of RANKL and CtsK gene. Therefore, this study contributes in ameliorating the excessive bone turnover in primary osteoporosis rat model [109].
The magnetic HA (MHA) scaffolds contributed to osteoblast formation in osteoporosis by modulating the exosomal cargo derived from osteoclasts thereby reducing the efficacy of exosomes taken up by osteoblast cells. MHA treatment reduced the expression of certain proteins such as ubiquitin, adenosine triphosphate (ATP), and ROS and simultaneously enhanced the Rho kinase activity in osteoclast derived exosomal cargo [110]. Sistanipour et al. [111] fabricated catechin-conjugated mesoporous HA nanoparticles that exhibited strong antioxidant properties, and enhanced the affinity towards hydroxyl and superoxide radicals when compared to free catechin. Catechin-mesoporous hydroxyapatite nanoparticles (Cat-MHAP) promoted the osteogenic differentiation in both the MSCs and tumor cells, accompanied by the attenuation of intracellular ROS. From these results, Adenosine triphosphate Cat-MHAP can be proposed as a novel “nano-antioxidant” having immense potential as a promising biomaterial for treating bone defects, particularly postsurgery osteosarcoma patients [111].
Silica nanoparticles display unique characteristics, which enables their use in biomedical application. They exhibit enhanced drug loading capacity due to their porous nature, increased biocompatibility and facilitate attachment of functional moieties on the surface for targeting and improved loading of a variety of cargos such as drugs, genes and proteins. Pinna et al. [112] developed ceria-encapsulated mesoporous silica nanoparticles (Ce-MSNs) that showed significant antioxidant properties for efficient therapy for osteoporosis by employing new bone formation and inhibiting osteoclast activity. The Ce-MSNs successfully reduced the ROS generation by t-butyl hydroperoxide, when exposed to MC3T3-E1 cells and RAW 264.7 cells. This study demonstrated that Ce-MSNs elicited mineralization and proliferation in MC3T3-E1 cells without supplementing with the osteogenic medium [113]. Gene silencing through siRNA has been successfully delivered by MSNs, wherein Sost-siRNA was used and loaded with osteostatin in functionalized MSNs with a PEG coating and alendronate (ALN) to deliver the siRNA to targeted site of osteoporosis. The Sost-siRNA is responsible for the knockdown of osteogenic markers (sclerostin) that achieved bone formation and bone remission in OVX mice [114]. Furthermore, that Silicone gel nanoparticles decorated with octacalcium phosphate (Silica/OCP) developed to study its viability effects through ROS induced autophagy on osteoblasts was reported. Silica/OCP induced autophagy by upregulating the ROS levels in osteoblast cells. In addition, the initiation of autophagy can inhibit the oxidative stress thereby inhibiting apoptosis and protecting the osteoblast proliferation. Overall, the outcome of this study suggested that Silica/OCP nanoparticles can enhance autophagy and promote the osteoblast proliferation. This study can be used to correlate the behaviour of osteoblast inducing bone growth with oxidative stress in treating osteoporosis [115]. Bioactive silica-based nanoparticles exhibit both activation of osteoblastogenesis and suppressed osteoclastogenesis by inhibiting the expression of NF-κB via downregulation of oxidative stress, both in in vitro and in vivo models [115].
Metallic nanoparticles have gained significant attention due to their flexibility in shape, size and exhibited potent antioxidant properties to combat bone disorder. Lee et al. [116] studied the effects of selenium nanoparticles (SeNPs) in osteogenic differentiation to treat osteoporosis by regulating the oxidative stress in MC3T3-E1 cells. SeNPs exhibited anti-inflammatory properties and regulated the ROS levels. SeNPs reduced the ROS levels and protected the cells posttreatment with H2O2 [117]. Platinum nanoparticles (PtNPs) displayed potent antioxidant activity. PtNPs ameliorate ovariectomy-induced bone loss by decreasing the osteoclastic activity and differentiation in vivo. The PtNPs impede osteoclast formation by disturbing the RANKL signaling. This caused inactivation of NF-κB ligand and reduced the level of nuclear factor in activated T-cells, cytoplasmic 1 [nuclear factor of activated T cells (NFAT2)]. Therefore, PtNPs inhibited RANKL induced long-lasting ROS as well as intracellular concentrations of Ca2+ accumulation [118]. Similarly, AuNPs reduced the osteoclastogenesis activity by inhibiting the RANKL, and also showed significant antioxidant activity by regulating the ROS generation. AuNPs upregulated RANKL-induced Gpx-1 in bone marrow derived macrophages [119]. Currently, Dou et al. [120] constructed pH-responsive cerium nanoparticles (CNS) which particularly targeted mature osteoclasts (mOCs) without affecting the preosteoclasts. Biocompatible CNS was directed to the acidic extracellular microenvironment produced by mOCs that resulted in reduction of ROS levels and bone regeneration. The nanoparticles were engineered in a critical sized defect using 2-N, 6-O-sulfated chitosan that increased the calcium deposition as confirmed from in vitro and in vivo OVX mice models [120] (Table 3).
Role of nanoparticles in bone remodelling
| NPs | Modifications | Therapeutic agents | Target (bone remodeling) | Experimental model | Inference | Reference |
|---|---|---|---|---|---|---|
| Liposomes | Gelatin methacryloyl-dopamine (GelMA-DOPA) | MT | Osteoblast | MC3T3-E1 (precursors osteoblast) and OVX mice model | MT promotes osteoblast differentiation and bone formation and has been effectively used to combat oxidative stress | [101] |
| PLGA | Nanodecoys | RAW cell membrane | Osteoclast | RAW 264.7 cells (preosteoclast) and OVX mouse model | Nanodecoys capable of scavenging RANKL and TNF-α produced by osteoclast cells and promote osteoblastogenesis | [103] |
| CS | Nano-HA (n-HA/resveratrol/chitosan) | Resveratrol | Osteoclast | RAW 264.7 cells and OVX rat model | CS microsphere implanted into bone defects in the osteoporotic rat femoral condyles, enhanced entochondrostosis and also possessed anti-inflammatory property to treat osteoporotic bone disorder | [106] |
| HA | IO NPs | miR-21 and miR-124 | Osteoblast and osteoclast | MC3T3-E1, 4B12 cells and RAW 264.7 cells | nHAp/IO/miR-21/miR-124 improves metabolism of preosteoblasts and promotes osteogenesis, simultaneously decreasing differentiation of preosteoclasts | [107] |
| Mesoporous silica NPs | nanoceria | Osteoblast and osteoclast | MC3T3-E1 cells and RAW 264.7 cells | Ce-MSNs exhibited antioxidant capability and stimulated cell proliferation and osteogenic responses | [114] | |
| Selenium NPs | Osteoblast | MC3T3-E1 cells | SeNPs in osteogenic differentiation in order to treat osteoporosis by regulating the oxidative stress | [118] | ||
| Gold NPs | EGCG | EGCG | Osteoclast | Bone marrow macrophages cells and in vivo LPS-induced calvarial bone erosion model | EGCG-GNPs exhibited anti-osteoclastogenesis by reducing the intracellular ROS generation and inhibited the MAPK pathway | [119] |
NPs: nanoparticles; CS: chitosan; EGCG: epigallocatechin gallate; GNPs: gold nanoparticles
In summary, this study concludes that osteoporosis is a socio-economic challenge, as the aging population has increased chances of developing fracture in any part of the body, primarily due to bone fragility. Bone loss in the later stage may be due to the most important biomarker—oxidative stress. ROS induces bone metabolism and disrupts bone remodelling by an overload or deficiency which would affect the proliferation and differentiation of osteoclasts and osteoblasts, resulting in declination of bone mass and enhanced risk for osteoporotic fractures. Therefore, neutralizing excess oxidative stress during aging or in postmenopausal women could act as an excellent preventive measure for osteoporosis. The involvement of ROS overload in osteoporosis along with nanotherapeutics to ameliorate osteoporosis has been discussed, as the precise cellular mechanism involved in osteoporosis is still poorly understood. Hence, exploiting nanotherapeutics for maintaining the differentiation and proliferation of osteoblasts and suppression of osteoclast activity is quintessential.
This review summarizes the currently developed nanotherapeutic delivery systems for treating bone disorders based on an in-depth understanding of the composition of bone, along with a basic awareness of pathogenesis of the disease and its progression. We conclude that a suitable combination approach by developing a multifunctional system will give possible cues for improving the efficacy of the treatment. Any therapy which improves the drug profile and delivery to bone tissue will be a promising approach.
•O2–: superoxide anion radical
ALP: alkaline phosphatase
BMP-2: bone-morphogenetic protein 2
Ce-MSNs: ceria-encapsulated mesoporous silica nanoparticles
CS-AuNPs: chitosan coated gold nanoparticles
Cys: cysteine
DDS: drug delivery system
ERK1/2: extracellular signal-regulated protein kinase 1/2
GSH: glutathione
GSSG: oxidised glutathione
H2O2: hydrogen peroxide
IL-1: interleukin-1
IO: iron oxide
JNK: c-Jun N-terminal kinase
LA: lipoic acid
LNPs: lipid nanoparticles
MAPK: mitogen-activated protein kinase
miR-21: microRNA-21
MT: melatonin
NAC: N-acetylcysteine
NADPH: nicotinamide adenine dinucleotide phosphate
nano-HA: nano-hydroxyapatite
NF-κB: nuclear factor kappa B
NO: nitric oxide
OA: osteoarthritis
OPG: osteoprotegerin
OVX: ovariectomized
PLGA: poly (lactic-co-glycolic acid)
PPi: pyrophosphate
PtNPs: platinum nanoparticles
RANKL: receptor activator of nuclear factor kappa B ligand
ROS: reactive oxygen species
Runx-2: runt-related transcription factor 2
SeNPs: selenium nanoparticles
siRNA: small interfering RNA
TiIs: titanium nanomaterials
TNF-α: tumor necrosis factor-α
Wnt: wingless
LB is thankful to University Grants Commission-Senior research fellowship (UGC-SRF) for her fellowship. KN acknowledges Council of Scientific & Industrial Research-Senior research fellowship (CSIR-SRF) for her fellowship.
LB, KN and AKV conceived and wrote the manuscript. AKV edited the manuscript. All authors contributed to manuscript writing, revision, read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
The work on siRNA was supported by the project grant “Small molecule functionalized biopolymeric nanoparticles encapsulating siRNA for targeted delivery to osteoblasts for managing bone disorders” (BT/PR 21645/NNT/28/1224/2017) from Department of Biotechnology, Government of India. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2022.
Copyright: © The Author(s) 2022. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Yuka Ikeda ... Satoru Matsuda
Jelena Radovanovic ... Esma R. Isenovic
Ranjeet Singh, Partha Pratim Manna
Anastasija Panic ... Esma R. Isenovic
Tao Wang, Haiyan Xu
Juanjuan Fei ... Jun Ren
Alberto Rubio-Casillas ... Raied Badierah
