Affiliation:
Department of Food Science and Nutrition, Nara Women’s University, Kita-Uoya Nishimachi, Nara 630-8506, Japan
Affiliation:
Department of Food Science and Nutrition, Nara Women’s University, Kita-Uoya Nishimachi, Nara 630-8506, Japan
Affiliation:
Department of Food Science and Nutrition, Nara Women’s University, Kita-Uoya Nishimachi, Nara 630-8506, Japan
Affiliation:
Department of Food Science and Nutrition, Nara Women’s University, Kita-Uoya Nishimachi, Nara 630-8506, Japan
Affiliation:
Department of Food Science and Nutrition, Nara Women’s University, Kita-Uoya Nishimachi, Nara 630-8506, Japan
Affiliation:
Department of Food Science and Nutrition, Nara Women’s University, Kita-Uoya Nishimachi, Nara 630-8506, Japan
Email: smatsuda@cc.nara-wu.ac.jp
Explor Med. 2021;2:443–454 DOI: https://doi.org/10.37349/emed.2021.00062
Received: July 30, 2021 Accepted: August 30, 2021 Published: October 31, 2021
Academic Editor: Esma R. Isenovic, University of Belgrade, Serbia
The article belongs to the special issue Reactive Oxygen Species (ROS) in Pathophysiological Conditions
Excessive reactive oxygen species (ROS) may cause oxidative stress which is involved in aging and in the pathogenesis of various human diseases. Whereas unregulated levels of the ROS may be harmful, regulated basal level of ROS is even necessary to support cellular functions as a second messenger for homeostasis under physiological conditions. Therefore, redox medicine could develop as a new therapeutic concept for human health-benefits. Here, we introduce the involvement of ROS on the crossroads of stemness, senescence, and carcinogenesis in a stem cell and cancer cell biology. Amazingly, the anti-proliferative (APRO) family anti-proliferative proteins characterized by immediate early growth responsive genes may also be involved in the crossroads machinery. The biological functions of APRO proteins (APROs) seem to be quite intricate, however, which might be a key modulator of microRNAs (miRNAs). Given the crucial roles of ROS and APROs for pathophysiological functions, upcoming novel therapeutics should include vigilant modulation of the redox state. Next generation of medicine including regenerative medicine and/or cancer therapy will likely comprise strategies for altering the redox environment with the APROs via the modulation of miRNAs as well as with the regulation of ROS of cells in a sustainable manner.
Reactive oxygen species (ROS) include a set of highly reactive radicals such as superoxide anion and hydroxyl radical as well as non-radical species such as hydrogen peroxide, largely derived from oxidative metabolism in cellular physiological processes [1, 2]. Main intracellular source of ROS is the mitochondrial electron transport chain during the ATP-synthesis in mammals [3]. Unnecessary ROS production may cause oxidative stress which is involved in aging and also in the pathogenesis of various human diseases including cancer [4]. The function of ROS as intracellular second messengers and as extracellular mediators is leading physiological signaling [5]. While ROS may also affect various pathophysiological conditions, they will be required as selective strategies for therapeutic intervention. Redox medicine is a new therapeutic concept targeting ROS for human health benefit. In particular, stem cells are responsible for regeneration supporting homeostasis of an organism through self-renewal, proliferation, and differentiation, whose function can be affected by ROS [6]. Initially, ROS were considered to act as molecules damaging to cellular components [7]. For example, high ROS levels are known to cause cellular DNA damages. However, many studies have accepted that ROS play a critical role in physiological processes in lower ROS levels [8]. In addition, different degrees of ROS would affect cell energetics and intracellular signaling pathways to regulate messenger RNA (mRNA) and protein expression, which determines cell fate modulating either cell survival or cell death [9]. Interestingly, ROS mediated significant responses including stemness of stem cells, cellular senescence, and carcinogenesis seem to be involved in the roles of anti-proliferative (APRO) family proteins [10]. In fact, expression of an APRO protein is frequently downregulated in many human cancers and in stem cells [11]. Furthermore, the APRO proteins have been implicated in a variety of cellular processes including cell division, DNA repair, transcriptional regulation and mRNA stability, which could regulate cell-cycle progression, apoptosis, and/or differentiation [12]. Therefore, APRO proteins might be a link between cellular senescence and carcinogenesis [10]. In this review, we would like to highlight the molecular mechanisms and biological consequences of APRO family proteins during stem cell-maintenance, senescence, and cancer progression with ROS motivations for the future superior medical application.
Stem cells are characterized by their ability to differentiate into several specific cells, which retain a high proliferative capability and plasticity [13]. In addition, stem cells are robust to collapse and protected against the responses to various stimulus [14]. However, ROS stimuli have been hypothesized to lead to the loss of the transplanted stem cells [15]. In general, ROS may inhibit the proliferation of stem cells and enhance the differentiation to the specific cells [16] (Figure 1). The impacts of ROS on stem cells proliferation and/or differentiation have attracted interests due to potential applications of stem cells for medical regenerative therapeutics. Antioxidant enzymes such as catalase and/or superoxide dismutase (SOD) have been shown upregulated upon the stem cells differentiation, which led to a dramatic decrease in the intracellular ROS level [17–19]. ROS metabolism might be definitely controlled by various antioxidant molecules involved in the redox machinery in cells. One mechanism by which ROS may exert their effects is via the regulation of target molecules in a phosphoinositide-3 kinase (PI3K)/AKT pathway [20]. Since stem cells are firmly regulated by oxidative stress, the control of ROS levels is important to maintain their self-renewal capability. In quiescent stem cells, very low levels of ROS may be indispensable for their stemness-maintenance; however, physiological ROS levels may encourage stem cells’ proliferation and/or differentiation [21]. High levels of ROS are mostly involved in programmed cell death and/or apoptosis [22]. For example, radiation therapy kills cancer cells by generating high levels of ROS, which contributes to inducing severe DNA damages and cancer cells death. Remarkably, members of APRO family transducer of ErbB-2 (TOB)1 and TOB2 may inhibit the proliferation of mouse embryonic stem cells [23]. It has been shown that embryonic stem cells in single knockout TOB1–/– or TOB2–/–, and double knockout TOB1–/– and TOB2–/– grow faster than wild type embryonic stem cells without losing pluripotency [23]. In addition, knockdown of TOB1 considerably increases the proliferative activity of mesenchymal rat stem cells in vitro [24]. B-cell translocation gene (BTG)1, the other member in APRO family, also seems to be necessary for maintaining stem cells’ quiescence and self-renewal [25]. Downregulation of Nanog has resulted in reduction of pluripotency markers such as Krüppel-like factor 2 (Klf2) and octamer-binding transcription factor 4 (Oct-4) in P19 stem cells, whereas expression of TOB1 has been upregulated by the Nanog-silencing [26]. In addition, another APRO member tetradecanoyl phorbol acetate-inducible sequences (TIS)21/BTG2 plays a pivotal role in maintaining the hematopoietic stem cells’ compartment and/or hematopoiesis [27]. Furthermore, physical exercise in BTG1-null mice may rescue the loss of proliferative capability arising in elder stem cells [11, 28].
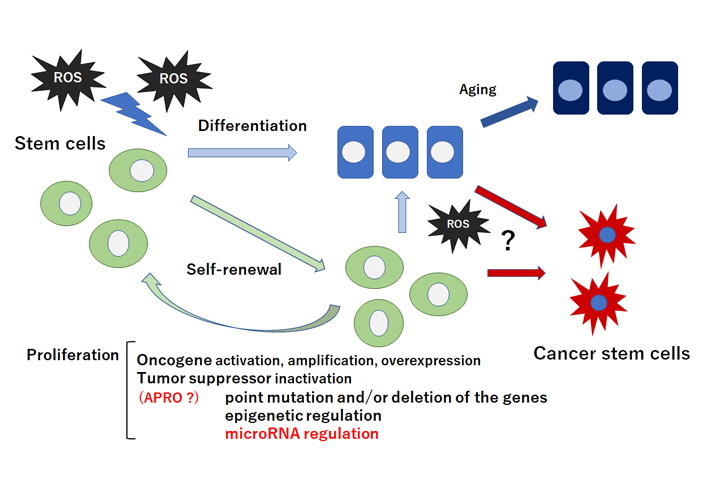
Schematic representation of stem cells self-renewal, proliferation, and differentiation. Stem cells and/or cancer stem cells are capable of maintaining the stem cells population through appropriate self-renewal and proliferation. Quiescent and/or self-renewing stem cells exhibit low ROS levels probably due to their robust antioxidant ability. Intermediate levels of ROS may prime stem cells for differentiation and/or cancer stem cell-formation. Tumor suppressor inactivation by microRNA-regulation could lead to the proliferation of stem cells and/or cancer stem cells. Note that some critical pathways have been omitted for clarity. ?: the phenomena have not yet been confirmed at present
During cellular aging, boosted ROS generation and reduced antioxidants may result in redox imbalance triggering age-related diseases [29]. ROS could weaken cellular function via the modification of proteins and lipids in cells and/or tissues [30]. For example, ROS-induced lipid peroxidation occurs in physical dysfunction in elderly individuals [31]. Consequently, aging may be connected with the accumulation of oxidized molecules categorized by elevated carbonyl residues [32, 33]. Enhanced oxidative stress-mediated protein carbonyls may contribute to age-related diseases [34]. Being declined antioxidant defense by aging, fragility to remove oxidative-damaged molecules could probably accelerate the aging further. Also, long-term exposure to oxidative stress conditions is harmful. It has been shown that chronic oxidative modification of proteins leads to protein aggregation, diminished cellular function, and finally to apoptotic cell death in elderly animal [35]. Glutathione-SH (GSH) antioxidants system is also indispensable for the cellular detoxification of ROS in cells. It has been shown a direct association between reduced levels of the GSH by severe oxidative stress and quick up-regulation of hemeoxygenase-1 (HO-1) in a different kind of cells [36, 37]. Eventually, increased HO-1 via the decrease of antioxidant defense systems appears to be as important as ROS production in the aging cells. Interestingly, BTG1 may also cause senescence by reducing mitochondrial membrane potential [38]. In addition, BTG1 overexpression may also induce G2/M arrest, differentiation, and senescence in a cell line cells [39]. Similarly, BTG2, another member of APRO family, is involved in a variety of biological processes including cell differentiation and cellular senescence, and its expression is deeply regulated by p53 [40]. However, oxidative stress upregulates the BTG2 expression via ROS-ΝFκΒ signaling cascade independent of p53 implying that it could be involved in mediating several biological phenotypes depending on the cellular context [40]. BTG2 may regulate posttranslational modification of p53 as contrasting to inhibiting sirtuin 1 (SIRT1) and B-cell/CLL lymphoma 2 (Bcl2) expression [41]. In addition, the expression of TOB1 also increases with cellular senescence [42].
Hypoxia may play an imperative role in cancer cells-microenvironment [43]. During deprivation of enough oxygen supply, cells cannot keep adequate antioxidant capability resulting in increased ROS levels [44]. Hence, cells must adjust to the consequences of reduced oxygen availability. ROS levels may activate redox-sensitive transcription factors that can enhance tumor formation [45]. It is possible that increased ROS levels, brought during chronic inflammation, may stimulate aberrant self-renewal in tumor cells [46, 47] (Figure 1). Tumor cells frequently overexpress catalase and produce enormous concentrations of hydrogen peroxide [48]. The tumor cell itself escapes the toxic action of hydrogen peroxide then destroys neighboring healthy normal cells [49]. Increased ROS is responsible for the oxidation of negative feedback loop and hence control the actions of other signaling pathways in tumor cells growth by the PI3K/AKT pathway [50]. Cell survival is also promoted by the oxidation and inactivation of the negative regulators of PI3K/AKT signaling [51]. Generation of ROS in cancer cells may also lead to the inactivation of phosphatase and tensin homologue deleted on chromosome 10 (PTEN) that leads to an increase in PI3K/AKT signaling which promotes tumor cell-proliferation [52]. PTEN has been found to be inactivated by hydrogen peroxide in a variety of cancers [53]. The major ROS-regulated hypoxia inducible factor (HIF) may downregulate mammalian target of rapamycin (mTOR) which is a key regulator of cell growth by controlling cell metabolism, mRNA translation, and control of autophagy [54]. The tumor suppressor genes in cells may produce proteins that play important roles as antioxidants [55]. For instance, p53 could regulate the expression of various antioxidant enzymes including catalase and SOD2 thereby decreasing ROS accumulation [56]. It seems that induction of these p53 target genes is a conserved expression response to oxidative stress in different cells or organs. As p53 is deleted and/or mutated in almost all cancers, relationship between ROS accumulation and oncogenic signaling has been shown [57]. Amazingly, BTG2 is often downregulated in several types of cancer [58]. High-level BTG2 protein expression may correlate with prolonged survival in patients with breast cancer [59]. In addition, cytoplasmic TOB1 expression has been important in angiogenesis and cell differentiation within gastric cancer and may be used as a potential prognostic marker of the cancer [60]. Likewise, significant prognostic effects of the several APRO family have been found in lung adenocarcinoma [61]. Potential correlations between some APRO family members and survival outcomes are also observed in ovarian, colorectal and brain cancer [61]. Genomic profiling of B-cell leukemia and lymphoma has pointed BTG1 towards a role of tumor suppressors, since BTG1 is frequently deleted and/or mutated in these malignancies [62, 63]. Additionally, exosome-derived BTG1 protein is a potential biomarker for the prognosis in patients with lung cancer [64]. These findings have suggested that APRO genes and/or proteins might work as potential tools for cancer therapy. In fact, tumor growth suppression by adenovirus-mediated introduction of TOB1 in pancreatic cancer had been described suggesting a medical application for chemotherapy-resistant cancer [65].
The APRO family anti-proliferative genes are characterized in immediate early growth responsive genes [66]. The gene products include pheochromocytoma cell 3 (PC3)/TIS21/BTG2, BTG1, TOB1, TOB2, abundant in neuroepithelium area (ANA)/BTG3, PC3B and others [66]. These APRO family proteins have been described to participate in diverse human diseases, which have also been implicated in a variety of cellular processes including cell-division, DNA repair, and mRNA stability [67]. Accordingly, APRO family members therefore may be involved in physiological and/or pathological processes including cell proliferation, cell differentiation, apoptosis/programmed cell death, and acting as potent tumor suppressors [67]. As for the regulation of ROS accumulation, BTG2 has been shown to render cancer cells more sensitive to doxorubicin treatment by upregulating SOD2 expression without regulating any other ROS scavengers [68]. BTG2 mediates crosstalk between PI3K/AKT and NF-κB pathways, which regulates p53-independent induction of G2/M arrest both within normal and cancer cells [68]. In addition, BTG2 enhances the G2/M arrest along with reduction of hydrogen peroxide levels [68]. TOB1 antagonizes the PI3K/AKT signaling then induces cancer cell apoptosis by activating BAX protein and inhibiting the BCL2 expression [69]. The AKT/PTEN and the tumor suppressor p53 pathways have been proven to play a central role in regulating cell apoptosis by regulating the oxidative stress and/or ROS reducing [70]. In addition, TOB1 may reduce the phosphorylation of AKT, resulting in decreased protein expression of β-catenin, which in turn declines the expression of cyclin D1 and/or cyclin-dependent kinase-4 (CDK4) [71]. BTG1 overexpression along with cancer therapy-irradiation may be involved in inhibition of the PI3K/AKT signaling pathway, suggesting that BTG1 promoted ionizing radio-sensitivity of breast cancer cells [72]. Low-levels of limited local ROS play an important role as redox-signaling molecules in pathways such as PI3K/AKT/mTOR signaling involved in the maintenance of cellular homeostasis [73]. Consistently, induction of p53 overexpression may bring cellular senescence, autophagy and apoptosis, which are dependent on the regulation of the PI3K/AKT/mTOR pathway with APRO family proteins and/or excess ROS production [74].
Given the diverse roles attributed to APRO family proteins, the molecular biological mechanisms of APRO family proteins might be quite intricate. The N-terminal conserved APRO domain is a protein-protein interaction module, which is capable of binding to DNA-binding transcription factors as well as the CNOT7 and CNOT8 deadenylase subunits of the carbon catabolite repression 4-negative on TATA-less (CCR4-NOT) complex [75]. Several members of the APRO family are shown to be implicated in transcription in the nucleus and cytoplasmic mRNA deadenylation and its turnover [76] (Figure 2). Likewise, TOB1 can concomitantly interact with the poly(A) nuclease complex CCR4-chromatin assembly factor-1(CAF1) and the cytoplasmic poly(A)-binding protein, suggesting a role of TOB1 in linking the deadenylation [77]. In addition, TOB1 and TOB2 proteins contain an extra-long C-terminal domain with two poly(A)-binding protein (PABP)-interacting motif 2 (PAM2) motifs [78]. These TOB proteins can interact with CAF1 and PABP simultaneously, which promotes general deadenylation of mRNA [77]. The interaction of TOB with CAF1 and PABP promotes deadenylation by recruiting the CAF1-CCR4 deadenylase complex to the 3’ end of mRNAs with a poly(A) tail [77]. Interestingly, the antiproliferative effects of TOB1 have been suggested to involve the CAF1-CCR4 deadenylase complex [79], suggesting that TOB proteins can exert their antiproliferative function by modulating the mRNA turnover [80]. BTG2 also interacts with CAF1 deadenylase through its APRO domain, a defining feature of APRO family, to control cell proliferation [81]. In fact, it has been shown that mRNA destabilization by BTG1 and BTG2 sustains cell quiescence [82].
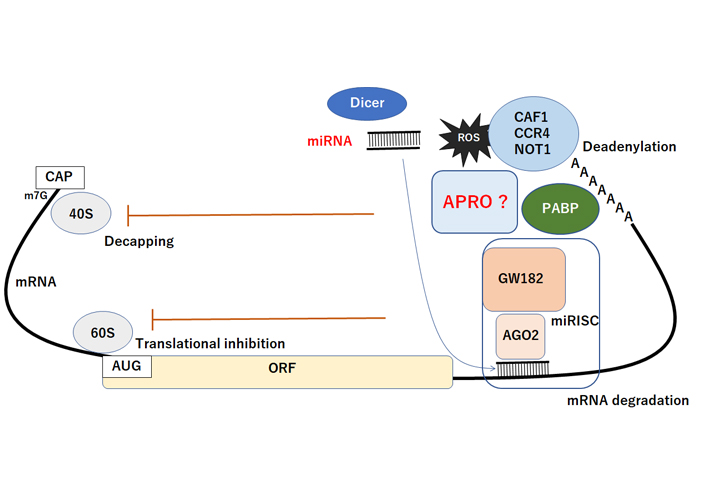
Schematic representation of miRNA-mediated functional inhibition of mRNA. The AGO2 protein interacts with GW182 constructing the miRISC complex, which may facilitate the deadenylation and mRNA degradation process by CAF1/CCR4/NOT1 with PABP and APRO complex. Consequently, the miRNA could play active roles in regulating posttranscriptional gene expression via the decapping, translational inhibition, deadenylation, and degradation of mRNA. The CAF1/CCR4/NOT1 complex is recruited to the 3′UTR of specific mRNAs through an interaction with PABP protein. APRO may also interact with PABP and recruits the CAF1/CCR4/NOT1 complex to initiate. Hammerhead represents inhibition. Note that some critical pathways have been omitted for clarity. CAF1: Chromatin assembly factor-1; CAP: 5’ Capping structure of mRNA; ORF: open reading frame; miRISC: microRNA-induced silencing complex; AUG: initiation codon (ATG); PABP: poly A binding protein; GW182: an 182 kDa protein with multiple glycine/tryptophan (GW) repeats; AGO2: Argonaute2; ?: the phenomena have not yet been confirmed at present
MicroRNAs (miRNAs) inhibit mRNA expression by base pairing to the 3’UTR of target mRNAs, which consequently inhibits translation by initiating poly(A) tail deadenylation and mRNA destabilization [83]. The miRNA-mediated mRNA deadenylation occurs subsequent to initial translational inhibition, indicating a two-step mechanism of miRNA action, which serves to consolidate repression. The miRISC interacts with the PABP and the CAF1 and CCR4 deadenylases [84]. In addition, the miRNA-mediated deadenylation is dependent upon CAF1 activity and PABP, which serves as a miRNA coactivator [84]. Importantly, a core component of the miRISC could interact with PABP via its C-terminal region, which is required for miRNA-mediated deadenylation [84]. Accordingly, APRO family proteins could be a key modulator of miRNAs-function, since APRO family proteins have a potential to interact with CCR4/CAF1 complex (Figure 2). The CCR4/CAF1 complex has been shown a multifunctional regulator that plays important roles in multiple cellular processes in eukaryotes [85]. In addition, the expression of APRO family (TOB1, BTG1) may also be regulated by certain miRNAs [24, 86].
There might be an unsolved paradigm in this field. Oxidative stress due to generation of ROS could cause damages to cellular proteins, lipids and DNAs, which is one of critical reasons responsible for carcinogenesis. Cancer or malignant tumor might have at least a cancer stem cell in its population, which can eventually render the disease immortality without senescence. Dicer, a key component of the miRNAs biogenesis, is a ribonuclease/helicase enzyme which involves in maturation of miRNAs. Dicer was found to be regulated by ROS/NF-E2-related factor-2 (NRF2) interaction to contribute to activation of damaged-DNA-repair machinery. NRF2 may indirectly control the damaged-DNA-repair and miRNAs processing machinery through the crosstalk between NRF2 and Dicer [87]. In addition, lack of Dicer has been shown to enhance ROS production and oxidative stresses [88]. Furthermore, it has been shown that the lack of dicer activity in plant cells make them unable to control excessive ROS production [89].
The relationship between APRO molecules and carcinogenesis in a cell may be reminiscent of the relationship between cytotoxic T cells and cancer cells in a body called “immune check points” (Figure 3). We would like to suggest here tentatively to use a term “stemness check points” for the former relationship. However, this has to be confirmed precisely and would be a part of future rigorous research. The field of redox and stem cells medicine has blossomed in recent decades, but further evolvement will depend on linking more firmly distinct ROS and oxidative stress to pathophysiological processes. Given the crucial roles of ROS for pathophysiological functions, upcoming therapeutics should include vigilant modulation of the redox state. New approaches to modify the redox processes should be dynamic for improving health and quality of life (QOL) of individuals. Next generation medicine will likely include strategies for altering the redox environment with APRO being one of the key targets.
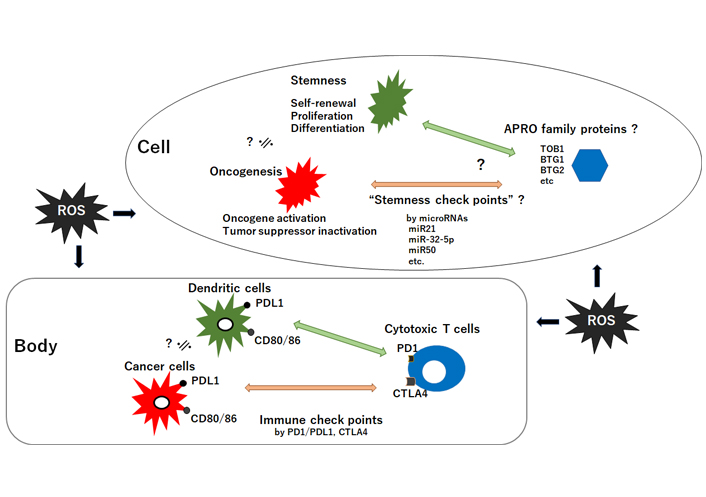
The relationship between tumor suppressor APRO family proteins and oncogenesis/stemness-homeostasis in a cell seems to look like the relationship between cytotoxic T-cells and cancer-cells/dendric-cells in a body. It could be hypothesized that the former may be regulated by “stemness checkpoints” microRNAs for targeting APRO family proteins, whereas the latter is regulated with “immune checkpoints” molecules such as PD1/PDL1 and CTLA4. ROS could affect both. CD: cluster of differentiation; PD: programmed cell death; PDL1: programmed cell death ligand 1; CTLA4: cytotoxic T-lymphocyte-associated protein 4; ?: the phenomena have not yet been confirmed at present; ≒: approximately equal
APRO: anti-proliferative
APROs: anti-proliferative proteins
BTG: B-cell translocation gene
CAF1: chromatin assembly factor-1
CCR4: carbon catabolite repression 4
miRISC: microRNA-induced silencing complex
miRNAs: microRNAs
mRNA: messenger RNA
mTOR: mammalian target of rapamycin
NOT: negative on TATA-less
NRF2: NF-E2-related factor-2
PABP: poly(A)-binding protein
PI3K: phosphoinositide-3 kinase
PTEN: phosphatase and tensin homologue deleted on chromosome 10
ROS: reactive oxygen species
SOD: superoxide dismutase
TOB: transducer of ErbB-2
YI and SM contributed conception of the study. YI, KT, NN, AT, YK and SM wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
©The Authors 2021.
Copyright: © The Author(s) 2021. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Jelena Radovanovic ... Esma R. Isenovic
Ranjeet Singh, Partha Pratim Manna
Anastasija Panic ... Esma R. Isenovic
Tao Wang, Haiyan Xu
Juanjuan Fei ... Jun Ren
Alberto Rubio-Casillas ... Raied Badierah
Largee Biswas ... Anita Kamra Verma
