Affiliation:
1Department of Critical Care Medicine, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong, China
2Guangdong Clinical Research Center for Critical Care Medicine, Guangzhou 510080, Guangdong, China
†These authors contributed equally to this work.
ORCID: https://orcid.org/0000-0001-9737-9886
Affiliation:
1Department of Critical Care Medicine, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong, China
2Guangdong Clinical Research Center for Critical Care Medicine, Guangzhou 510080, Guangdong, China
†These authors contributed equally to this work.
Affiliation:
1Department of Critical Care Medicine, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong, China
2Guangdong Clinical Research Center for Critical Care Medicine, Guangzhou 510080, Guangdong, China
†These authors contributed equally to this work.
Affiliation:
1Department of Critical Care Medicine, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong, China
2Guangdong Clinical Research Center for Critical Care Medicine, Guangzhou 510080, Guangdong, China
†These authors contributed equally to this work.
Affiliation:
1Department of Critical Care Medicine, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong, China
2Guangdong Clinical Research Center for Critical Care Medicine, Guangzhou 510080, Guangdong, China
Affiliation:
1Department of Critical Care Medicine, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong, China
2Guangdong Clinical Research Center for Critical Care Medicine, Guangzhou 510080, Guangdong, China
Affiliation:
1Department of Critical Care Medicine, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong, China
2Guangdong Clinical Research Center for Critical Care Medicine, Guangzhou 510080, Guangdong, China
Affiliation:
1Department of Critical Care Medicine, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong, China
2Guangdong Clinical Research Center for Critical Care Medicine, Guangzhou 510080, Guangdong, China
Affiliation:
1Department of Critical Care Medicine, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong, China
2Guangdong Clinical Research Center for Critical Care Medicine, Guangzhou 510080, Guangdong, China
ORCID: https://orcid.org/0000-0001-7880-0719
Affiliation:
1Department of Critical Care Medicine, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong, China
2Guangdong Clinical Research Center for Critical Care Medicine, Guangzhou 510080, Guangdong, China
3Clinical Trial Unit, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong, China
Email: wujianf@mail.sysu.edu.cn
Explor Immunol. 2022;2:200–210 DOI: https://doi.org/10.37349/ei.2022.00045
Received: November 22, 2021 Accepted: March 17, 2022 Published: April 22, 2022
Academic Editor: Dominique J. Charron, Hospital Saint Louis, France
The article belongs to the special issue The Sepsis induced Immune Conundrum
Aim: Thymosin alpha 1 (Tα1) is a promising treatment for the improvement of sepsis patients. Until now, its function in reducing acute organ damage of sepsis patients is still unclear. The aim of this study was to determine whether Tα1 can alleviate organ dysfunction in sepsis patients.
Methods: This study retrospectively enrolled sepsis patients from a multicenter randomized controlled trial [efficacy of Tα1 for severe sepsis (ETASS)]. The sequential organ failure assessment (SOFA) score on day 0 (initial), day 3, and day 7 was collected. Absolute SOFAday07 was defined as initial SOFA score minus SOFA score on day 7 (initial SOFA–SOFA day7). Delta SOFA score (ΔSOFAday07) was provided by the formula: (initial SOFA–SOFA day7) × 100/initial SOFA, and it was expressed as a percentage. After propensity score matching (1:1 ratio), baseline characteristics were well-balanced between the Tα1 group and placebo group. The primary outcome was evaluated with a comparison of ΔSOFAday07 decline between patients treated with or without Tα1 therapy.
Results: Among 288 enrolled patients, 149 patients received both Tα1 and standard therapy (Tα1 group), and 139 patients received both placebo and standard therapy (placebo group). Compared with the placebo group, the Tα1 group had significantly lower Absolute SOFAday07 [95% confidence interval (CI) 0.8 (0–1.7), P = 0.049]. Among 111 pairs of patients matched by propensity score, the Tα1 group still had lower Absolute SOFAday07 [95% CI 1.0 (0.1–1.9), P = 0.029]. Meanwhile, Tα1 treatment could significantly improve ΔSOFAday07. When the amplitude of ΔSOFAday07 was graded, one third of patients in the Tα1 group had an increase of more than 60%, compared with 22% in the placebo group. Subgroup analysis found that the ΔSOFAday07 improved significantly after Tα1 therapy in sepsis patients with no immunoparalysis at baseline, no complications, and early intervention.
Conclusions: For sepsis patients, Tα1 treatment can alleviate organ dysfunction, and ΔSOFAday07 can be used as an indicator of its therapeutic effect (ClinicalTrials.gov identifier: NCT00711620).
Sepsis is defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection [1]. Nowadays, immunotherapy draws increasing attention as a novel therapeutic option in patients with sepsis [2, 3]. Immune suppression in patients with sepsis not only undermines the ability of anti-infection, but also aggravates multiple organ injuries. Because of the rising emergence of antibiotic-resistant bacterial strains, there is an increase in demand for new treatment options.
Thymosin alpha 1 (Tα1) acts as an endogenous regulator of immune homeostasis, and it has been approved in different countries for the treatment of certain cancers, hepatitis, and other infections in recent years [4–6]. Its vital role in the course of sepsis has been reported in some human studies in which mortality was often used to evaluate the efficacy of Tα1 [7–9]. Though mortality is an objective endpoint, it can easily be influenced by multiple factors and cannot be used to predict outcomes in clinical practice. Therefore, an earlier indicator that can be used during the early stage of the course of sepsis could be of clinical importance. Though monocyte human leukocyte antigen-DR (mHLA-DR) and other immune indicators are ideal indicators to evaluate the efficacy of Tα1, their use in everyday clinical practice is limited by access to resources [10, 11].
According to sepsis and septic shock (Sepsis-3) definitions, immune dysfunction can cause a series of organ damage, indicating that the recovery of organ damage has the potential to be an appropriate indicator when seeking alternative and probably safer therapeutic solutions targeting the immune system. Sequential organ failure assessment (SOFA) score is used to clinically evaluate organ dysfunction, and its score is positively correlated with the mortality of sepsis patients [12, 13]. A recent study demonstrated that the trend of SOFA scores within a week was an appropriate indicator for efficacy of sepsis treatment [14]. This retrospective cohort study was implemented to determine the value of Tα1 treatment in alleviating organ dysfunction measured by SOFA score in sepsis patients.
The efficacy of Tα1 for severe sepsis (ETASS) trial, a multicentre randomized controlled trial, was conducted to test the effect of Tα1 and placebo in patients with severe sepsis between 2008 and 2010. This trial was approved by the ethics committee in all six tertiary teaching hospitals. A full description of the methods of the ETASS trial, including study protocol, case report form, sample size, quality control, and main results can be found in the original paper [9]. Patients with autoimmunity or immunodeficiency diseases or those in need of long-term immunosuppression therapy were excluded. Antibiotic, fluid, and ventilator care were managed by the physician according to the Surviving Sepsis Campaign (SSC) guidelines [15]. In this trial, severe sepsis was defined as the presence of a proven or suspected infection in at least one site, two or more signs of a systemic inflammatory reaction, and at least one severe or acute sepsis-related organ dysfunction. Therefore, the term “severe sepsis” in our previous study is similar to the definition of sepsis in Sepsis-3, and “sepsis” was used to replace “severe sepsis” in our study.
SOFA score was collected on day 0 (initial), day 3, and day 7. Absolute SOFAday07 was defined as initial SOFA score minus SOFA score on day 7 (initial SOFA−SOFA day7). Delta SOFA score (ΔSOFAday07) was provided by the formula: (initial SOFA−SOFA day7) × 100/initial SOFA, and it was expressed as a percentage. Then, ΔSOFAday07 was grouped according to overall interquartile range (IQR). “Free days” were calculated as the number of days that the patient was alive and free of specified intervention [ventilator use and intensive care unit (ICU) stay] during the 28-day study period. When using dichotomous variable for subgroup analysis, SOFA score and the Acute Physiology and Chronic Health Evaluation II (APACHE II) score was classified by the median. The elderly was defined as aged those 60 years or over in our previous study [16]. Intervention time was defined as the h from the onset of sepsis to randomization of subjects, and it was then divided into two groups: early (≤ 24 h) and delayed (> 24 h). To assess baseline immune status, we divided mHLA-DR into two categories: immunoparalysis (≤ 30%) and no immunoparalysis (mHLA-DR > 30%), and patients were divided into three groups according to their lymphocyte count according to the previous study [17].
Continuous variables with normal distribution were summarized as mean standard deviation (SD) and compared by t-test; while non-normal distributed variables were described as median IQR and compared by the Wilcoxon rank-sum test. Logistic regression analysis was used to evaluate the association between Tα1 and ΔSOFAday07. The odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were reported. Propensity score matching (PSM) was performed to account for confounding by indication bias in two groups by age, sex, initial SOFA score, initial mHLA-DR, study drug initial therapy time, and microorganism species. We also analyzed the efficacy parameters of Tα1 in different prespecified subgroups. The heterogeneity of treatment effects among subgroups was assessed with interaction tests. Two side P values were reported and a P value less than 0.05 was considered statistically significant. All analyses were conducted using IBM SPSS software version 26.0 (IBM Corp., Armonk, New York, USA) and GraphPad Prism 9.0 (GraphPad Software, Inc., San Diego, USA).
Among 288 enrolled sepsis patients, 149 patients received both Tα1 therapy and standard therapy (Tα1 group), and 139 patients received both placebo and standard therapy (placebo group) (Figure 1). There was no statistical difference in baseline characteristics between the two groups (Table 1). As for outcomes, no statistically significant difference was found between the Tα1 group and placebo group in survival rate. Compared with the control group, the Tα1 group had longer 28-day ICU-free days (17.8: 11.3–20.4 vs. 11.8: 0.9–18.3, P = 0.001).
Baseline clinical characteristics of patients with sepsis
| Characteristics | Total (n = 288) | P value | |
|---|---|---|---|
| Tα1 group (n = 149) | Placebo group (n = 139) | ||
| Age (years) | 65.1 ± 13.9 | 66.8 ± 12.4 | 0.276 |
| Sex (male) | 118 (79) | 100 (72) | 0.152 |
| Pre-existing conditions | 125 (84) | 113 (81) | 0.561 |
| Congestive cardiomyopathy | 3 | 4 | |
| Hypertension | 69 | 66 | |
| Coronary heart disease | 18 | 13 | |
| Liver disease | 7 | 7 | |
| COPD | 24 | 25 | |
| Diabetes | 34 | 28 | |
| Recent trauma | 7 | 5 | |
| Cancer | 50 | 42 | |
| Recent surgical history | 0.702 | ||
| No history of surgery | 77 | 78 | |
| Elective surgery | 39 | 35 | |
| Emergency surgery | 33 | 26 | |
| Other indicators of disease severity | 0.201 | ||
| MV | 118 | 108 | |
| Shock | 47 | 47 | |
| Use of vasopressor | 55 | 46 | |
| RRT | 24 | 13 | |
| Low dose corticoid | 16 | 12 | |
| Blood transfusion | 51 | 42 | |
| Acute organ dysfunctions | 0.303 | ||
| Pulmonary | 142 | 132 | |
| Renal | 41 | 34 | |
| Cardiovascular | 100 | 81 | |
| Hematological | 55 | 49 | |
| Hepatic | 23 | 24 | |
| Number of acute organ dysfunctions | 0.798 | ||
| 1 | 24 | 30 | |
| 2 | 64 | 59 | |
| 3 | 40 | 32 | |
| 4 | 16 | 14 | |
| 5 | 5 | 4 | |
| Site of infection | 0.598 | ||
| Lung | 113 | 105 | |
| Abdomen | 38 | 36 | |
| Positive blood culture | 9 | 7 | |
| Urinary tract | 2 | 5 | |
| Other | 11 | 11 | |
| Result of pathogens | 0.542 | ||
| Gram negative | 33 | 30 | |
| Gram positive | 9 | 13 | |
| Fungus | 15 | 18 | |
| Mixed | 59 | 56 | |
| No | 33 | 22 | |
| APACHE II score | 22.4 ± 6.3 | 21.2 ± 7.6 | 0.169 |
| C reactive protein (mg/L) | 129.0 (73.9, 189.5) | 114.0 (66.8, 175.5) | 0.153 |
| White blood cell (× 109/L) | 14.6 (10.2, 19.9) | 14.5 (11.0, 17.7) | 0.526 |
| Neutrophil (%) | 86.4 (80.8, 90.7) | 86.6 (80.9, 91.1) | 0.772 |
| Monocyte (%) | 5.0 (2.9, 7.5) | 4.9 (3.3, 7.4) | 0.865 |
| Lymphocyte (× 109/L) | 0.9 (0.5, 1.5) | 0.9 (0.5, 1.4) | 0.909 |
| Platelet (× 109/L) | 165.0 (86.3, 253.8) | 170.0 (116.0, 272.3) | 0.452 |
| Lactate (mmol/L) | 2.1 (1.3, 3.1) | 2.0 (1.3, 2.8) | 0.419 |
| Creatinine (μmol/L) | 102.9 (71.5, 196.3) | 93.0 (61.2, 180.2) | 0.183 |
| Total bilirubin (μmol/L) | 14.5 (9.2, 23.6) | 13.0 (8.4, 23.2) | 0.375 |
| ICU mortality | 7 (14.0) | 13 (13.1) | 0.884 |
| Hospital mortality | 10 (20.0) | 23 (23.2) | 0.652 |
| 28-day mortality | 8 (16.0) | 19 (19.2) | 0.630 |
| ICU-free days (median, IQR) | 17.8 (11.3, 20.4) | 11.8 (0.9, 18.3) | 0.001 |
| MV free days (median, IQR) | 21.0 (16.3, 25.5) | 19.4 (10.8, 24.9) | 0.275 |
Values are described by number (percentage), mean ± SD or median IQR. COPD: chronic obstructive pulmonary disease; RRT: renal replacement therapy; MV: mechanical ventilation
In our study, there is no statistically difference between the Tα1 group and placebo group in initial SOFA score (7.7 ± 3.4 vs. 7.1 ± 3.5, P = 0.108) and SOFA score on day 7 (5.0 ± 3.9 vs. 5.2 ± 3.9, P = 0.684) (Table S1). However, the Tα1 group had significantly lower Absolute SOFAday07 [95% CI 0.8 (0–1.7), P = 0.049] compared to the placebo group, but no remarkable difference was found in each individual organ score.
To further verify the results, 1:1 PSM was used to balance the baseline characteristics of patients (Table S2). Among 111 pairs of patients matched by propensity score, the Tα1 group had remarkably lower Absolute SOFAday07 [95% CI 1.0 (0.1–1.9), P = 0.029] (Table 2). The score of respiratory system was significantly decreased in the Tα1 group.
The change of Absolute SOFAday07 in patients with sepsis after PSM
| Characteristics | After PSM (n = 222) | Mean difference (95% CI) | P value | |
|---|---|---|---|---|
| Tα1 group (n = 111) | Placebo group (n = 111) | |||
| SOFA on day 0 (mean, SD) | 7.5 ± 3.2 | 7.4 ± 3.6 | 0.1 (–0.8, 1.0) | 0.859 |
| SOFA on day 3 (mean, SD) | 5.3 ± 3.0 | 5.8 ± 3.4 | –0.5 (–1.3, 0.4) | 0.263 |
| SOFA on day 7 (mean, SD) | 4.4 ± 3.3 | 5.3 ± 3.8 | –0.9 (–1.9, 0) | 0.051 |
| Absolute SOFAday07 (mean, SD) | 3.2 ± 3.1 | 2.2 ± 3.8 | 1.0 (0.1, 1.9) | 0.029 |
| Respiratory | 1.1 ± 1.3 | 0.7 ± 1.2 | 0.4 (0, 0.7) | 0.024 |
| Coagulation | 0.2 ± 1.0 | 0.2 ± 1.2 | 0 (–0.3, 0.3) | 0.765 |
| Cardiovascular | 0.7 ± 1.7 | 0.6 ± 1.5 | 0.1 (–0.3, 0.5) | 0.613 |
| Hepatic | 0.1 ± 0.7 | 0 ± 0.9 | 0.2 (–0.1, 0.4) | 0.150 |
| Neurologic | 0.7 ± 1.2 | 0.5 ± 1.4 | 0.2 (–0.2, 0.5) | 0.381 |
| Renal | 0.3 ± 0.9 | 0.2 ± 0.9 | 0.2 (–0.1, 0.4) | 0.161 |
Among enrolled sepsis patients, ΔSOFAday07 of the Tα1 group was higher than that of the placebo group (37.1, 14.9–62.7 vs. 33.1, –3.1–58.8, P = 0.12) (Figure 2). After PSM, the Tα1 group had significantly higher ΔSOFAday07 (45.5, 19.4–68.7 vs. 32.6, –3.3–59.8, P = 0.012). When the amplitude of ΔSOFAday07 was graded, one third of patients in the Tα1 group had an improvement in SOFA score by more than 60%, and 14% of them had no change or even worse SOFA score (Figure 3), while in the placebo group only 22% patients had an improvement in SOFA score by more than 60% and one third had no change or even worse SOFA score in the placebo group. Logistic regression further demonstrated that Tα1 therapy contributed to the higher ΔSOFAday07 (Table 3).
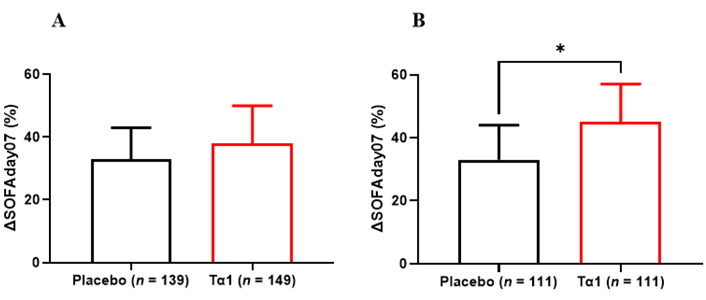
ΔSOFAday07 between Tα1 and placebo group. A: Total patients; B: PSM patients. *: P < 0.05
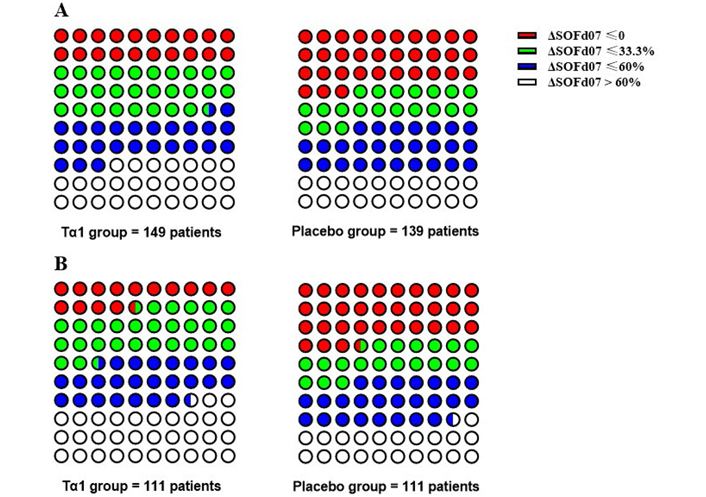
ΔSOFAday07 classification in patients with sepsis. A: Total patients in Tα1 group (left) and placebo group (right); B: PSM patients in Tα1 group (left) and placebo group (right)
Tα1 treatment was associated with higher ΔSOFAday07 in patients with sepsis
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Sample size (n) | 288 | 288 | 222 |
| Tα1 treatment | 0.510 (0.299–0.869) | 0.523 (0.301–0.909) | 0.336 (0.170–0.663) |
Values are ORs (95% CIs) unless stated otherwise. aAdjusted for sex, age, pre-existing condition, initial SOFA score, and baseline mHLA-DR; badjusted for covariates in model 2 after PSM
Subgroup analysis after PSM found that sepsis patients with no immunoparalysis had significantly higher ΔSOFAday07 after Tα1 therapy (Table 4). Meanwhile, patients with no complications and early clinical intervention also gained more higher ΔSOFAday07 after Tα1 therapy.
Subgroup analysis of ΔSOFAday07 after PSM
| Tα1 (n = 111) | Placebo (n = 111) | P value | |||
|---|---|---|---|---|---|
| n | Median, IQR | n | Median, IQR | ||
| Age | |||||
| Non-elderly | 37 | 40 (22, 72) | 39 | 22 (–8, 61) | 0.062 |
| Elderly | 74 | 47 (17, 67) | 72 | 35 (1, 57) | 0.066 |
| Sex | |||||
| Male | 84 | 43 (16, 66) | 87 | 33 (–2, 58) | 0.056 |
| Female | 27 | 54 (29, 75) | 24 | 31 (–8, 66) | 0.117 |
| Initial mHLA-DR | |||||
| Immunoparalysis | 26 | 47 (13, 73) | 29 | 31 (–1, 60) | 0.116 |
| No immunoparalysis | 85 | 45 (21, 67) | 82 | 33 (–6, 60) | 0.047 |
| Lymphocyte day 0 | |||||
| < 0.7 × 109/L | 38 | 56 (30, 78) | 39 | 44 (3, 62) | 0.102 |
| 0.7–1.1 × 109/L | 33 | 44 (16, 62) | 31 | 27 (–1, 59) | 0.225 |
| > 1.1 × 109/L | 39 | 39 (15, 67) | 41 | 26 (–12, 59) | 0.093 |
| Pre-existing conditions | |||||
| No | 18 | 55 (36, 71) | 21 | 0 (–19, 51) | 0.006 |
| Yes | 93 | 42 (18, 68) | 90 | 38 (7, 61) | 0.207 |
| Initial SOFA score | |||||
| ≤ 8 | 70 | 43 (18, 70) | 73 | 30 (–11, 60) | 0.033 |
| > 8 | 41 | 45 (26, 67) | 38 | 35 (17, 60) | 0.198 |
| APACHE II score | |||||
| ≤ 21 | 55 | 48 (19, 76) | 61 | 32 (–10, 60) | 0.048 |
| > 21 | 56 | 43 (20, 62) | 50 | 33 (4, 57) | 0.124 |
| Intervention time | |||||
| Early | 41 | 58 (32, 78) | 48 | 39 (15, 62) | 0.041 |
| Delayed | 70 | 36 (16, 63) | 63 | 27 (–16, 56) | 0.055 |
In this study comparing change in SOFA score in sepsis patients treated with Tα1 or placebo, we found that Tα1 therapy can alleviate organ dysfunction in sepsis patients, and change in SOFA from initial to day 7 (ΔSOFAday07) can be used as an alternative indicator of its therapeutic effect.
It is well-established that sepsis is a complicated illness with extremely high heterogeneity, causing multi-organ dysfunction [18, 19]. The updated definition of sepsis highlighted the bridging function of immune disorder during infection and multiple organ dysfunction syndrome (MODS) [20]. The connection between immune and other systems in sepsis needs to be further explored. In our study, we found that immune therapy can accelerate the improvement of SOFA score in the first week, indicating that treatments targeting immune disorders contribute to the reverse of organ dysfunction.
Tα1 is a peptide separated from thymus, which modulates the immune response via several pathways and helps to boost immunity [21, 22]. Studies have indicated that Tα1 interacts preferentially with negative regions of the membrane [sodium dodecyl sulfate (SDS) mixed with dodecylphosphocholine] due to the phosphatidylserine exposure, after that it may interact with nearby proteins and/or receptors acting as an effector and causing a biological signaling cascade [23, 24]. To date, several reports have attested that low serum Tα1 levels are associated with different pathological conditions such as hepatitis B, psoriatic arthritis, multiple sclerosis and sepsis. Tα1 is able to target different cell types by increasing the expression of major histocompatibility complex class I (MHC class I), MHC class II, and β2-microglobulin [25–27]. Unlike other immunotherapeutic drugs such as anti-programmed cell death protein 1 (anti-PD-1) antibodies or granulocyte-macrophage colony-stimulating factor (GM-CSF), its target receptor stays unknown, and many researchers still rely on the mortality rate to evaluate its therapeutic effect [28, 29]. In recent years, mHLA-DR acts as an ideal marker for the immune function of sepsis [16, 30]. However, though equipment for simple point-of-care testing of mHLA-DR is under rapid development, we still encounter various difficulties to use mHLA-DR as the primary endpoint in a multicenter study [31, 32].
The ΔSOFA score has been selected as the primary outcome in several clinical trials involving patients with sepsis and septic shock, along with mortality reported [33, 34]. It allows doctors to compare the trajectory of organ dysfunction from baseline in the trial, which can not only predict mortality, but also indicate prognosis and guide following therapies. Compared with traditional mortality endpoints, ΔSOFA provides an earlier and simpler assessment of sepsis treatment. Soo et al. [35] conducted a cohort study involving 20,007 critically ill patients and found that compared with the average rate of change at later time points, the slope of the SOFA score on day 1 and day 7 was higher, and was better correlated with the endpoint results (ICU and hospital mortality). Iba et al. [36] investigated patients with sepsis-related diffuse intravascular coagulation (DIC) and found that ΔSOFA between day 1 and day 7 was an effective early predictor of 28-day mortality [area under the curve (AUC): 0.81]. Karakike et al. [14], using the data from two randomized controlled trial (RCT) studies, further confirmed that ΔSOFA on day 7 was an early prognostic indicator of the 28-day mortality [area under the receiver operating characteristic curve (AUROC) 95% CI 0.84 (0.80–0.89); P < 0.001]. Our results also showed that Tα1 group had significantly higher ΔSOFAday07, which suggests that the change in ΔSOFA can be used as one of the early indicators of the therapeutic effect of Tα1.
This study had some limitations. First of all, this is a post hoc analysis of an RCT trial. Secondly, this study only collected SOFA scores for 3 time points within a week. Further prospective and longitudinal studies and clinical trials are necessary to further our understanding of Tα1’s effect on organ function during longer clinical course in sepsis patients. However, the research data was carefully selected from an RCT and was strictly implemented, and thus the results are somewhat representative. The SOFA score can easily be obtained in the ICU settings, and its continuous monitoring may be more conducive to the evaluation of therapeutic efficacy of Tα1.
In conclusion, the present study demonstrated the value of Tα1 in reducing organ damage in sepsis patients by monitoring the dynamic changes of SOFA score, indicating the need for further explorations of the interaction between immune disorders and organ damage. In addition, ΔSOFAday07 can be used as an appropriate indicator for evaluating the efficacy of Tα1.
APACHE II: Acute Physiology and Chronic Health Evaluation II
CIs: confidence intervals
ETASS: efficacy of thymosin alpha 1 for severe sepsis
ICU: intensive care unit
IQR: interquartile range
mHLA-DR: monocyte human leukocyte antigen-DR
PSM: propensity score matching
RCT: randomized controlled trial
SD: standard deviation
SOFA: sequential organ failure assessment
Tα1: thymosin alpha 1
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/100345_sup_1.pdf.
We would like to thank all of the doctors, nurses, technicians, and patients involved at the six participating hospitals for their dedication to the ETASS trial.
FP, YL and JW drafted the manuscript. FP, LZ and LW carried out the data analysis. BG, LL, MC, YN and LW collected and discussed the data. JW, FP and XG designed all research, interpreted data and edited the manuscript. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
The authors declare that they have no conflicts of interest.
The ETASS trial was approved by the ethics committee of the First Affiliated Hospital of Sun Yat-sen University (200815). Written informed consents were obtained from the patients or next of kin.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
Requests for accessing the datasets should be directed to contact the corresponding author Prof. Jianfeng Wu. The supplementary tables can be found in the Supplemental materials section.
This work was funded by Guangdong Clinical Research Center for Critical Care Medicine (2020B1111170005), by Sun Yat-sen University Clinical Research Program 5010 (2007015, 2019002), and by Program for the Natural Science Foundation of Guangdong Province (2016A030313269). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2022.
Copyright: © The Author(s) 2022. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Michael Bauer, Reinhard Wetzker
Francisco Javier Martín Oncina
Markus Blaess ... Hans-Peter Deigner
Dablu Lal Gupta ... D. N. Rao
Nicholas Daering, Majdi N. Al-Hasan
