Affiliation:
Department of Microbiology Immunology and Parasitology (DMIP), EPM-UNIFESP Federal University of São Paulo, São Paulo (SP) 04023-900, Brazil
Email: vbueno@unifesp.br
ORCID: https://orcid.org/0000-0001-8954-129X
Explor Immunol. 2023;3:341–360 DOI: https://doi.org/10.37349/ei.2023.00106
Received: January 27, 2023 Accepted: April 25, 2023 Published: August 31, 2023
Academic Editor: Tamas Fulop, Université de Sherbrooke, Canada
The article belongs to the special issue Immunosenescence: Mechanisms and Its Impact
Changes occurring in the immune system along the ageing process increase the risk of infection, susceptibility to tumor development, and autoimmunity. Interventions such as physical exercise, supplements, and probiotics have been proposed in order to circumvent these conditions. Vitamin D supplementation could contribute to the immune system homeostasis in older adults since a large proportion of this population has low levels of circulating vitamin D. Additionally, observational studies have shown the association between vitamin D status and infections, chronic diseases such as cancer, diabetes, and cardiovascular disease. Recently it was observed that old patients with COVID-19 and vitamin D deficiency had enhanced severity of lung damage, longer stay at the hospital, and increased risk of death, suggesting that vitamin D plays an important role in the patient outcome from COVID-19. A high dose of vitamin D supplementation improved clinical recovery in a case-series report but in another study, no evident link between levels of vitamin D and risk of COVID-19 infection was found. Results also remain debatable for vitamin D supplements and improvement of immune response after vaccination, tuberculosis, pneumonia, and sepsis. It has been hypothesized that vitamin D could modulate the immune system and thus provide both efficacies in the immune response to pathogens/vaccinations and reduction of the inflammatory phenotype. This review will discuss vitamin D and homeostasis of the immune system; the literature-based clinical data on vitamin D and infections; and the possible link between vitamin D and immune response after vaccination.
The worldwide growth in the number of individuals over 65 years of age presents a challenge for public health. A recent confirmation was severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection when deaths related to COVID-19 in the USA (2020–2023) according to age were 73.547 (0–49 years old), 200.067 (50–64 years old), 251.321 (65–74 years old), 290.862 (75–84 years old), and 300.134 (85 years old and older). These data show a positive correlation between age and death for COVID-19 infection and a similar trend was observed in the same period for deaths due to pneumonia and influenza [1]. Older adults likely present chronic diseases (i.e. cardiovascular and degenerative diseases, sarcopenia, metabolic disorders, and cancer) which in addition to the hallmarks of ageing (telomere shortening, aberrant intracellular signaling, mitochondrial dysfunction, damaged protein accumulation, altered nutrient sensing, and epigenetic alterations) have been suggested to influence the health span in several ways, including via effects on the immune system [2–4]. The disturbance in the immune system function may contribute to the higher incidence and severity of infections, poor response to vaccines, and cancer development in the aged population [5, 6]. In order to improve the function of immune cells in older adults, some therapies such as physical activity, supplements (i.e. vitamin D), and probiotics have been proposed [7–11].
The first line of human defense against pathogens is based on the production of antimicrobial factors such as cathelicidin, defensins, hepcidin, neutrophil peptides, and interferon-gamma (IFN-γ). Vitamin D has been linked to the increased production of these factors in human infections and in human cell culture [12–15]. Vitamin D receptor (VDR) has been found by us (Figure 1) and others to be expressed in immune cells suggesting that these cells can respond to the variations in the levels of local or circulating vitamin D [16, 17].
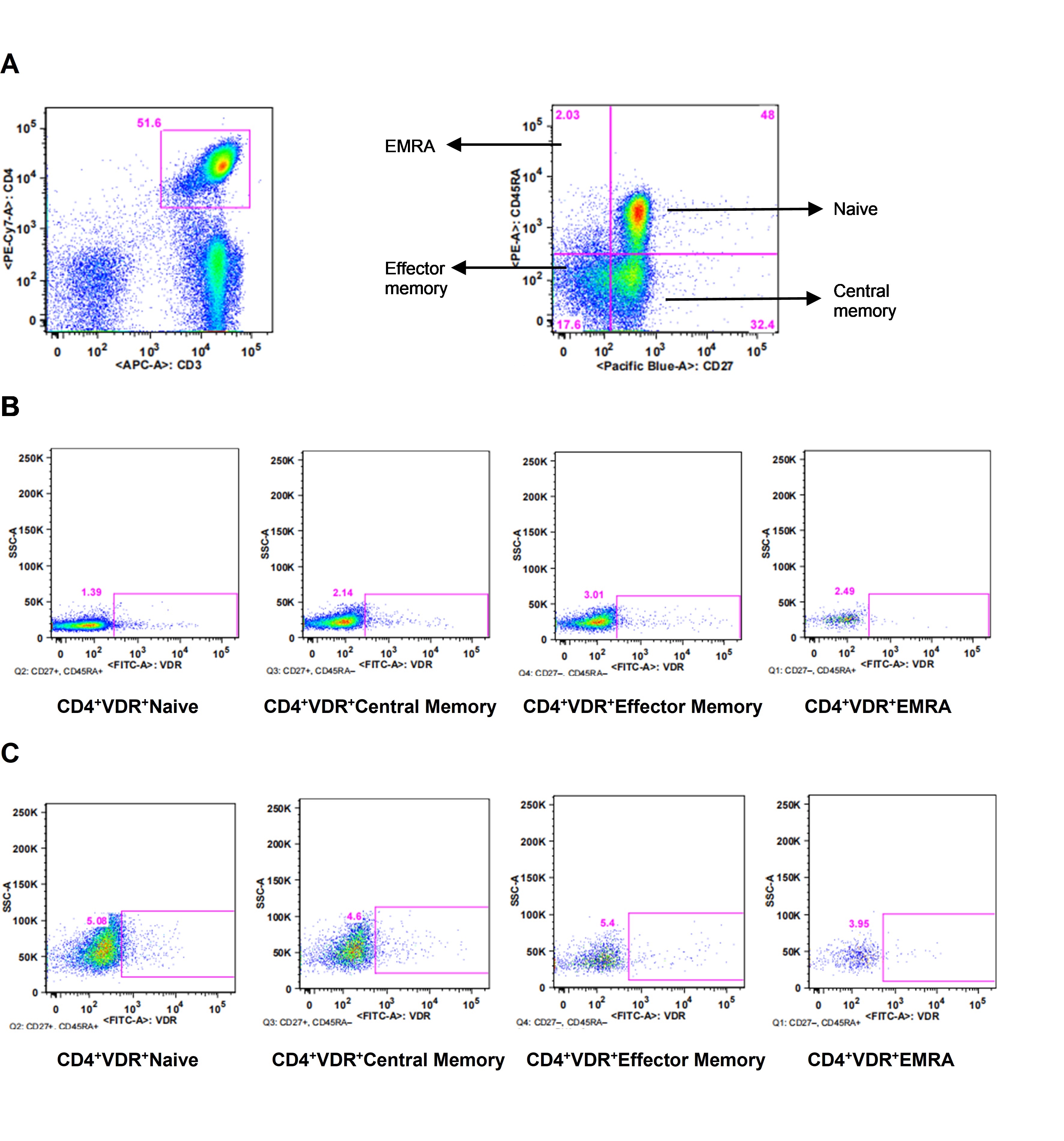
VDR expression. A. Representation of CD4+ T cells (CD3+CD4+) and phenotypes; B. expression of VDR on CD4+CD27+CD45RA+ (naive), CD4+CD27+CD45RA– (central memory), CD4+CD27–CD45RA– (effector memory), CD4+CD27–CD45RA+ [effector memory re-expressing CD45RA (EMRA)] without stimulus; C. expression of VDR on CD4+ T cells phenotypes after stimulus
Erdoğan and Fındıklı [18] found that in ageing patients with sepsis the serum levels of vitamin D and VDR were significantly decreased in non-survivors (73.4 9.0 years old). They concluded that in patients with sepsis, VDR low levels are predictors of poor outcomes. However, there are controversial results regarding to the benefits of vitamin D supplementation for older adults. Recently, Goncalves-Mendes et al. [19] found no difference in serum cathelicidin, antibody titers, and ROS production after influenza vaccination in individuals older than 65 years old (vitamin D deficient) supplemented with cholecalciferol (n = 19) or receiving placebo (n = 19). In opposition, Dramé et al. [20] reported in a systematic review of observational studies that vitamin D supplementation for COVID-19 older patients was associated with better rates of primary clinical outcomes (survival, less severe disease, and less requirement of oxygen therapy). It was also observed that older patients without vitamin D deficiency presented better primary clinical outcomes regarding to death rate, disease severity, and requirement of oxygen therapy or invasive mechanical ventilation [20]. An Australian study with 21.315 older adults randomized to 60.000 IU of vitamin D or placebo monthly for 5 years showed a slight reduction in the number of episodes for antibiotic prescriptions, total prescriptions, and repeated prescriptions, suggesting a particular benefit for those with low vitamin D status [21].
The aim of this review was to overview some of the cellular mechanisms associated with the ageing process and the immune system decreased efficacy. In addition, it was pointed out the possible effects of vitamin D on immunity focusing mainly on infections and vaccination. It was searched on PubMed for literature-based observational data about vitamin D and ageing in: infections, disease severity, patient outcome after infectious diseases, and immune response after vaccination. This review has limitations since the search was restricted to PubMed and there was a lack of discussion on differences between gender, early ageing, and octagenarians/centenarians, comorbidities, active and sedentary older adults.
The conclusion is that despite vitamin D has been suggested as a supplement for older adults, the role played by this vitamin in host resistance to infection and improved response after vaccination is still controversial.
During the ageing process, a significant number of older adults presents alterations in tissues and organs with a consequent decrease in physiologic and metabolic reserves culminating thus in comorbidities and poor quality of life. In opposition, centenarians seem to have developed adaptive strategies to deal with age-related physiological and pathological changes. The immune system can impact the ageing process and/or be impacted by biological ageing which will lead to changes in the efficacy of the immune response [22, 23]. Targets for immune system improvement have been evaluated and one candidate is vitamin D since its functions go beyond osteoblast differentiation and matrix calcification in bone. Potential actions of vitamin D have been reported in bone marrow, brain, colon, breast, malignant cells, and immune cells [24]. Considering that lower levels of vitamin D are common in older individuals, some health professionals have recommended vitamin D supplementation for the general ageing population and mainly for aged-care residents and critically ill patients [25–27]. However, results are still controversial and further studies are needed to confirm the benefit of vitamin D for the ageing immune system.
Vitamin D sources are cod liver, swordfish, and salmon oils, ultraviolet (UV)-exposed mushrooms, fortified foods (milk, margarine, cereals, and orange juice), supplements, and sunlight. Solar UV radiation leads 7-dehydrocholesterol (skin) conversion to pre-vitamin D3 and later vitamin D3. Vitamin D2 and D3 from diet and skin are hydroxylated in the liver by 25-hydroxylase (CYP27A1) to the precursor 25 hydroxyvitamin D [25(OH)D] [25(OH)D3; 25OH]. Next, hydroxylation by 1α-hydroxylase (CYP27B1, stimulated by the parathyroid hormone—PTH) in the kidneys leads to the biologically active form of vitamin D 1,25-dihydroxyvitamin D [1,25(OH)2D; 1,25 dihydroxycholecalciferol (1,25OH)]. The biological active 1,25OH exhibits a half-life of 4–6 h whereas the precursor 25OH has a half-life of 3 weeks [28, 29]. Vitamin D actions are triggered by its binding to VDR followed by the dimerization with the retinoid X receptor (RXR). The translocation of the heterodimer (1,25D-VDR-RXR) to the nucleus and its binding to vitamin D-responsive elements (VDRE) in the promoter regions leads to gene expression [30]. In the active form, vitamin D stimulates calcium reabsorption in the intestine and promotes osteoblast differentiation and matrix calcification in bone. Homeostasis of calcium and bone is not the only function of vitamin D as the expression of VDR occurs in bone marrow, brain, colon, breast, malignant cells, and immune cells [24]. In addition, vitamin D acts in endocrine, paracrine, and autocrine manner since other tissues than the kidney express 1α-hydroxylase and could thus convert 25OH to 1,25OH [31]. This plethora of biological actions has potential to regulate the development of chronic diseases such as hypertension and cardiovascular diseases (renin expression and inhibition of vascular smooth muscle division), cancer (cell growth and angiogenesis), diabetes (insulin sensitivity and secretion), cognitive loss, and Alzheimer’s disease (inflammation and plaque formation) [29].
Cells from the immune system contain 1α-hydroxylase and can therefore activate 25OH. Gottfried et al. [32] showed that human macrophages and in a less extent dendritic cells (DCs) express the CYP27A1 (bioactivator of vitamin D3) and are capable to convert vitamin D3 into the active metabolite 1,25OH. Immune cells also express the VDR and thus 1,25OH may act in the immune system (paracrine or autocrine pathways). However, the regulation of immune cells activating vitamin D is probably different since non-renal 1α-hydroxylase expressed by macrophages is not similar to the renal hydroxylase and it is not regulated by PTH [33]. Instead, circulating levels of 25OH or cytokines [IFN-γ, interleukin-1 (IL-1), tumor necrosis factor-alpha (TNF-α)] can regulate 1α-hydroxylase [34], suggesting that the immune system has a different control of vitamin D activation.
Protection to the organism facing pathogens is dependent on several factors such as chronic diseases, nutritional status, hormonal dysregulation, and immunity impairment (immunosenescence) during the ageing process. Immunosenescence is a complex process in which the immune system is remodeled to cope with the infections and damages to the organism over time. The dynamic of this process is not completely understood and it can be influenced by several factors [35]. In this scenario, vitamin D’s possible effects on the immunosenescence have been proposed since its receptor (VDR) is expressed in immune cells [16, 17]; the culture of peripheral blood mononuclear cells in the presence of vitamin D has shown modulation of cell phenotype and cytokine production [36, 37]; innate immune cells can secrete antimicrobial peptides [cathelicidin antimicrobial peptide (CAMP)/LL-37] in the presence of vitamin D [38–40]. However, results are still controversial regarding the possible benefits of vitamin D supplementation in host resistance to infection.
Considering that innate and adaptive immunity play an important role in the defense against pathogens and that vitamin D status has been associated with up- and down-regulation of the functions performed by the immune system (Figure 2), further knowledge on how vitamin D could modulate the immune response is essential to determine whether the supplementation in ageing individuals represents a benefit.
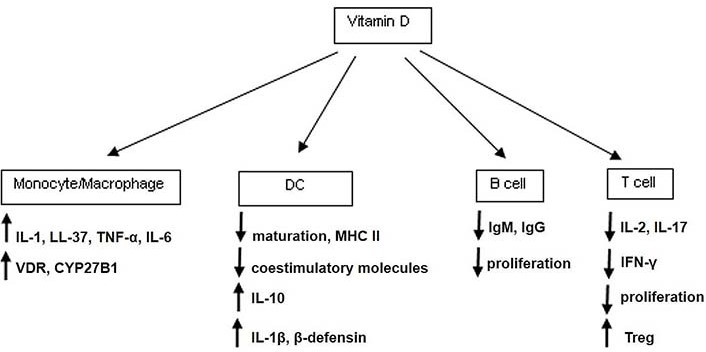
Vitamin D effects on immune cells. The activation of the innate immune response by pathogens occurs mostly through pattern recognition receptors (PRRs) such as toll-like receptor (TLR). In human monocytes, the activation caused by pathogens via TLR up-regulates genes associated with the conversion of 25OH to 1,25OH and VDR. The addition of vitamin D to monocytes in culture causes the up-regulation of VDR downstream genes such as cathelicidin [38–40]. The peptide cathelicidin (LL-37) has antibacterial activity but also, is able to neutralize lipopolysaccharide (LPS), to stimulate leukocyte chemotaxis, and to promote angiogenesis. Liu et al. [38] showed that a TLR2/1-binding synthetic peptide derived from Mycobacterium (M.) tuberculosis when added to human primary monocytes and DCs causes up-regulation of VDR gene in monocytes and genes of activation in DCs. MHC II: class II major histocompatibility complex; IgM: immunoglobulin M; IgG: immunoglobulin G; Treg: T regulatory cell
Alvarez-Rodriguez et al. [41] found in health controls that 25OH levels were lower than 20 ng/mL in 5% of the young (n = 27; 26.8 2.5 years old), 21.7% of the middle-aged (n = 23; 44.1 7.9 years old), and 31.6% of the older adults (n = 21, 70.6 8.4 years old). In addition, age was associated with lower levels of vitamin D and cathelicidin (LL-37), and circulating LL-37 showed a positive correlation with 25OH levels. After stimulation of monocytes with specific TLR activation there was a significant negative correlation between age and production of pro-inflammatory cytokines (TNF-α, IL-6). Vitamin D levels correlated positively with the production of IL-1-β, TNF-α, and IL-6 after monocytes activation with imiquimod (TLR7). Alvarez-Rodriguez et al. [41] concluded that age and vitamin D affect the innate immune response.
In a laboratorial simulation of virus infection Telcian et al. [42] exposed human primary bronchial epithelial cells in culture to rhinoviruses and respiratory syncytial virus (RSV) and observed that the infection down-regulated VDR expression on these cells. The addition of vitamin D in the culture reduced the rhinovirus replication and release, increased the expression of rhinovirus-induced IFN-stimulated genes, and the secretion of cathelicidin. In addition, vitamin D (1,000 nmol/L) decreased RSV-induced pro-inflammatory cytokines (IL-6, IL-8) expression in human primary bronchial epithelial cells. These findings show that even though rhinovirus caused the reduction of VDR expression in epithelial cells, exogenous vitamin D acted via cathelicidin and IFN to increase antiviral defenses.
Considering that both innate and adaptive immunity are important in the defense against infections, Olliver et al. [36] studied both branches of immune response in cells from healthy controls stimulated with pneumococci products. Vitamin D (1,25OH; 100 nmol/L) increased the expression of the costimulatory molecule CD86 and MHC II in DCs cultured with pneumococci products confirming that vitamin D affects DC maturation. The authors also observed the up-regulation of factors related to the migration of DCs to draining lymph nodes for antigens presentation to T cells. DCs exposed to both vitamin D and pneumococci products had an enhanced expression of pattern recognition receptors [PRRs—nucleotide-binding oligomerization domain 2 (Nod2) and TLR2] but acquired a non-phagocytic phenotype. Vitamin D increased both gene/protein expression of IL-1β and β-defensin in pneumococci products-stimulated DCs. In the adaptive response, memory T-helper cells cocultured with pneumococci products-stimulated DCs showed up-regulation of IFN-γ, IL-17, and L-10, whereas vitamin D addition abrogated IFN-γ and IL-17 production and increased IL-10 production. The authors concluded that in a cell culture model, vitamin D enhances key elements of innate immunity while dampens adaptive immune responses against pneumococci.
Rode et al. [37] found that human CD4+ T cells stimulated in the presence of 25OH or 1,25OH displayed reduced IFN-γ production. However, the addition of IL-12 to the culture partially rescued IFN-γ production in the presence of vitamin D. To clarify whether vitamin D prevents the polarization to adaptive effector response [T helper-1(Th1)], CD4+ T cells were stimulated with IL-12 and anti-IL-4 in the absence or presence of 25OH and after 24 h it was observed a discrete decrease in the downstream activation factor T-box transcription factor 21 (TBX21) but not after 48 and 72 h in culture. The same was found for IL-12Rβ2 suggesting that vitamin D does not inhibit differentiation of Th1 but affects the transcription of IFN-γ. Both signal transducer and activator of transcription 1 (STAT1) and STAT4 phosphorylation were not significantly affected by vitamin D during T cell activation. Th1-inducing conditions were essential to counteract the inhibitory effect of vitamin D on IFN-γ production. The co-culture of CD4+ T cells with DCs for 6 days caused cathelicidin upregulation that was dependent on vitamin D but independent of Th1-inducing conditions. DCs cultured with M. tuberculosis (heat-killed) showed reduced cathelicidin expression through TLR signaling. A positive effect of vitamin D addition was observed as the expression of cathelicidin was less inhibited in the presence of vitamin D than in its absence. These findings suggest that vitamin D can act to decrease IFN-γ production by CD4+ T cells but helps DCs to overcome the inhibitory effect of M. tuberculosis.
Considering the ageing scenario, an important aspect is the low-grade inflammatory status (inflammageing), which has been characterized by increased circulating levels of inflammatory factors such as C-reactive protein (CRP), interleukin (IL)-6, and TNF-α. To identify whether vitamin D levels were associated with the inflammageing profile, the InCHIANTI study evaluated individuals (n = 867, mean age 75.1 years old) according to 25OH status and found that levels lower than 31.4 nmol/L were associated with higher circulating IL-6 but not TNF-α, IL-1α, and IL-18 [43]. The English Longitudinal Study of Ageing evaluated 25OH levels and inflammatory markers in community-dwelling individuals (n = 5,870, 50–80 years old). Low levels of 25OH (≤ 30 nmol/L) were negatively associated with CRP [44].
Regarding to Treg cells, patients with chronic heart failure (CHF) were compared to healthy young and old donors (79–90 years old) for the evaluation of Treg and inflammatory Th17 cells in peripheral blood. Healthy young and old donors presented higher Treg and lower Th17 in comparison with CHF patients. Additionally, there was a positive correlation between the decreased Treg and 25OH status in CHF patients. Purified CD4+ T cells (CHF and healthy young) were cultured in the presence of 1,25OH and after activation (anti-CD3, monoclonal antibody binding to CD3 on T cells /anti-CD28, monoclonal antibody binding to CD28 costimulatory molecule/rhIL-2) it was observed an increased frequency of Treg in healthy donors. In CHF patients, there was a decrease in IL-17 and IFN-γ expression along with an increase in Treg cells after culture in the presence of vitamin D. The expression of VDR increased in healthy donors and CHF patients. The authors concluded that, in vitro, vitamin D modulates CD4+ T cells through VDR and could benefit CHF patients [45].
In older individuals, the observed inflammageing profile, its correlation with ageing-related chronic diseases, and the suboptimal vitamin D levels association with chronic degenerative diseases/overall mortality [46–49], suggest that adequate levels of 1,25OH could modulate the immune system in this population.
The gold standard measurement for vitamin D status is the blood concentration of 25OH since 1,25OH (active form) could be normal or even elevated in adults with deficiency [50]. On the other hand, 1,25OH levels have been correlated with the severity of infections and thus could predict the patient outcome in some conditions [51, 52]. The USA Endocrine Society defined 20 ng/mL level of vitamin D as deficiency, 21 ng/mL to 29 ng/mL as insufficiency, and 30 ng/mL or more as sufficiency. The maintenance of 40 ng/mL to 60 ng/mL serum concentration of vitamin D is considered ideal and up to 100 ng/mL is safe [53].
The Committee of Scientific Advisor Nutrition Working Group reported in a review of 25OH status in different regions (Asia, Europe, Latin America, Middle East/Africa, North America, and Oceania) that vitamin D sub-optimal levels is a common finding worldwide. Authors also pointed out that depending on lifestyle and environmental conditions, hypovitaminosis D could be observed in all age groups [54]. In older adults, the decreased solar exposure, the skin atrophy with reduced amount of the precursor 7-dehydrocholesterol, and the reduced content of natural vitamin D in diet lead to lower serum levels of vitamin D [55, 56]. Indeed, a study from Switzerland [57] reported that 15% of community-dwelling older adults remained with insufficient levels of vitamin D even during summer and 30% reached 30 ng/mL levels. These findings suggest that even though higher levels of vitamin D are expected during summer, age can be a determinant of vitamin D status. Thus, it is a consensus that concentrations of 25OH tend to decline with age [58] but it is a debate whether vitamin D status is correlated with increased risk for mortality. A Baltimore USA study [59] evaluated the bioactive form of vitamin D 1,25OH in 70–79 years old women and found that after six years of follow-up, levels lower than 15.3 ng/mL were significantly associated with higher risk of all-cause mortality compared with higher serum concentrations (> 27 ng/mL). In opposite, the Octabaix study in Spain [60] evaluated 328 community-dwelling individuals (male and female; 85 years old) and more than half of them presented hypovitaminosis D. However, 25OHD blood levels lower than 14 ng/mL to 33.4 ng/mL were not statistically associated with increased overall or cardiovascular mortality after a three-year follow-up. For hospitalized patients, the American Society for Parental and Enteral Nutrition recommends 200 IU of daily vitamin D administration [61] but it has been shown that even double dose (400 IU) is not enough for patients at the hospital to reach levels considered sufficient of vitamin D [62, 63].
As the conversion of skin 7-dehydrocholesterol to pre-vitamin D3 depends on the sunlight, vitamin D levels are affected by season (fluctuates seasonally) and thus could impact both the immunity and the outcome after infectious diseases. Berry et al. [64] evaluated the association of serum vitamin D concentration with seasonal infections and lung function in 6,789 individuals (45 years old) from UK. Levels of 25OH increased from February to September (mean 72.5 nmol/L) and the prevalence of respiratory infections decreased from January to August (late summer). Vitamin D status was inversely associated with seasonal infections and authors suggest that the adequate intake of vitamin D could prevent hypovitaminosis contributing thus to the respiratory health. In individuals with no pathogen (n = 101, mean age 59.4 years old), influenza pneumonia (n = 50, mean age 60.1 years old), Legionella pneumonia (n = 49, mean age 62.6 years old), and Streptococcus (S.) pneumonia (n = 100, mean age 57.4 years old), Pletz et al. [51] observed a season association for 25OH but not for 1,25OH. After statistical adjust for seasons, age had a significant impact on 1,25OH but not on 25OH levels. Both 25OH and 1,25OH lower levels were associated with patients needing hospitalization and longer period of stay at the hospital. There was an inverse correlation between serum levels of 1,25OH and severity of community-acquired pneumonia without significant association to specific pathogens. In another study, individuals with active tuberculosis (n = 107; 29–53 years old) presented lower levels of 25OH and higher levels of inflammatory factors (CRP, IL-6, TNF-α). 25OH lower levels were also observed in household contacts (n = 144; 26–52 years old) than in healthy controls (n = 31; 26–48 years old). Season of sampling (winter/spring versus summer/autumn) was the major factor related to lower levels of 25OH and vitamin D deficiency in household contacts. Considering that these individuals were confirmed as latent tuberculosis infected 8–10 weeks after last exposure it suggests that the exposition to active tuberculosis during winter (vitamin D lowest levels) increases the susceptibility to disease [65]. Khoo and Koenen et al. [66] followed up healthy male donors (n = 15; 28–60 years old) for a year and evaluated levels of 25OH, 1,25OH, CD4+, and CD8+ T cells. The median concentration of 25OH and 1,25OH was 43 nmol/L and 219 pmol/L in winter whereas it was 89 nmol/L and 237 pmol/L in summer respectively. In summer it was observed increase in CD4+ naive T cells along with CD4+ T cells expression of C-C motif chemokine receptor 4 (CCR4) and CCR6 (skin homing), CCR9 (gut homing), CCR7 (lymphoid tissue homing). In summer, there was a reduction in the level of IFN-γ expression by CD4+ T cells and a decrease in the percentage of IL-2 and IL-17-secreting CD4+ T cells. Levels of IFN-γ production by CD8+ T cells reduced from spring to autumn as well as the percentage of IL-2-secreting CD8+ T cells.
Vitamin D status has been correlated with the incidence and outcome after infections, not exclusively, but mainly in ageing individuals (Table 1). In a study from Finland [67], 723 men and 698 women (53–73 years old) were evaluated according to 25OH levels and pneumonia incidence. In a follow-up of 9.8 years old, authors found that individuals with the lowest vitamin D level had a 2.6-fold higher risk of hospitalization by pneumonia than the group with the highest level.
Vitamin D levels and risk of death in healthy older adults and in patients
| Condition | Age/years old | Study | Results | Reference |
|---|---|---|---|---|
| Healthy older adults: women | 70–79 | Six-year follow-up | Vitamin D levels ≤ 15.3 ng/mL—higher risk of all-cause mortality | [59] |
| Healthy older adults: women/men | Mean 85 | Three-year follow-up | Vitamin D levels 14 ng/mL to 33.4 ng/mL not associated with higher risk of overall or cardiovascular mortality | [60] |
| Healthy adults: women/men | 53–73 | Follow-up of 9.8 years | Vitamin D levels: 8.9 nmol/L to 33.8 nmol/L presented 2.6-fold higher risk of hospitalization by pneumonia | [67] |
| Healthy older adults: women/men | 64 ± 12 | Follow-up of 9.5 years | Vitamin D levels < 54 pg/mL: increased risk for all-cause of death and for cardiovascular and respiratory infection deaths | [52] |
| Pneumonia patients | 16–97 | 30 days hospital admission | Vitamin D levels <30 ng/L: higher mortality | [69] |
| Sepsis patients | Mean 66 | 30 days hospital admission | Severe sepsis patients with lower vitamin D levels (15.7 nmol/L) presented a correlation with positive bacteria growth and death | [68] |
| Sepsis/septic shock patients | Mean 66 | 30 days hospital admission | Vitamin D levels < 7 ng/L: sepsis-related mortality; severe deficiency in pneumonia and septic shock: mechanical ventilation and prolonged vasopressor support | [70] |
| Intensive care unit (ICU)/sepsis | Mean 54 | Hospital admission | Lower levels of 25OH and LL-37 than controls | [71] |
A study in Hisayama, Japan with an average of 9.5-year follow-up evaluated the bioactive form of vitamin D (1,25OH) in serum of 3,292 individuals (64 12 years old). Levels of 65.4 to 78.1 (n = 819) and ≥ 78.2 pg/mL vitamin D were associated with similar crude cumulative survival rate (89%) whereas the lowest level (< 54.0 pg/mL) was linked to a decreased (76%) survival range. The age- and sex-adjusted hazard ratio for all cause of death increased significantly with the lower serum of 1,25OH and the same was observed for specific-cause (cardiovascular and respiratory infection) deaths [52].
In a study from UK in patients (mean age of 66 years old) with sepsis (n = 20), severe sepsis (n = 41) and health controls (n = 20), the etiology with higher incidence was community-acquired pneumonia. Vitamin D levels were significantly higher in healthy controls and in patients with mild sepsis (66.5 nmol/L and 49.5 nmol/L respectively) than in patients with severe sepsis (15.7 nmol/L). 25OH lower levels were also associated with positive bacteria growth (blood/urine/sputum/bronchoalvelolar) and death within 30 days of hospital admission [68].
In a New Zealand hospital, during the winter, 112 individuals with pneumonia (16–97 years old) were evaluated and 30-day mortality rates were higher in patients with levels of vitamin D < 30 nm/L. However, the antimicrobial cathelicidin and β-defensin serum levels were not associated significantly with 30-day mortality [69].
In a study from Rome, patients with severe sepsis/septic shock (n = 107, mean age 66 years old) were evaluated for serum 25OH. Severe hypovitaminosis D (< 7 ng/L, n = 57) was associated with higher rates of sepsis-related mortality (50.9% versus 26%) and microbiological positivity (80.7% versus 58%) along with more days using vasopressors (4–10 days versus 2–7.25 days) and lower eradication of pathogens (35% versus 68%). In the pneumonia and septic shock population, severe vitamin D deficiency was correlated with mechanical ventilation for long periods and vasopressor support [70]. ICU patients/sepsis (n = 24, mean age 54 years old), ICU/controls (n = 22, mean age 51 years old), and healthy controls (n = 21, mean age 46.5 years old) were evaluated for levels of 25OH, vitamin D binding protein, and cathelicidin (LL-37). Critically ill patients presented 25OH, and LL-37 levels significantly lower than healthy controls. Vitamin D binding protein in patients with sepsis was significantly lower than in subjects without sepsis. Vitamin D binding protein is the major carrier protein for circulating 25(OH)D and its lower levels results in further loss of urinary 25OHD, exacerbating the already low level of circulating 25OHD. Hypovitaminosis was associated with lower systemic levels of cathelicidin suggesting that vitamin D regulates the production of antimicrobial peptides such as LL-37. Authors hypothesized that vitamin D optimal status could increase the levels of cathelicidin, enhancing thus the clearance of infections [71].
Considering that vitamin D insufficiency is associated with higher incidence of infections and its action in immunity has been reported, some authors evaluated whether vitamin D status is correlated with the immune response after vaccination. Healthy, community-dwelling individuals (≥ 50 years old) were evaluated for the hemagglutination inhibition (HI) titer from pre- to post-vaccination against influenza in 2 years (n = 591 in year 1, n = 509 in year 2). Based on ≥ 4-fold rise in HI titer, for H1N1 it was observed 33% and 20% seroconversion in year 1 and year 2 respectively. In year 1, 68% seroconverted to H3N2 vaccine strains whereas 45% was observed in year 2. Influenza B vaccine was associated with 34% of conversion in year 1 and 28% in year 2. Authors did not find a consistent association between vitamin D status on the odds of seroprotection or seroconversion for any of the vaccine strains [72]. Sadarangani et al. [73] evaluated healthy individuals (n = 159, 50–74 years old) receiving seasonal trivalent (A/California/H1N1- like virus) vaccine. After 28 days it was found increased humoral response based on hemagglutination antibody inhibition [hemagglutination-inhibition antibody (HAI) titer], virus neutralizing antibody (VNA) titer, and B-cell ELISPOT. Vitamin D status at baseline correlated with day 28 HAI but not with VNA or B-cell ELISPOT across time points (28 and 75 days). Dendritic and T cells (regulatory, CD4 and CD8) and their products were not correlated with baseline vitamin D status at any time points. Authors reported that at the time of vaccination, 75% of the individuals were already immune to A/H1N1 based on a cut-off HAI titer of 1:40 and VNA titers were similar at baseline compared to H1N1 HAI titer.
Vitamin D insufficiency and higher incidence of infections are common and related findings, mainly in older adults, suggesting that the supplement could be beneficial. However, there is no consensus in the literature on whether vitamin D supplement protects ageing individuals against infections (Table 2).
Vitamin D supplementation and incidence of infections
| Condition | Age/years old | Study | Results | Reference |
|---|---|---|---|---|
| Healthy community | Mean 59 | Supplementation 50 mg/day in winter | No decrease in the severity and duration of upper respiratory infection | [74] |
| Residents (skilled nursing/assisted living facilities) | 80 ± 10 | High dose of 4,000 IU/day or standard dose of 400–1,000 IU/day followed for 12 months | Upper acute respiratory infection (ARI), skin and soft tissue infections are lower in the high dose group. All-cause hospitalization and death similar in both groups | [75] |
| Bone fracture | Mean 77 | Supplementation of 800 IU vitamin D daily | Fewer infection events, and decreased use of antibiotics than the placebo group but the results were not statistically different | [77] |
| Patients: antibody deficiency/increased susceptibility to infections | 18–75 | 12 months supplementation (4,000 IU/day oral) | Decrease in respiratory tract infections and prolonged time to the first respiratory tract infections (RTI) x placebo | [79] |
| Sepsis/septic shock patients | 55–70 | Supplementation 200,000 IU or 400,000 IU vitamin D single bolus | Higher levels of LL-37 and IFN-γ, reduced hospital length stay, and 30-day readmission | [80] |
A New York’s community received during the winter season the active supplementation with vitamin D (n = 78, mean age 59 years old) or placebo (n = 70, mean age 58 years old). The dose of 50 mg/day increased vitamin D levels from 64.3 25.4 nmol/L to 88.5 23.2 nmol/L but did not decrease the severity and duration of the reported upper respiratory infection (URI) in comparison with the placebo group in winter. Authors suggested that the results are associated with: supplement should initiate at least 3 months before winter; the dose of 50 mg/day may not be enough to stimulate innate immunity; and the baseline of 25OH was already adequate in the evaluated population [74]. Older long-term care residents are more susceptible to ARI due to immune response impairment and low functional reserve. Ginde et al. [75] hypothesis was based on the role of vitamin D in the immune system, the risk of vitamin D deficiency in older adults, and the association between vitamin D deficiency and ARI. Residents (skilled nursing or assisted living facilities) from USA received high dose (n = 55; 80 10 years old; 3,000–4,000 IU/day) or standard dose (n = 52; 82 10 years old; 400–1,000 IU/day) of vitamin D and were followed for at least 12 months. Participants in the high (31%) and standard (46%) dose groups had at least one ARI but the incidence was lower in the high dose group for upper ARI, skin, and soft tissue infections. Groups were similar for the incidence of lower ARI, urinary tract infections or other infections, and hospitalizations for ARI. There was similarity between groups for all-cause hospitalizations (46% high and 43% standard dose) and death (22% high and 21% standard). In the same line, a UK study showed that the active intervention with vitamin D3 (n = 137, 2.4 mg every 2 months + 10 µg daily) in residents of sheltered-accommodation had no effect on the time to first ARI in comparison with residents receiving placebo (n = 103). In 1-year follow-up the supplement group showed increase in upper respiratory infections and symptoms duration whereas no altered risk or duration of lower respiratory infections were observed [76]. Individuals with bone fractures (mean age 77 years old) from 21 centres in England and Scotland that were or were not (placebo) taking 800 IU vitamin D daily were evaluated. In a follow-up of 11 to 25 months, vitamin D users reported lower infection events (17.2% versus 18.8%) and decreased use of antibiotics (6.4% versus 7.5%) than the placebo group but the results were not statistically different [77]. Middle-aged adults (n = 161, 47 years old) receiving vitamin D or placebo (n = 161, 48 years old) with a mean baseline vitamin D level of 29 ng/mL showed similar incidence and severity of upper RTI (URTI). Vitamin D monthly dose of 100,000 IU was associated with similar rates of rhinoviruses, coronaviruses, parainfluenza viruses, RSV, and others, in both groups in 18 months follow-up. Murdoch et al. [78] concluded that in European adult individuals with near-normal vitamin D levels, the dose administered had no effect on the URTI prevention. However, in the cases of individuals with immune deficiency or critically ill conditions, the supplement with vitamin D could be protective mainly due to its action on the innate immune response. In agreement, Bergman et al. [79] found that patients (n = 124, 18–75 years old) with and without antibody deficiency and increased susceptibility to RTI, randomized to 12 months’ treatment with oral vitamin D3 (4,000 IU/day) had decreased number of RTI and prolonged time to the first RTI in comparison to placebo. The increased serum levels of 25OH (around 120 days) coincided with the appearance of beneficial effects of vitamin D.
Patients with sepsis or septic shock were treated at the hospital admission with a placebo (n = 10, 58–70 years old), 200,000 IU (n = 10, 55–66 years old) or 400,000 IU (n = 10, 59–67 years old) of vitamin D single bolus. At day 5, the 400,000 IU group presented a significant increase in serum levels of vitamin D and a higher level of LL-37 (cathelicidin). This group had significantly reduced IL-1β and increased IFN-γ in the same period. Hospital length of stay and 30-day readmission were significantly reduced in the vitamin D group [80]. Peelen et al. [81] investigated vitamin D levels and antibody response in a cohort (≥ 40 years old) of community-acquired pneumonia by S. pneumoniae. Individuals defined as responders (R) or hypo-responders (HR) based on antibody levels presented similar 25OH status (25–66 nmol/L in R and 25–65 nmol/L in HR). Another cohort of splenectomised patients (≥ 40 years old) vaccinated against protein-conjugated S. pneumoniae (n = 40) or Neisseria meningitides type C MenC (n = 77) presented similar levels of vitamin D for R (66 nmol/L—S. pneumoniae and 66 nmol/L—MenC) and HR (63 nmol/L—S. pneumoniae and 60 nmol/L—MenC). Peelen et al. [81] concluded that vitamin D status in the 2 cohorts evaluated does not inhibit the adequate antibody response but it is otherwise pivotal in immune homeostasis.
Regarding to vaccination (Table 3), the administration of 1,25OH (1 mg) at the site adjacent to the influenza vaccine was tested in 175 healthy volunteers. HAI titers against H1N1, H3N2, and influenza B measured 1 or 3 months after vaccination were not significantly different in comparison with placebo. Kriesel and Spruance [82] concluded that calcitriol administration was not associated with humoral immunity enhancement. For a combined tetanus/diphtheria toxoid vaccine, the oral administration of 2,000 IU vitamin D3 oil/day (n = 20, 26–34.5 years old) or placebo (n = 12, 26–32.7 years old) for 9 weeks (winter months) was associated with marginally higher efficacy for specific IgG concentrations in vitamin D group. Serum concentrations of specific IgA at baseline and after immunization were independent of vitamin D or placebo. Nevertheless, the levels of vitamin D increased significantly in the supplemented group (28.4–80.3 nm vitamin D, 13.1–29.1 nm placebo). Seven days after immunization, ex vivo stimulated peripheral blood cells presented similar frequencies of CD4 T-helper cells (CD154 upregulation) and of IL-2, IL-10, IFN-γ, and TNF-α secretion in both groups. In young adults, vitamin D supplement was considered efficient for specific IgG secretion after tetanus/toxoid vaccination [83].
Vitamin D and response to vaccination
| Condition | Age/years old | Study | Results | Reference |
|---|---|---|---|---|
| Healthy, community-dwelling individuals | ≥ 65 | Influenza vaccination H1N1, H3N2, or B virus | Levels of Vitamin D were not correlated with seroprotection or seroconversion | [72] |
| Healthy individuals | 50–74 | Influenza vaccination A/California/H1N1-like virus | Vitamin D levels at baseline correlated with day 28 hemagglutination inhibition assay | [73] |
| Healthy individuals | 18–49 | 1 mg 1,25OH at the site adjacent to influenza vaccine | Hemagglutination inhibition assay (H1N1, H3N2, B virus) is not different in comparison with placebo | [82] |
| Healthy individuals | 26–35 | 2,000 IU vitamin D/day for 9 weeks, vaccination tetanus/ diphtheria toxoid | Discrete higher IgG levels and no difference in specific IgA in comparison with placebo | [83] |
| Splenectomised patients | ≥ 40 | Vaccinated against S. pneumoniae or Neisseria meningitides | Same levels of vitamin D at baseline, similar production of antibodies | [81] |
It has been shown that older age is an independent risk factor associated with mortality for COVID-19 [84–86] (Table 4).
Vitamin D and COVID-19
| Condition | Age/years old | Study | Results | Reference |
|---|---|---|---|---|
| COVID-19 and vitamin D levels | 65 ± 13 70 ± 14 | Levels of vitamin D 12 ng/mL or 8 ng/mL | Survivors presented higher levels of vitamin D than non-survivors | [87] |
| COVID-19 and vitamin D levels | Median 63 | Levels of vitamin D 17 ng/mL to 33 ng/mL | No correlation between prehospitalization vitamin D and clinical outcomes | [91] |
| COVID-19, vitamin D supplement | 66 ± 11 65 ± 11 | 200,000 IU vitamin D intramuscularly 1 mcg/day vitamin D orally | Reduced incidence of mechanical ventilation, ICU, death, sepsis and atrial fibrillation in higher dose intramuscularly | [92] |
| COVID-19, vitamin D supplement | 59 ± 10 | High single oral dose 500,000 IU x placebo | No prevention in the respiratory worsening and no significant effects on the length of hospital stay | [93] |
| COVID-19, vitamin D supplement | 56 ± 14 | High single oral dose 200,000 IU x placebo | Similar length of hospital stays, in-hospital mortality, ICU, mechanical ventilation | [94] |
| COVID-19, vitamin D, and vaccination | 53–69 | 800 IU/day or 3,200 IU/day x placebo | No difference on breakthrough SARS-CoV-2 infection, post-vaccination titers of anti-S or neutralizing antibodies | [95] |
| COVID-19, vitamin D, and vaccination | 40 ± 11 | Vitamin D levels higher than 50 nmol/L | Higher levels of anti-S post-vaccine | [96] |
Infante et al. [87] evaluated 137 hospitalized patients (34–89 years old) with COVID-19 and observed that 100% presented vitamin D levels lower than 30 mg/dL. In addition, levels of vitamin D were significantly higher in survivors (12 ng/mL) than in non-survivors (8 ng/mL). Patients in the survivors’ group presented lower mean length of stay in the ICU, significantly lower levels of markers associated with inflammation, coagulation, and sepsis. These findings suggest that deficiency in vitamin D could contribute to the worse clinical outcome and prognosis of hospitalized patients with COVID-19. In a meta-analysis, Oscanoa et al. [88] evaluated 23 studies (n = 2,692 patients, mean age 60.8 15.9 years old) and found that vitamin D deficiency was associated with increased risk of severe SARS-CoV-2 disease and mortality. In patients with COVID-19 (51.8 16.42 years old), it was observed significantly lower levels of vitamin D than in healthy controls (48.2 15.64 years old). In addition, patients with COVID-19 displayed the frequency of total lymphocytes, T CD4+, T CD8+, and NK cells significantly lower than healthy individuals. TNF-, IL-12, and IFN-γ presented a significant negative correlation with vitamin D levels, and these factors were significantly higher in patients than in controls [89]. Another study also found decreased levels of vitamin D in patients with COVID-19 and increased serum levels of cytokines [90]. However, Szeto et al. [91] found no correlation between prehospitalization serum levels of vitamin D and COVID-19 clinical outcomes even when evaluated in subgroups based on age, race, and gender. Moreover, patients older than 50 years old or over 30 kg/m2 BMI did not present significant correlation between vitamin D levels and inflammatory markers or COVID-19 clinical outcome.
Considering that the cytokine storm syndrome is a hallmark of COVID-19 disease severity and since vitamin D is fundamental for the immune system homeostasis and lung functions against pathogens, it has been hypothesized that vitamin D may be capable to reduce the disease burden, but results are controversial. Patients with COVID-19 received vitamin D orally (low-dose 1 mcg/day) or intramuscularly (high-dose 200,000 IU) and were evaluated during the hospital stay. High-dose of vitamin D (n = 58, 66.1 ± 11.2 years old) led to significantly lower levels of CRP, low-density lipoprotein (LDL), D-Dimer, ferritin, total leukocyte count (TLC), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) compared to low-dose vitamin D (n = 58, 65.7 ± 12.6 years old). In addition, the high-dose group presented a reduced incidence of mechanical ventilation, ICU hospitalization, death, sepsis, and atrial fibrillation [92]. In hospitalized patients with mild-to-moderate COVID-19 and risk factors, a single high oral dose of vitamin D3 (n = 115, 59.8 ± 10.7 years old, single oral dose of 500,000 IU of vitamin D3 soft gel capsules—5 capsules of 100,000 IU) was compared with placebo (n = 103, 58.3 ± 10.6 years old). Vitamin D did not prevent the respiratory worsening and there were no significant effects on the length of hospital stay or other outcomes [93]. In another study, patients (n = 120, 56.2 ± 14.4 years old) with COVID-19 receiving a single oral dose of vitamin D3 (200,000 IU) presented similar length of hospital stay, in-hospital mortality, admission to the ICU, and need for mechanical ventilation than the placebo group [94].
Regarding to vaccination, Jolliffe et al. [95] showed that vitamin D supplement for adults (53–69 years old, baseline vitamin D mean level—39.9 nmol/L) caused increase in 25(OH)D concentration but it was not associated with differences in the risk of breakthrough SARS-CoV-2 infection, post-vaccination titers of anti-S or neutralizing antibodies, frequency of T cells (naive, central memory, effector memory, EMRA), cytokines produced by T cells after in vitro stimulation. However, Piec et al. [96] found correlation between SARS-CoV-2 post-vaccine anti-S higher titers and 25(OH)D levels of more than 50 nmol/L in a cohort of health care workers (mean age 40.9 ± 11 years old). The loss of antibody after peak in 8 weeks was slower for individuals with 25(OH)D > 50 nmol/L [96]. In young adults (n = 101, 34–52 years old), the levels of vitamin D at baseline were significantly correlated with anti-S IgG levels and neutralizing titers six months after vaccination against COVID-19 (BNT162b2). However, the same association was not observed for the number of T-cells producing IFN-γ [97].
As cancer patients have an increased risk of severe COVID-19, it was investigated in 119 patients with solid and hematologic cancer whether the levels of 25(OH)D correlated with the anti-SARS-CoV-2 spike’s protein receptor binding domain IgG (S-RBD IgG) and neutralizing antibody (Nab). It was found that the S-RBD level was significantly higher in the vitamin D sufficient group than in the deficient group and the same was observed for Nab. However, S-RBD IgG showed no such correlation with vitamin D levels for patients older than 60 years old [98]. Therefore, it is possible that under specific conditions, vitamin D levels could predict patient outcomes from COVID-19 and/or the supplement of vitamin D could improve the immune response against the infection or vaccination. In agreement, Velikova et al. [99] suggest that patients with compromised immune systems such as the frail elderly, malnourished individuals, and those using immunosuppression could benefit from vitamin D supplementation to reach an improvement in the immune response to COVID-19 vaccines.
The reduced level of vitamin D in older adults has been suggested to contribute to the higher incidence and severity of infections in addition to the less efficient immune response after vaccination. However, several factors and not only vitamin D may interfere with changes occurring in the immune system during the ageing process that leads to impaired immunity. There is a consensus about the decrease in the levels of vitamin D with ageing whereas it is not fully agreed that vitamin D levels in healthy older adults are correlated with increased risk of infections, hospitalizations, and all-cause deaths. In opposition, older patients with sepsis and septic shock displaying lower levels of vitamin D present a higher risk of mortality. Vitamin D supplementation in healthy aged individuals has not been associated with decreased severity and duration of respiratory infection whereas some benefit has been found in older patients with specific conditions (deficiency in antibody, bone fracture, and sepsis). Regarding to vaccination (influenza) in healthy older adults, it was observed no correlation or a discrete association between vitamin D and antibody levels. Recently SARS-CoV-2 infection showed a positive correlation between age and death. In this context, some reports have shown the association between vitamin D levels and COVID-19 clinical outcomes. In some reports, Vitamin D supplementation was positive in reducing the incidence of mechanical ventilation, ICU requirement, sepsis, atrial fibrillation, and death whereas there are also reports showing no significant effects on patient outcomes. It was shown recently that for the vaccination against COVID-19, vitamin D levels > 50 nmol/L are relevant for higher titers of anti-S antibodies. On the other hand, Vitamin D supplementation showed no difference in post-vaccination titers of anti-S antibodies or neutralizing antibodies. In summary, for older adults with impaired health (frailty, immunosuppression, cancer, and malnourishment), vitamin D supplementation may promote host resistance to infection and improve response after vaccination.
1,25OH: 1,25 dihydroxycholecalciferol
25(OH)D: 25 hydroxyvitamin D
ARI: acute respiratory infection
CCR4: C-C motif chemokine receptor 4
CHF: chronic heart failure
CRP: C-reactive protein
DCs: dendritic cells
HAI: hemagglutination-inhibition antibody
HR: hypo-responders
ICU: intensive care unit
IFN-γ: interferon-gamma
IgG: immunoglobulin G
IL-1: interleukin-1
M.: Mycobacterium
MenC: Neisseria meningitides type C
RSV: respiratory syncytial virus
RTI: respiratory tract infections
S.: Streptococcus
SARS-CoV-2: severe acute respiratory syndrome coronavirus 2
S-RBD: spike’s protein receptor binding domain
Th1: T helper-1
TLR: toll-like receptor
TNF-α: tumor necrosis factor-alpha
Treg: T regulatory cell
UV: ultraviolet
VDR: vitamin D receptor
VNA: virus neutralizing antibody
VB: Writing—original draft, Writing—review & editing.
The author declares that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
The study was supported by CAPES PrInt UNIFESP [88881.310735/2018-01]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Fidaa Bouezzedine ... Georges Herbein
Pitu Wulandari
Rafael Moura Maurmann ... Brandt D. Pence
Roberto Paganelli, Angelo Di Iorio
Natasa Strbo ... Alessia Paganelli
