Affiliation:
MARE—Marine and Environmental Sciences Centre, Department of Life Sciences, University of Coimbra, Calçada Martim de Freitas, 3000-456 Coimbra, Portugal
Email: leonel.pereira@uc.pt
ORCID: https://orcid.org/0000-0002-6819-0619
Explor Foods Foodomics. 2023;1:15–31 DOI: https://doi.org/10.37349/eff.2023.00003
Received: January 03, 2023 Accepted: February 16, 2023 Published: April 27, 2023
Academic Editor: Jose Mendiola, Academic researcher at the Institute of Food Science Research (CIAL-CSIC), Spain
The article belongs to the special issue The food (r)evolution towards food quality/security and human nutrition
Among the species of the rich algological flora of the North Atlantic, some can be used for direct consumption in human food, although few are currently cultivated on a large scale and/or marketed for this purpose. The European tradition regarding this custom is practically nil and the expression of current eating habits is little different from the past. In Europe, only in times of hunger (for example, during the Great World Wars) was seaweed consumed by the populations closest to the coastline. In addition to the multiple applications described, which expanded enormously in the 1970s, based on phycocolloids (agar, carrageenans, and alginates)—used as thickeners in the food industry, in soups, meat preserves, dairy products, and pastries—there is currently a trend of increasing consumption, both in North America and Europe.
Currently, society in Western countries said to be developed, lives immersed in an illusory abundance and food diversity. We are driven towards consumption without rules or dietary care and towards fast food, rich in calories and unsaturated fats. This appears as the miraculously adequate answer to the frenetic pace of urban life—so much, so that we have even adopted the designation of “ready-to-eat food”, or “fast food” as a style and erroneous perception of reality, in which food is seen merely as doses of fuel organic to meet our most immediate energy needs. The consequences of a diet of this type (antagonistic to traditional “slow food”, or homemade and regional food, refined with greater precept and care), where the lack of essential nutrients is evident, translates into diseases related to obesity (and collateral diseases, derived from it), as well as those related to excessive intake of sugars (diabetes) and fats (arteriosclerosis), among others [1, 2].
On the other hand, this illusion is not expressed with the same impact in developing countries or in transition countries, seen from an economic perspective, or even in those so-called emerging—although in the latter the tendency is more towards their imposed consolidation, than towards its eradication. Countries like Brazil, with a considerable coastline, face the same dilemma and have before them the path that European countries can follow, where food practices can and should be adapted to local resources. Less developed countries, but with an appreciable coastline, such as Angola and Mozambique (Africa), within the Portuguese-speaking belt, for example, may adopt new food strategies as a way of suppressing the strong shortages still felt [3, 4].
The criteria for the search and selection of edible species with commercial value are based, in the first place, on the texture and flavor of each seaweed (more than on the nutritional value) and, in the background, on the creation of new dietetic eating habits in the West, that is, in caloric value or beneficial to health [5].
The question that arises, having reached this point of awareness, is simple—what contribution or benefits can seaweed bring to the human diet, in terms of food, gastronomy, or diet? The answer seems simple given current knowledge—they represent exactly the opposite of the concept of “fast food”: a natural food, wild and abundant for the time being (and with a growth rate capable of sustaining an intensive culture), capable of providing a high nutritional value, but reduced caloric value. Low in fat, seaweed has polysaccharides that behave, for the most part, as fibers with no caloric value. Macroalgae seem to be, therefore, the best way to correct not only the lack of food for ingestion, but also the nutritional deficiencies of the current diet felt worldwide (in developed, emerging, and/or underdeveloped countries), due to its varied range of essential constituents—minerals (iron and calcium), proteins (with all essential amino acids), vitamins and fiber—absolutely necessary nutrients for human primary metabolism. They are, therefore, a guarantee of survival, to which human beings, sooner or later, will resort, now more on a whim and curiosity (thanks to some pioneering work and investments that are beginning to bear fruit) and later, out of obvious necessity, and to meet the demands of an explosively growing human population that already exceeds 8 billion people, increasingly concentrated in Asia and Africa [4, 6].
In fact, marine macroalgae represent a food treasure with high potential; its analytical composition of seaweed stands out (see Table 1) [4, 7–9].
Nutritional composition of selected edible algae (% dry weight)
| Species | Proteins | Ashes | Dietary fiber | Carbohydrates | Lipids | |
|---|---|---|---|---|---|---|
| Chlorophyta (green macroalgae) | Codium sp. | 10 | - | 5 | 53 | 1.0 |
| C. fragile | 8–11 | 21–39 | 5.1 | 39–67 | 0.5–1.5 | |
| Ulva compressa | 21–27 | 18.6 | 33–45 | 48.2 | 0.3 | |
| U. intestinalis | 6.15 | - | - | 30.58 | 7.13 | |
| U. lactuca | 10–25 | 12.9–36.8 | 10.28–38 | 36–43 | 0.6–3.9 | |
| U. rigida | 18–19 | 28.6 | 38–41 | 43–56 | 0.9–2.0 | |
| Phaeophyceae (brown macroalgae) | Fucus spiralis | 10.77 | - | 63.88 | - | - |
| F. vesiculosus | 3–14 | 14–30 | 45–59 | 46.8 | 1.9 | |
| Himanthalia elongata | 5–17 | 30–36 | 9.48–37 | 44–61 | 0.5–3.1 | |
| Laminaria ochroleuca | 9–17.2 | 38.0 | 11.7–36 | 43.0–52 | 1.5–1.8 | |
| Saccharina japonica | 4.1–7.5 | 9.1–26.63 | 10–22.8 | 51.9–84.4 | 1.0–2.4 | |
| S. latissima | 6–26 | 34.78–37.3 | 9.89–30 | 44.1–61 | 0.5–1.8 | |
| Sargassum vulgare | 9.2–19.9 | 13.01–30.35 | 4.8–10.5 | 52.6–68.5 | 0.15–0.79 | |
| U. pinnatifida | 12–23 | 26–41.2 | 8.81–46 | 30.1–51 | 1.5–10.1 | |
| Rhodophyta (red macroalgae) | C. crispus | 11–21 | 21.08 | 10–34 | 55–68 | 0.9–3.0 |
| Osmundea pinnatifida | 20.64 | - | 33.82 | - | - | |
| P. palmata | 8–35 | 15–30 | 28.57–38 | 46–56 | 0.7–3 | |
| Porphyra sp. | 25.80 | - | 40.98 | - | - | |
| Porphyra umbilicalis | 29–39 | 12 | 29–35 | 43 | 0.3 | |
| Pterocladiella capillacea | 20.52 | - | 52.08 | - | - | |
| N. tenera | 7.9–47 | 17.5–20.5 | 12–35 | 44.3–72.3 | 0.7–2.3 | |
| Neopyropia yezoensis | 31–44 | 7.8 | 48.6 | 44.4 | 2.1 | |
sp.: species; C. fragile: Codium fragile; U. intestinalis: Ulva intestinalis; U. lactuca: Ulva lactuca; U. rigida: Ulva rigida; F. vesiculosus: Fucus vesiculosus; S. latissima: Saccharina latissima; U. pinnatifida: Undaria pinnatifida; C. crispus: Chondrus crispus; P. palmata: Palmaria palmata; N. tenera: Neopyropia tenera; *: species do not present in the Atlantic flora, but indicated here for comparison
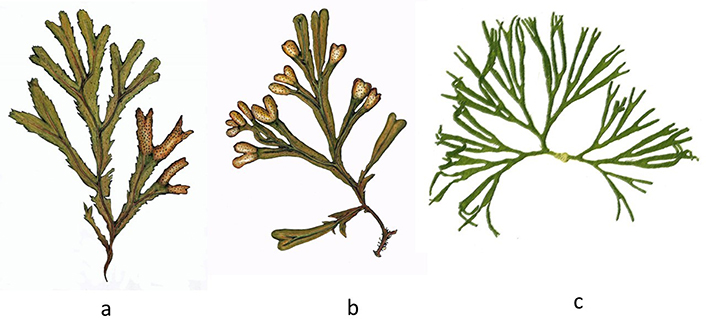
Presence of minerals (trace elements) with values about ten times higher than those found in terrestrial plants, as in the case of iron in Himanthalia elongata (“Sea spaghetti”) (Figure 1a), compared to Lens esculenta (lentil) or, in the case of calcium present in U. pinnatifida (Wakame) (Figure 1b) and C. crispus (“Irish moss” or simply “moss”) (Figure 1c), in relation to cow’s milk, which is so consumed and advertised as a bony fortifier.
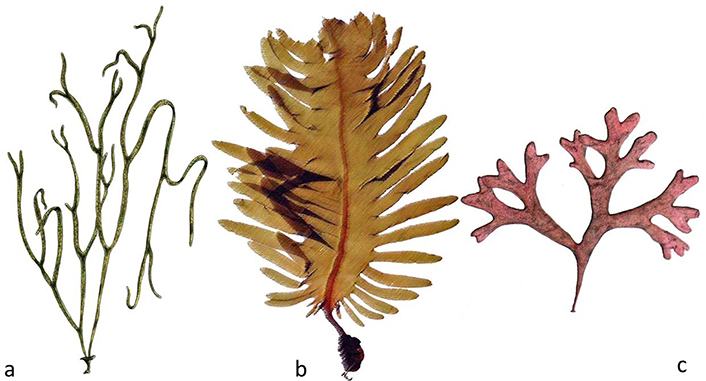
Edible algae. a. Himanthalia elongata; b. U. pinnatifida (Phaeophyceae); c. C. crispus (Rhodophyta)
Presence of proteins, the very important macromolecules for the construction of new animal tissues, which contain all the essential amino acids, constituting a protein model of high biological value, comparable in quality to those present in chicken eggs.
Presence of vitamins in significant amounts. Special mention should be made of the presence of vitamin B12, which is absent in higher plants, and which is essential for the formation of blood cells (erythrocytes) and maintenance of the nervous system.
Presence of fiber in quantities greater than that found in Lactuca sativa and similar to that of Brassica oleracea (lettuce and cabbage, respectively) and, therefore, with a digestive regulatory potential that surpasses them.
Their low-fat content and caloric value make them suitable foods for slimming regimes if integrated into a strategically programmed diet.
To date, more than 150 edible species of algae for human consumption have been identified in Europe, of which 86% are considered macroalgae (seaweeds). Of the total number of algae, only 30 species are approved as novel foods by European Union (EU) legislation [10]. Currently, diverse protein-rich seaweeds are approved by the European Food and Safety Authority (EFSA) for human consumption, such as U. lactuca (Chlorophyta; 29% of proteins), C. crispus (Rhodophyta; 20% of proteins), P. palmata (Rhodophyta; 19% of proteins), and N. tenera (Rhodophyta; 44% of proteins); and Fucus serratus (F. serratus; Phaeophyceae; 17% of proteins) and U. pinnatifida (Phaeophyceae; 29%), among others [11, 12].
U. pinnatifida (“Wakame”, Figure 1b) is native to the Northwest Pacific, from Vladivostok, Russia, southward on the coasts of Japan, Korea, China (including the coast of mainland and Hong Kong). It is a widespread invader with non-native populations on the Pacific coast of North America (California to Mexico), Europe (Northern Ireland to the Canary Islands), Argentina, New Zealand (including the Sub-Antarctic Islands), and Australia [13]. U. pinnatifida is a brown seaweed (Phaeophyceae), which lives in deep waters (up to 25 m) and can reach 1.5 m in length. Wakame is the second most consumed seaweed in food worldwide. Coming almost entirely from the seas of Japan, Korea, and China aquaculture (or more specifically phyco-culture), reaches an annual production volume of 2,563,477 tons, in 2019 (fresh weight) [14]. With regard to its culinary value, it is one of the recommended species to start tasting prepared seaweed, due to its smooth texture and pleasant taste. This seaweed is available on the market in dry form, so it must first be soaked (10 min), watered with lemon, and served raw (in salads). Its proteins are highly digestible, and the percentage of calcium is the highest among edible and commercial seaweeds. Although all algae are excellent sources of iodine, U. pinnatifida is one of the richest [4, 9].
P. palmata (“Dulse”, Figure 2a) is a red alga (Rhodophyta), typically Atlantic, small (up to 50 cm), which lives in relatively deep, cold, and agitated waters. P. palmata often grows attached to other algae (adhering to the strains of Laminaria hyperborea, for example) (Figure 2b)—a frequent phenomenon in algae, called epiphytism [15]. P. palmata is one of the most beautiful red algae on our coast and was the first species to be historically referenced as human food, knowing that it was traditionally used by the coastal peoples of Iceland, Norway, Ireland, Scotland, and French Brittany. It is currently used fresh, in northern Europe, as a substitute for vegetables and dried as an aperitif and condiment for various dishes. About 30% of its weight is made up of oligo elements (iron, potassium, and iodine) and proteins of high nutritional value [circa (ca.) 18%]. P. palmata also has high values of vitamin C, which facilitates the absorption of iron, and phycoerythrin, a red pigment precursor of vitamin A [4, 9].
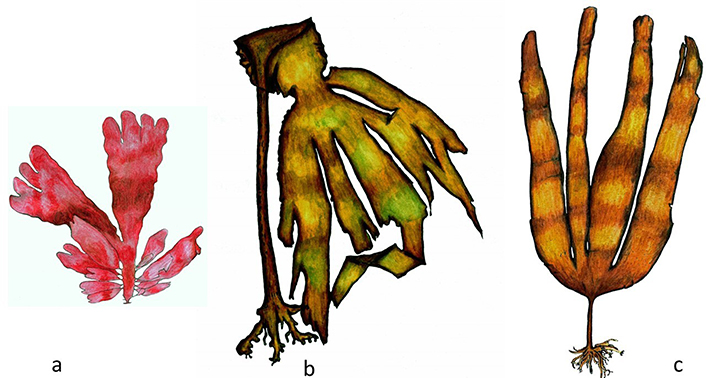
Edible algae. a. P. palmata (Rhodophyta); b. Laminaria hyperborea; c. Laminaria ochroleuca (Phaeophyceae)
Himanthalia elongata (“Sea spaghetti”, Figure 1a) is a brown seaweed (Phaeophyceae), olive-yellow in color, consisting of a small perennial basal structure, cup-shaped, measuring 2 cm to 3 cm. In spring, narrow and long bands develop from it, which give the commercial name to this seaweed (“sea spaghetti”), measuring up to 3 m in length. Its geographical distribution covers the North Atlantic, the Iberian coasts, and the English Channel. Commercially unknown in Asian countries, it is increasingly valued in Europe, both in restaurants and in specialized bakeries. Pies, pizzas, pasta, pâtés, bread, fried appetizers, and tinned cans have been made for several years, as their flavor is reminiscent of some cephalopods (cuttlefish). It is, among the Atlantic species, one of the most successful and accepted seaweeds, and, at the same time, one of the cheapest (due to its large biomass and ease of collection in coastal areas) [15]. Due to its excellent nutritional value, fleshy consistency, and smooth taste, “sea spaghetti” is considered one of the delicacies of our seas.
Laminaria ochroleuca (Figure 2c) and S. latissima (“Atlantic Kombu”) (Figure 3a). The “Japanese Kombu”, the original “Kombu”, consists only of Saccharina japonica, an alga native to the Sea of Japan and which is already the subject of cultivation practices in this country, Korea and China, with an annual production volume of 12,273,519 tons, in 2019 (fresh weight) [14].
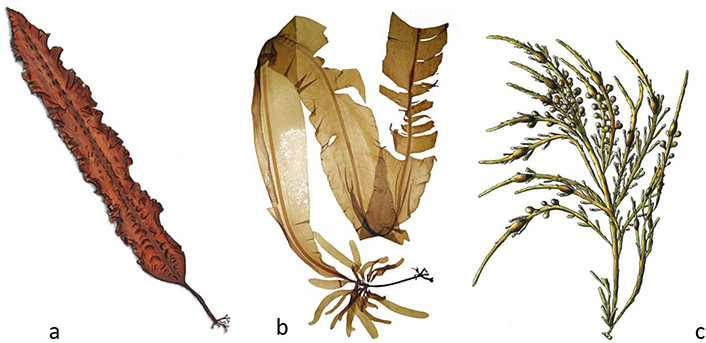
Edible algae. a. S. latissima; b. Alaria esculenta; c. Ascophyllum nodosum (A. nodosum; Phaeophyceae)
Other species are also grouped, in terms of commercial designation, under the epithet “Kombu”. The species S. latissima (formerly called Laminaria saccharina), despite being a deep seaweed and with a preference for areas with calm waters (low hydrodynamics), is present in the North Atlantic, from Norway to the North of Portugal (Viana do Castelo). Commercially, this seaweed is called “Real Kombu”, and its composition is very similar to that of Laminaria ochroleuca, commercially known as “Atlantic Kombu”. The latter “Kombu” is a little harder than the “Japanese Kombu” and is distributed in the Iberian Peninsula from Santander, in Cantabria, to Cape Mondego, in Portugal. Regarding the other species of the Atlantic flora that are normally called “Kombu”, Laminari digitata (a “tangle”) and Laminaria hyperborea (“North European kelp”), were collected in 2019, from wild populations, 40,100 and 51,624 tons (fresh weight), respectively [14]. With a fleshy consistency, it is used in the kitchen to soften, increase digestibility and prevent flatulence, as well as to intensify the flavor, due to the presence of glutamic acid (or glutamate, a non-essential amino acid, often used as an additive and flavor enhancer and which is quickly absorbed from the intestine). It is used in making bread and vegetable burgers, using ground Laminaria (or Saccharina) in the form of flour. The tasty broths in which “Kombu” was cooked are the basis of many traditional Japanese dishes (“dashi”) and in them, pasta or cereals can then be cooked, as a complement to hydrocarbons. Any of the algae gathered under the trade name “Kombu” also stands out for its high content of essential trace elements such as magnesium, calcium, and iodine [9, 15].
Alaria esculenta (“Atlantic wakame”; Phaeophyceae) (Figure 3b) is an attractive alga, whose name literally means “edible wings”. It attaches to a rock or other hard substrate by claw shaped holdfast (haptera), from which a short, distinct narrow, cylindrical, flexible thallus (stipe) arises, which then forms the very distinct strong, flattened, midrib of the leafy part of the plant (frond). These large, narrow, elongated, ribbed, flattened, ribbon-like, and slightly wavy fronds have a distinct midrib, supple to the touch, and are very flexible. The base of the stipe also bears wavy membranous lamina up to 7 cm wide on either side. Its color can be yellowish, olive-green, olive-brown, yellow-brown, or rich brown. It can sometimes grow huge, with fronds up to 5 m long and 25 cm wide, although they are typically not longer than 1 m or 2 m long [4]. This species is used to be commonly served either as a vegetable or a leaf in Iceland, Ireland, Scotland, and is also consumed in Siberia, and Norway [4, 6].
A. nodosum (“Knotted Wrack” or “North Atlantic Rockweed”; Phaeophyceae) (Figure 3c) forms a single bladder centrally in long, flattened strap-like fronds. The fronds hang downwards, draping sheltered intertidal rocks. Many fronds grow from the base and the plant generally regenerates new fronds from the base when one of the larger fronds is damaged. There is evidence that clumps can be over 400 years old and maybe even older. Ascophyllum is currently confined to the North Atlantic basin. These macroalgae species are used as packing for shellfish from the North Atlantic and when discarded may take hold [4]. This species is harvested in Ireland, Scotland, and Norway for the manufacture of seaweed meal. A. nodosum is historically consumed in Iceland and Greenland, and used also to make herbal tea. The species is harvested commercially in Canada, the USA, Scotland, France, Norway, Iceland, and Ireland [4, 7]. The total harvested in 2019, from wild populations, was 75,155 tons (fresh weight) [14].
The original “Nori” is made from red algae (Rhodophyta) Neopyropia yezoensis and N. tenera, cultivated in Japan since the 15th century. The word “Nori”, in its origin, means seaweed. However, with the passage of time, this word came to designate the product made with seaweed sheets of the genus Porphyra, Pyropia, Neoporphyra, and Neopyropia. “Nori” then consists of thin sheets made from crushed seaweed, which serve as the casing of the well-known Japanese “Sushi” [4]. “Atlantic Nori”, made from wild algae of the genus Porphyra or Neopyropia (Porphyra umbilicalis, Neopyropia leucosticta, Porphyra linearis, and Porphyra dioica), is traditionally consumed in the Celtic countries of the north and also in the Azores, as well as in Wales and Ireland, generally as an ingredient in the preparation of unleavened bread known as “laverbread” [3]. Porphyra umbilicalis (Figure 4a) is a laminar alga, translucent and mucilaginous to the touch, with a wavy circular contour, and can reach 40 cm in diameter. Due to its mineral and protein richness, intense flavor, characteristic aroma, and smooth texture, “Nori” is not only one of the most appreciated and sought-after seaweeds but also the most commercially expensive [15].
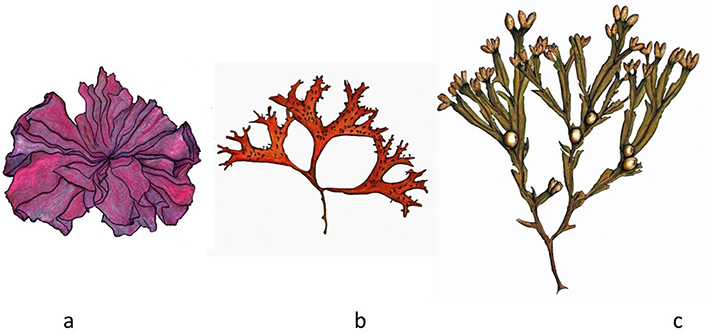
Edible algae. a. Porphyra umbilicalis; b. Mastocarpus stellatus (Rhodophyta); c. Fucus vesisculosus (Phaeophyceae)
C. crispus (“Irish moss”)—this small-sized red alga (Rhodophyta), with a fan-shaped thallus, dichotomously divided, grows on the rocks of the mid littoral plateau. Its color can vary from an iridescent purple-red to a greenish color (which appears in the summer period and in areas of lesser depth), as a chromatic adaptation to the increase in luminosity (the intensity of the color decreases inversely). C. crispus is a species with a distribution in the North Atlantic, being common in the coastline of Great Britain, Ireland, Iceland, and between Norway and Spain; the possibility of existence in Morocco and in the Cape Verde islands [16]. In the western Atlantic, this species is found from Newfoundland (Canada) to Delaware (USA). The most luxuriant populations, which for that reason are the object of intensive commercial exploitation, extend along the coasts of Nova Scotia, Prince Edward Island, Marine, and Massachusetts, as far as the western Atlantic is concerned; along the French coasts (from Cherbourg to the island of Noirmoutier), from Spain (coasts of Galicia) and from Portugal, to the East Atlantic [4]. Together with Mastocarpus stellatus (“False Irish Moss”) (Figure 4b), which occupies the same habitat, it is harvested in northern Portugal and Galicia for industrial purposes [15].
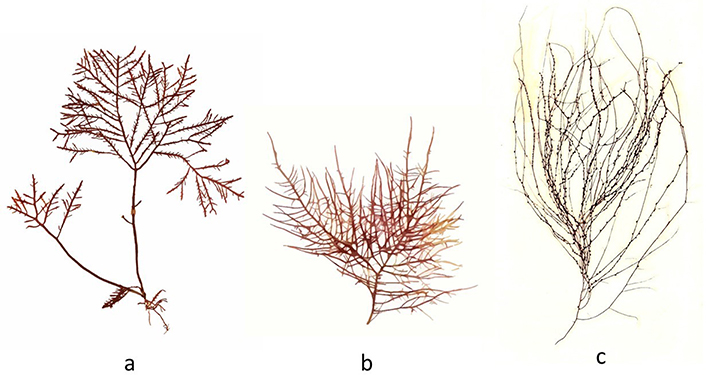
Fucus spp. (“Wrack”)—are brown algae (Phaeophyceae) and are characterized by the presence of a dichotomously divided thallus, which can reach 60 cm in length and have blades 1 cm to 2 cm wide. It is dark brown or olive green in color, leathery in consistency, and attaching to the substrate by means of a basal disc. The blades have a prominent midrib and may have air vesicles or aerocysts (present in F. vesiculosus) (Figure 4c), which allow the thalli to float when immersed [15]. F. serratus (Figure 5a) is flat, olive-brown to golden-brown algae with serrated edges. This seaweed is approved by EFSA for human consumption in the EU [11]. Powdered down it makes a great stock for stews. Stored dried it makes a very nutritious tea as well as is used in soups [4]. F. spiralis (Figure 5b) possess well-grown frons, usually easily recognizable by flattened, twisted, dichotomously branched thallus, lacking bladers, and the large, oval receptacles at the frond tips, each receptacle being surrounded by a narrow rim of the vegetative frond. This species, commonly known as “tremoço-do-mar” (“sea lupin”), in the Atlantic Islands of Azores, is considered a snack—the swollen reproductive parts (receptacles) of the frond are picked and consumed fresh [17]. F. spiralis has been used historically for the treatment of obesity, gout, goiter, and corns, and also in weight reducing and revitalizing bath treatments. It has been used for cattle feed, and as organic manure [4].
Edible algae. a. F. serratus; b. Fucus spiralis (Phaeophyceae); c. Codium tomentosum (Chlorophyta)
Codium tomentosum (“Chorão”, “chorão-do-mar”, “Imose-miru” or “Pingarelhos”) (Figure 5c)—green alga with a cylindrical, regularly dichotomous, dark green, spongy and elastic consistency. The thalli of this alga can reach, in autumn, dimensions of 30 cm to 50 cm in length, and may be covered by epiphytic algae. It has non-mucronate utricles. This species is sold in Makassar markets and is used as food in various parts of Malaysia. It is eaten raw in salads. The seaweed is used as a food in India, Indonesia, Thailand, Japan, and in Hawaii it is consumed in soups or with soy sauce or vinegar. In Portugal, the species C. tomentosum is cultivated using the integrated multi-trophic aquaculture (IMTA) method by the company Algaplus (https://www.algaplus.pt/), in Ílhavo, Aveiro [4].
Agarophytes (Gelidium corneum, Pterocladiella capillacea, and Gracilaria gracilis)—there are several red algae that produce Agar, as discussed in previous chapters. Gelidium corneum (Figure 6a) is a red alga (Rhodophyta), with a dark red, cartilaginous thallus, measuring up to 35 cm and having a rigid consistency. This alga forms dense populations on the infralittoral plateau of the central Portuguese coast and on the lower horizon of the midlittoral plateau of the coastal zone between Lisbon and the Algarve, and on the Azorean islands, together with another agarophyte for industrial use, Pterocladiella capillacea (Figure 6b). This species is collected mainly in the Azores archipelago. This last species has an erect, dark red, cartilaginous, and very branched thallus, 4 cm to 20 cm long and 2 mm thick, which is attached to the substrate by means of small rhizoids. It is a perennial species, like Gelidium corneum, abundant in the lower part of the midlittoral plateau and in the infralittoral plateau. It normally forms extensive monospecific zones, but it is common to occur in close association with fronds of G. corneum, on the mainland coast, and with fronds of the coralline red macroalgae Ellisolandia elongata, in the Azores archipelago [4, 17]. Gracilaria gracilis (Figure 6c) is an agarophyte, purple in color with greenish tones, cartilaginous consistency, and with a size that can reach 50 cm to 60 cm in length. This alga has thalli fixed to the substrate by means of a small basal disc, cylindrical and with prominent cystocarps on the surface. G. gracilis is found in protected and semi-exposed areas on the mid- and infra-littoral levels. It needs the presence of sand to develop, and it tolerates changes in salinity well. Although this seaweed is not harvested in Portugal for the agar industry, it is extensively cultivated for agar extraction in Namibia and South Africa [4].
Edible algae. a: Gelidium corneum; b: Pterocladiella capillacea; c: Gracilaria gracilis (Rhodophyta)
A phycocolloid [phyco, phyco (gel) = algae + colloid] is a compound (formed by large molecules or small particles) that forms colloidal systems if in an aqueous medium, being in an intermediate state between a solution and a suspension. Phycocolloids are large molecules, made up of simple sugars, arranged in long chains, and which are part of, as functional constituents present in intercellular spaces and also to confer consistency/flexibility to the cell walls of a large number of algae, fundamentally chestnuts and the red ones. They are formally polysaccharides, in chemical terms, which due to their properties cannot be digested or assimilated by the animal organism [8, 10].
The different phycocolloids used in the European food industry, generally as natural additives, are in Table 2.
Phycocolloids used in the European food industry
| Phycocolloids | E number |
|---|---|
| Alginic acid | E400 |
| Sodium alginate | E401 |
| Potassium alginate | E402 |
| Ammonium alginate | E403 |
| Calcium alginate | E404 |
| Propylene glycol alginate | E405 |
| Agar | E406 |
| Carrageenan | E407 |
| Semi-processed carrageenan | E470a |
E numbers (“E” means “Europe”) are codes for food additives
Phycocolloids are commonly used in the food industry to replace, with enormous advantages for the health of the body that ingests them, artificial thickeners, gelling agents, and stabilizers in suspensions and emulsions. Their main characteristics are the fact that they lack taste, smell, and color, they are soluble in water and are compatible with most foods without promoting organoleptic changes (without altering their chemical stability, although they interfere with their consistency) and for allowing fat to be replaced in dairy derivatives, pâtés, and sauces, without ceasing to be equally tasty and creamy [18].
Agar, carrageenan (extracted from red macroalgae), and alginates (extracted from brown macroalgae) are the three main principal component of phycocolloids and are found in many commercially available products on the market. The extraction of phycocolloids from algal biomass is a physical and chemical process, which begins with washing the algae (agarophytes, carrageenophytes, and alginophytes) to remove contaminants (sand and small stones; other organic elements). The next step is drying to preserve the quality of the phycocolloids, as well as to reduce the weight of the algae (largely due to the water contained in them) and maximize its conservation, preventing its rotting. In tropical regions, drying is usually carried out in litter boxes exposed to the sun, but in colder climates or with low exposure to sunlight, rotary air dryers are used, heated by the controlled burning of fossil fuel (fuel or diesel). The seaweed thus dried is transported to factories or warehouses and can be used in response to intermittent market demands. The factories strategically located next to the harvesting areas, eliminate this part of the process (onerous in terms of time and resources), and use fresh seaweed, which allows a substantial reduction in costs in the extraction and purification of phycocolloids. Alginates, in turn, are extracted by macerating alginophyte species (Phaeophyceae) in an acid medium, followed by grinding and dissolution in an alkaline aqueous medium, which ends with its precipitation in an acid medium [19, 20].
Due to their stability in media subject to wide variations in pH and salinity (such as passing through the digestive tract, for example), alginates (Figure 7) are recurrently used as excipients for drugs and also for active principles, or even compounds suitable for cosmetics (for example, in sodium alginate microspheres, for example in a micro-encapsulation process). However, its use in the medical field is not restricted to that.
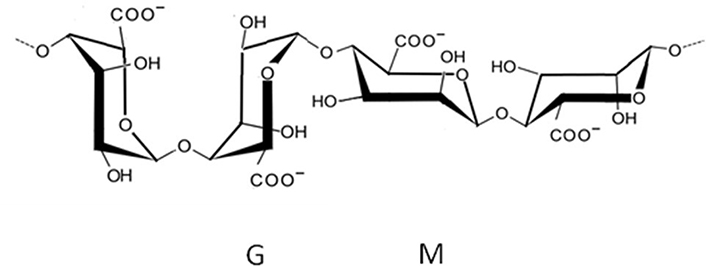
Idealized structure of the chemical units of alginic acid. G: α-L-guluronate; M: β-D-mannurate
Alginates are also very effective laxatives since, by molecularly absorbing considerable amounts of water as they pass through the intestine, they increase in volume and actively contribute to facilitating intestinal transit. They are also used in dentistry, namely in the preparation of dental molds, for later reversal in implants or prostheses. Bandages suitable for burns are also impregnated with alginate, as this phycocolloid facilitates healing and/or regeneration (keeping the skin hydrated or dry) and contributes to less painful healing. They are also used as agents to neutralize certain heavy or radioactive metals, in cases of intoxication by ingestion. In biotechnology, alginate is also used in physical immobilization techniques—enzymes, microorganisms, and cells are immobilized inside small spheres of alginate [21].
Agar is extracted mainly from the genera Gelidium and Gracilaria and is a mixture of three types of polysaccharides: agarose (the main component), agaropectin, and sulfated galactan. This phycocolloid is sometimes called agar-agar or Japanese gelatin [18, 22].
Agar (Figure 8) is essential in biotechnology studies, as it is used in the preparation of gelled culture media (culture of bacteria, algae, fungi, and tissue culture). It is also fundamental in obtaining monoclonal antibodies, interferons, alkaloids, and steroids. On the other hand, it is still recurrently used in the preparation of macromolecule separation matrices by means of electrophoresis (enzymes, for example), chromatography, or even in DNA sequencing processes (deoxyribonucleic acid) [18].
Carrageenans are mucilaginous substances present in the wall of red algae belonging to the order Gigartinales (C. crispus being one of its typical examples). Galactans (that is, galactose polymers) differ from Agar by their distinctly ionic character, as a result of the high content of sulfate OSO3− radicals. Galactans also differ from alginates in that the latter owe their ionic character to the carboxyl groups COO−.
Although with some dubious duality as to the origin of the designation, the name “carrageenan” is a derivation of a foreign word that probably comes from the word “carraigeen” (which in Gallic means “seaweed”), or from the name “carraghen”, a district from the Irish coast, where for five centuries now, there has been a tradition of harvesting the red seaweed C. crispus (due to its particular characteristic which allows it to gel milk). This same seaweed assumes several common names, many of which refer to this particularity. In France, a country with a great tradition in the collection and study of algae, C. crispus is called “lichen Blanchi” (white lichen) in northern Brittany, “picot” (twig) in the south, and “Goemon blanc” in Vendée. In one of the traditional French recipes, a mixture of milk and C. crispus is heated for 5 min to 80°C; on cooling, the milk gels and gives rise to a flan called “blanc mange”. In Portugal, C. crispus (Figure 1c) is known as botelha, cuspelho, moss, and leaf-lime, and in other parts of the world, it is known under different names: “pearl moss”, “Irish moss”, “lichen curly moss”, “lichen” and “jelly moss” [19, 20].
The industrial extraction of carrageenans began in 1930, in New England (USA), from the thalli of C. crispus and Mastocarpus stellatus, for the preparation of chocolate milk. Later and during World War II, thanks to the interruption of imports of Agar, it was replaced by carrageenan and this situation was the starting point of a flourishing industry. The product extracted in its pure state is called “carrageenan”, but it is extremely unstable and difficult to obtain; consequently, carrageenan binds to one or more cations, to form various carrageenan salts and originating “carrageenans” (Figure 9) [16, 19, 20].
Appearance—carrageenans, in their pure dry state, are presented in the form of a powder, odorless and tasteless. The carrageenan solution and gel are usually translucent and their incorporation into other solutions does not change either their taste or their original color. In solution, carrageenan polymers behave as anionic hydrophilic colloids [16, 23].
The molecular chains of these sulfated polysaccharides have two fundamental characteristics: they are made up of a monomer of galactose and contain a high proportion of OSO3− radicals (24–26%), to which the negative charge is due presented by the compound. Carrageenans come in different fractions, depending on the position in which they are esterified by the sulfate radical: beta (β), kappa (κ), iota (ι), and lambda (λ) (Figure 9), and respective hybrids. Of these, both kappa and iota form gels, with different characteristics that are reversible by heat—kappa consolidates into a harder, more brittle gel (the most used commercially), while iota forms a softer, more elastic gel. The carrageenans where the lambda fraction prevails (non-gelling) are characterized by having a high viscosity [16, 24].
Carrageenans are the main constituents of the skeletal wall and intercellular matter of macroalgae of the order Gigartinales. The content and quality change according to the season, the species, the medium, and the age of the plant. In Brittany (France), infralittoral plants of C. crispus contain more carrageenan than midlittoral thalli [25]. There is, for this species, a significant variation in the total amount of this compound between winter and summer, as the alga produces more phycocolloids during winter. Paradoxically, although it is understandable due to the fact that the climate was milder at that time, the collection of this algae is done, above all, between May and September. For the other species, the quantitative variation, according to the season of the year, is not very pronounced [16].
During the period of seaweed growth, the molecular chain of these polysaccharides remains relatively little polymerized; in this period the carrageenan produced is of average quality. Its quality (more polymerized chain) increases in the winter period, when the growth of the stalks is reduced or null—so, at first sight, the two processes seem to be, in a certain way and in metabolic terms, antagonistic. In fact, and for the same plant, the carrageenan obtained from the young parts (terminal branches), subjected to large growth, is of lesser quality than that found in the other older portions and at the base of the plant—the reason that motivates, in the collection of Eucheuma in Asia, the organization of the collection according to this stratification [26].
But what is the role of carrageenans inside seaweed? Do they constitute a reserve of carbohydrates? Are they support elements that only polymerize at the end of cell elongation? Are they ion exchangers? Are they calcium or potassium regulators? Are they metabolic precursors? Percival [27] put forward the thesis that carrageenans are the metabolic products that maintain the “flexible strength” of the plant. However, according to Fuller [28], if the previous hypothesis were correct, a higher carrageenan content should be found in plants in areas exposed to waves. However, this is not the case, as the rate of polymers is higher in quiet areas than in exposed areas. It was also suggested that the hydroscopic properties of carrageenans would allow C. crispus and other carrageenans to resist desiccation when emerging at low tide. In this case, the polysaccharide content should vary inversely with the depth of the algae. In reality, the opposite is true: coastal populations have a lower carrageenan content than infralittoral populations [25].
Nowadays, after the work of Christiaen [29] on Gracilaria gracilis Agar, it is admitted that all phycocolloids play a fundamental role in the secondary metabolism of the algal cell (the one that synthesizes compounds without apparent use for the survival of the species, but which are only found in some of them and not in all, such as those produced by primary metabolism), in the regulation of relations, more or less open, with the external environment for harmonizing the vital system. The synthesis of carrageenans, as secondary compounds, was studied by McCandless and Richter [30] using the carbon-14 isotopes technique, introduced as a marker in the nutrient medium in the form of NaH14CO3 and where the seaweed tissue was cultivated and promoted cell growth. It was found that its incorporation is much faster in lambda carrageenan than in kappa-carrageenan (Figure 9). After an interval of 24 h, the radioactivity measured in lambda carrageenan was ten times greater than that observed in kappa-carrageenan. It is, therefore possible to conclude, by analyzing the results obtained, that these two carrageenans have different metabolic synthesis pathways (or anabolic reactions).
The main food industry, with regard to the use of carrageenans, is the dairy industry. In general, small amounts of carrageenan are used, ranging from 0.01% to 0.05% [31]. For example, the addition of kappa-carrageenan (0.01–0.04%) to cheese prevents whey separation; an identical amount of carrageenan, added to ice cream, also prevents the separation of its different components and prevents the formation of ice crystals. The cocoa present in chocolate milk can be kept in suspension by adding small amounts of kappa-carrageenan. The soluble mixtures of cocoa or chocolate have lambda carrageenan in their composition so that, when added to milk or water, they dissolve easily, giving a more viscous character, which makes the liquid velvetier. In addition, lambda carrageenan stabilizes the cocoa particles in milk and increases (enhances) its flavor, making the drink more enjoyable. Lambda carrageenan (0.2–0.3%) can improve dairy drinks with coffee by preventing fat separation. Some small packages of ultra heat treated (UHT) milk have kappa carrageenan in order to avoid separation of the protein fraction. Lambda or kappa carrageenan can be added to natural dairy creams (milkshakes) to help lower the caloric value by incorporating air when these are stirred [32].
The research and production of new products with high added value is a constant challenge for carrageenan producing companies. A typical example of the exploration of niches and/or new markets in the food sector emerged in the 90s of the last century when alternatives were sought to replace gelatin of animal origin, due to the spread of mad cow disease [8, 33]. Although carrageenan cannot replace gelatin in all applications, the iota variant makes an excellent substitute for gelatin in confectionery (see Table 3).
| Use | Phycocolloid | Function |
|---|---|---|
| Water-based gelled desserts | Kappa + iotaKappa + iota + LBG | Jellify |
| Jellies | Kappa + iota | Jellify |
| Flans | Kappa, kappa + iota | Jellify and improve mouthfeel |
| Cold-prepared puddings | Kappa, iota, lambda | Thicken, jellify |
| Chocolate milk | Kappa, lambda | Keep the cocoa in suspension |
| Condensed milk | Iota, lambda | Emulsify |
| Dairy creams | Kappa, iota | Stabilize and emulsify |
| Dairy ice cream | Kappa + GG, LBG, X | Stabilize the emulsion and prevent the formation of ice crystals |
| Dairy shakes | Lambda | Stabilize and emulsify |
| Soy milk | Kappa + iota | Keep the components in suspension and improve the flavor |
| Juices | AgarKappa, lambda | Increase viscosity, emulsify |
| Cheeses | Kappa | Give texture |
| Canned and processed meats | Kappa | Retain liquids inside the meat and give texture |
| Salad dressings | Iota | Stabilize the suspension |
| Sauces and condiments | AgarKappa | Give body |
| Fillings for pies and cakes | Kappa | Give body and texture |
| Pie jellies | Kappa | Jellify |
| Gummies and candies | AgarIota | Gelling and texture |
| Frozen fish | Alginate | Moisture adhesion and retention |
| Wine | Kappa | Promotes flocculation and sedimentation of suspended solid particles |
| Beer | Kappa | To clarify and produce foam |
| Gums | Iota | Gelling and texture |
| Oven-baked products | Agar, kappa, iota, lambda | Improve quality and control moisture |
LBG: locust bean gum (E410); GG: Guar gum (E412); X: xanthan (E415)
As previously mentioned, with the appearance of bovine spongiform encephalopathy (BSE; or mad cow disease), efforts were made to find substitutes for gelatin of animal origin [33]. Animal-based gelatins were very successful in the dessert industry, mainly due to the smooth texture of the gels they form and the ease in releasing flavors and aromas, due to their characteristic structural and melting temperature (lower than human body temperature, between 25°C and 35°C, which ends up being a very pleasant sensation in the mouth itself), these interesting thermo-reversible gels. Jellies based on iota carrageenan have, compared to the former, the disadvantage of having a higher melting temperature and, therefore, are not as pleasant when ingested as they do not melt in the oral cavity. However, they have the advantage of not melting on hot days and also of not needing a refrigerator to gel—a very advantageous feature in tropical countries. Furthermore, iota carrageenan gelatins (Figure 9) do not harden over time (which, on balance, maximizes food shelf life and minimizes storage costs) [34].
In the last decade, food ingredient companies have developed hydrocolloid blends that mimic the properties of animal-based gelatin. Pressured by market pressures and the need for alternative solutions, they discovered that by combining various types of carrageenan with carob flour and starch, they managed to obtain a wide variety of gelatins/jellies with different melting temperatures and different textures. Mousses and long-lasting desserts based on carrageenan and pectin are now valid options for certain ethnic groups and/or vegetarians, as they do not contain animal-based gelatin—which has created a new market niche with added value [19].
Recently, commercial exploitation has extended to the market for low-calorie, or light, products—conventional fruit jellies, based on pectin and high sugar content, are transformed into light jellies when pectin is replaced by mixtures of kappa and iota carrageenan. A combination of carrageenan and xanthan is also used to impart texture and consistency to light mayonnaise. Seasonings and salad dressings, whether low or fat-free, contain kappa or iota carrageenan to keep aromatic herbs in suspension and enhance flavor [35].
Drinks simulating fruit juices can be reconstituted by dissolving and/or suspending their constituents reduced to powder in cold water, but if lambda carrageenan is added to that preparation, it ends up giving body to the drink (increase in viscosity) and simultaneously potentiates and enlivens their flavor [19]. Ice creams in which mixtures of kappa and iota carrageenan are used, in addition to carob flour or pectin, have interesting and smooth textures, constituting a creamy and fat-free alternative to dairy-based ice creams [19].
In the preparation of hams, the addition of carrageenan to the brine improves the quality of the product, as the carrageenan absorbs water and interacts with soluble proteins, promoting its retention and preventing its leaching from the meat mass. In recent years there has been an increase in demand for pre-cooked poultry products, such as chicken and turkey. So that this type of product does not lose weight and maintains a pleasant texture, it is usual to “inject” a brine with salt, phosphate, and carrageenan into the muscle mass of the chickens [36].
The use of hydrocolloids in obtaining food products has been tested as a fat substitute. When fat and salt are reduced, the meat becomes less tender, less juicy, and, consequently, loses flavor. To avoid this, phosphates and carrageenan are added in order to restructure the original appearance and flavor, that is, its organoleptic characteristics (those that impress the senses). Kappa carrageenan has been used, with some success, to replace half of the normal fat present in “Frankfurt” sausages. Mixing iota carrageenan with minced beef makes it possible to obtain a low-calorie hamburger, as is the case of the “MacLean” sold by a well-known chain of fast-food restaurants [37]. The applications of carrageenan in precooked meat-based foods are another area with great potential for expansion [18, 19].
In recent years, a constant outbreak of some emerging or remitting viral diseases has caused serious harm to human health. During the last decade, the number of antiviral products approved for clinical use has increased from 5 to over 30 [20, 38].
The potential antiviral activity of seaweed polysaccharides was first shown by Gerber et al. [39], describing that the polysaccharides extracted from Gelidium robustum [formerly called Gelidium cartilagineum variety (var.) robustum; Rhodophyta] protected chicken embryos against the influenza B virus or the mumps virus. Many seaweed species contain significant amounts of complex structural sulfated polysaccharides that have been shown to inhibit the replication of enveloped viruses, including members of the flavivirus, togavirus, arenavirus, rhabdovirus, orthopoxvirus, and herpesvirus families. Polysaccharides extracted from Rhodophyta have been shown to exhibit antiviral activity against a broad spectrum of viruses, including important human pathogens such as human immunodeficiency virus (HIV), herpes simplex virus (HSV), vesicular stomatitis virus (VSV) and Cytomegalovirus (CMV). The chemical structure, including the degree of sulfation, molecular weight, constituent sugars, conformation, and dynamic stereochemistry are responsible for the potential antiviral activity of sulfated polysaccharides extracted from algae. Furthermore, both the degree of sulfation and the distribution of sulfate groups in polysaccharides play an important role in the antiviral activity of these sulfated polysaccharides. Algal polysaccharides with low degrees of sulphation are generally little active or even completely inactive against viruses [20, 38].
Carrageenans are selective inhibitors of several viruses with and without a viral capsule, acting predominantly by inhibiting the binding or internalization of the virus into host cells. Carrageenans are an exceptionally potent inhibitor of human papilloma virus (HPV) in vitro, inhibiting the early stage of infection. Notably, they are also extremely effective against a variety of sexually transmitted HPV types that promote cervical cancer and genital warts. Several in vitro studies suggest that carrageenan may also have antiviral properties, inhibiting the replication of herpes and hepatitis A viruses, genital human papilloma (HPVs) and there are indications that carrageenan-based sexual lubricant gel may offer protection against transmission of HPV [9, 18].
A nasal spray containing only carrageenan (iota) provides an easy-to-apply treatment for upper respiratory tract infections in patients with suspected influenza A (H1N1) infection. Patients tested with this spray had a quick and efficient cure for the influenza virus. The composition of this spray, based on iota carrageenan, can reduce the severity and/or duration of the flu. Furthermore, due to the broad antiviral efficacy of carrageenan, patients receive treatment against concomitant viral infections in parallel. The pharmaceutical company “Boehringer Ingelheim” sells this nasal spray under the trademark Bisolviral® (Carragelose) [9, 18, 38].
The Bisolviral® spray reduces cold viruses (rhinoviruses) by more than 90% and should be used to relieve the initial phase of colds, the nose being the main entry point for viruses. Thus, Bisolviral® forms a soft protective layer, which helps to prevent the cold virus from multiplying and spreading. It can also be used to help moisturize the nasal passages [38]. New in vitro data show the virus-neutralizing effect of Carragelose® on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [40].
This review highlighted the historical consumption of diverse marine macroalgae species and their applications as food, extracts (phycocolloids), and food additives in the North Atlantic area. Concerns regarding the establishment of seaweed products on the market, such as production constraints (such as large-scale production technology limitations and sustainable cultivation challenges), and consumer perceptions (acceptance and knowledge) are issues that must be addressed in the future, as well as the legislative process followed to submit algae to the EU Novel Foods Catalogue. Current legislation is not broad enough for the seaweed sector, with specific regulations within each country and several species being produced, eaten, and traded beyond the approved catalogue. Consequently, an update of the authorized species list is urgently needed. The seaweed market has strong motivations and enormous potential, due to the nutritional and health benefits of seaweed, the likelihood of sustainable production, and most importantly, the need to meet the increasing demand for food from the growing population.
A. nodosum: Ascophyllum nodosum
C. crispus: Chondrus crispus
EU: European Union
F. serratus: Fucus serratus
HPV: human papilloma virus
P. palmata: Palmaria palmata
S. latissima: Saccharina latissima
U. pinnatifida: Undaria pinnatifida
LP: Conceptualization, Investigation, Writing—original draft, Writing—review & editing.
The author declares that he has no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Leonel Pereira is grateful for the support of the FCT—Foundation for Science and Technology, I.P., within the scope of the project of the Associate Laboratory ARNET [LA/P/0069/2020], and MARE—Marine and Environmental Sciences Centre [UIDB/04292/2020]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Micaela Antón ... Rafael Borneo
Rut Domínguez ... Manuel Domínguez
Eliseo Sánchez-Loredo ... Juan A. Ascacio-Valdés
Ayla Elmi Kashtiban ... Sayna Zahedinia
Shafa’atu Giwa Ibrahim, Roselina Karim
Brice Ulrich Foudjo Saha ... Lifoter Kenneth Navti
Olamide Akande ... Daniel Ajewole
Evans Ntim Amedor ... James Owusu-Kwarteng
Luís M.G. Castro ... Manuela Pintado
José Pinela, José Ignacio Alonso-Esteban
