Affiliation:
1Department of Health Science, University of the People, Pasadena, CA 91101, USA
ORCID: https://orcid.org/0009-0008-9424-9355
Affiliation:
1Department of Health Science, University of the People, Pasadena, CA 91101, USA
Email: elodiesepde831@gmail.com
ORCID: https://orcid.org/0009-0005-3008-3104
Affiliation:
1Department of Health Science, University of the People, Pasadena, CA 91101, USA
2Department of Biomedical Engineering, Federal University of Allied Health Sciences, Enugu, Nigeria
ORCID: https://orcid.org/0009-0005-4372-6068
Affiliation:
1Department of Health Science, University of the People, Pasadena, CA 91101, USA
3Department of Dental Technology, Federal University of Allied Health Sciences, Enugu, Nigeria
Explor Drug Sci. 2026;4:1008139 DOI: https://doi.org/10.37349/eds.2026.1008139
Received: October 05, 2025 Accepted: November 12, 2025 Published: January 07, 2026
Academic Editor: Michio Kurosu, University of Tennessee Health Science Center, USA
Background: The root cause of diabetes is dysregulated pathways, including those involving AMP-activated protein kinase (AMPK), GLUT-mediated glucose transport, and the PI3K/AKT pathway. There has been a notable increase in research on phytoconstituents as pathway-specific treatments for diabetes; however, the comprehensiveness of this evidence remains unclear.
Methods: This systematic review followed PRISMA guidelines and was registered on PROSPERO (CRD420251073083). Databases searched included PubMed, Scopus, Google Scholar, and Europe PMC for experimental studies (in vivo, in vitro, and in silico) published between 2015 and 2024. The final search was conducted in April 2025, and 2025 publications available as “early access” before this date were included. Only English-language studies were included. Animal studies (in vivo) were assessed for risk of bias using the SYRCLE tool, while in vitro studies were evaluated using the ToxRTool, based on test substance characterization, test system description, study design, and data reporting. Narrative synthesis was employed due to the heterogeneity of the data.
Results: Out of 3,222 articles, 177 articles met the inclusion criteria. Study types included in vitro (92; 52%), in vivo (66; 37.3%), in silico (15; 8.5%), and other experimental types (4; 2.3%). Phytoconstituents predominantly targeted PI3K/AKT (44.6%), GLUT transporters (19.8%), and AMPK (14.1%) pathways. Rodent models were most used (48.02%). Primary outcomes included improved insulin sensitivity, enhanced glucose homeostasis, and reduced oxidative stress and inflammation. The risk of bias analysis revealed 68.93% of the studies carried a moderate risk, 29.94% a low risk, and 1.13% a high risk.
Discussion: Phytoconstituent activity was consistent with the activation of diabetes-relevant signaling pathways, particularly PI3K/AKT, GLUT transporters, and AMPK cascades. However, most evidence was correlative, with limited loss-of-function validation. Methodological irregularities, moderate risk of bias, and limited translational research reduce the strength and generalizability of these findings.
Diabetes is a chronic disease characterized by elevated blood sugar levels [1]. The normal fasting blood sugar range is 72–108 mg/dL; 100–125 mg/dL is considered prediabetes, and a level above 126 mg/dL is classified as diabetes [2]. There exist two types of diabetes: type 1 and type 2. In type 1 diabetes, pancreatic β-cells are destroyed by CD4+ and CD8+ T cells and macrophages, leading to insulin deficiency. Islet cell antibodies are found in nearly 85% of patients, and most target glutamic acid decarboxylase (GAD) within β-cells of the pancreas. Insulin refers to a hormone produced by beta islet cells of Langerhans in the pancreas. It plays a significant role in regulating blood sugar levels by converting excess blood sugar into glycogen and enhancing glucose metabolism [3]. Type 2 diabetes is a chronic condition characterized by high blood sugar levels, also referred to as hyperglycemia [4]. It is associated with decreased physical activity and exercise, as well as increased sedentary habits, which are linked to elevated markers of chronic systemic inflammation [4]. Proinflammatory molecules, such as interleukin-6 (IL-6), C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), and IL-1, are released into the bloodstream and within specific organs in this scenario, causing metabolic inflammation [5]. The most common cause of morbidity and death for individuals with type 1 and type 2 diabetes is vascular complications, which are caused by vascular abnormalities brought on by a persistently high blood sugar level that raises oxidative stress and inflammatory reactions [5].
The most common type of diabetes is type 2 diabetes, with adults being the most affected. In the past thirty years, there has been a considerably high prevalence of type 2 diabetes in countries of all income levels in the world [6]. From 200 million people in 1990 to 830 million people worldwide have diabetes, with the majority living in low and medium-income countries, and about half of them living without any medication, with diabetes coverage being very low in these countries [7]. Given that these individuals do not take medication, they are very much susceptible to diseases such as blindness, kidney failure, heart attacks, strokes, and lower limb amputations. This situation resulted in millions of deaths in the year 2022, in addition to 11% of cardiovascular deaths caused by high blood sugar levels [6]. In the year 2019, there were over 463 million diabetic patients globally, with about 4.2 million diabetes-related deaths recorded [8]. About 537 million adults aged between 20 and 79 years worldwide suffer from diabetes. By the year 2030, it is estimated that over 643 million individuals, and by 2045, over 783 million individuals within this range are projected to be living with diabetes. In brief, while the world’s population is projected to grow by 20% from 2021 to 2045, the number of diabetic patients is expected to rise by 46% [9].
This study systematically analyzes how phytoconstituents target specific molecular pathways in experimental diabetes models to inform therapeutic management strategies, and explores current trends and emerging perspectives for their clinical translation. In line with this, the primary aim is to systematically analyze and synthesize evidence on how phytoconstituents target specific molecular pathways in experimental diabetes models, evaluate their potential for informing targeted therapeutic management strategies, and identify current trends and future perspectives for clinical translation through qualitative synthesis of in vivo, in vitro, and in silico studies published between 2015 and 2024, including 2025 studies available as “early access” before the final search date in April 2025.
To achieve this aim, the study sets out several specific objectives. First, it seeks to identify and categorize the molecular pathways [including AMP-activated protein kinase (AMPK) activation, glucose transporter 4 (GLUT4) translocation, phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) signaling, and enzymatic inhibition] targeted by phytoconstituents in experimental diabetes models. Following this, it will analyze the therapeutic mechanisms through which phytoconstituents modulate glucose metabolism and insulin signaling pathways across different experimental approaches. Additionally, it will assess the translational potential of phytoconstituent-based interventions, moving from experimental models to clinical therapeutic management strategies. Along with highlighting information gaps that guide future research directions, the study also aims to identify current trends in phytoconstituent-diabetes research. Ultimately, it will assess the potential for clinical translation and provide evidence-based recommendations for translating phytoconstituent treatments from the laboratory to the patient’s bedside.
Nutraceuticals and phytomedicines offer a low incidence of adverse effects that can be a fantastic alternative to regular drugs in combating diabetes and its related complications. Diabetes mellitus is a metabolic disorder characterized by abnormal glucose metabolism, accompanied by distinct long-term complications. The complications that are specific to diabetes include retinopathy, nephropathy, and neuropathy. Patients with all forms of diabetes of sufficient duration, including insulin-dependent diabetes mellitus (IDDM) and non-IDDM (NIDDM), are vulnerable to these complications, which cause severe morbidity. Retinopathy occurs in all forms of diabetes. Several high-quality studies, including the population-based Wisconsin Epidemiologic Study of Diabetic Retinopathy, have defined the natural history of retinopathy in IDDM and NIDDM using stereoscopic fundus photography. Nephropathy is the diabetes-specific complication associated with the most significant mortality. Diabetes remains a major risk factor for coronary artery disease. Dupuytren’s contractures and periarticular thickening of the skin leading to decreased mobility of the fingers are also more common in patients with diabetes [10].
Diabetes, if diagnosed at its early stage, can empower individuals and healthcare providers to initiate timely interventions, which would help prevent complications and improve the overall quality of life. Timely interventions, regular screening, and symptom awareness collectively can lead to better management and an enhanced quality of life [11]. Studies have shown that patients with diabetes tend to have higher all-cause mortality and morbidity due to cardiovascular disease, cancer, chronic lower respiratory diseases, cerebrovascular disease, influenza and pneumonia, and kidney disease [12].
This review’s strengths include its multi-database strategy (PubMed, Scopus, Google Scholar, and Europe PMC) and its focus on temporal publication trends (2015–2025).
A systematic search was carried out across four databases (PubMed, Scopus, Google Scholar, and Europe PMC) using these six keyword combinations:
Phytoconstituents AND Diabetes Mellitus AND Molecular Mechanisms
Plant-derived Compounds AND Antidiabetic Activity AND Signal Transduction
Herbal Medicine AND Diabetes Management AND Cellular Pathways
Natural Products AND Insulin Resistance AND Gene Expression
Botanical Extracts AND Glucose Metabolism AND Therapeutic Targets
Phytochemicals AND Diabetes Therapy AND Inflammatory Pathways
The search results are broken down as follows: PubMed (893 identified, 871 after duplicates removal), Scopus (329 identified, 325 after duplicates removal), Google Scholar (1,000 identified, 798 after duplicates removal), and Europe PMC (1,000 identified, 821 after duplicates removal). The overall workflow [13] of study identification, screening, eligibility, and inclusion is summarized in Figure 1. All studies summarized in Table S1 are collectively cited here for numerical continuity [14–190].
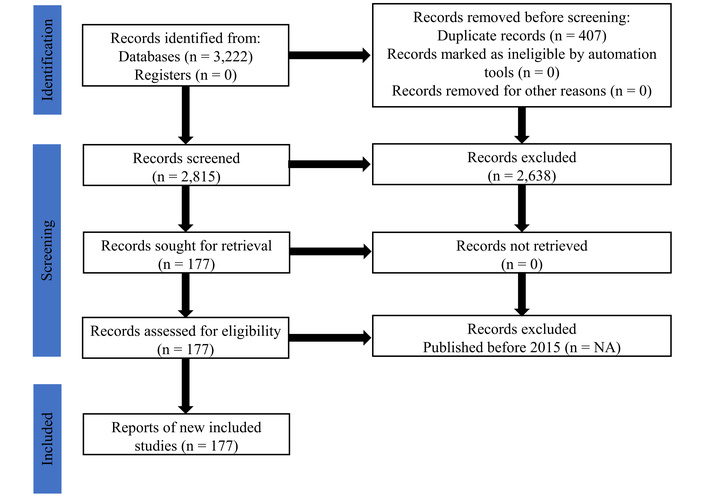
PRISMA flow diagram showing the selection of studies. Adapted from [13]. © 2019 The Authors. Licensed under a CC BY 4.0.
The inclusion criteria for this review were original experimental research publications published between 2015 and 2025 that addressed molecular pathways associated with diabetes, including glycemic control pathways (AMPK activation, GLUT4 translocation, PI3K/AKT signaling, and inhibition of α-amylase or α-glucosidase). Models of insulin resistance, hyperglycemia, or diabetes mellitus, whether in vitro, in vivo, or in silico, were included. Studies examining phytoconstituents (bioactive compounds derived from plants) or herbal extracts with potential antidiabetic benefits were considered. Articles published in English that are entirely accessible.
The exclusion criteria for this review included reviews, meta-analyses, editorials, commentaries, and conference abstracts. Articles that were not written in English, those published before 2015, and those without full-text accessibility were also excluded. Studies that did not address diabetes mellitus, hyperglycemia, or insulin resistance, or did not contain phytoconstituents or bioactive compounds derived from plants, were excluded. Duplicate or retracted publications, as well as those that did not investigate particular molecular processes like PI3K/AKT, AMPK, GLUT4, or enzymatic inhibitory pathways, were also excluded.
Studies that used experimental models explicitly created to research diabetes were included in this review. In vivo models that met the criteria were genetically diabetic db/db mice, mice induced by a high-fat diet (HFD), and rats induced by streptozotocin (STZ). For in vitro research, only papers that modeled diabetes circumstances utilizing insulin-resistant adipocytes, hepatic cells, or pancreatic β-cells were included. In silico research was considered if it targeted diabetes-related proteins, such as protein tyrosine phosphatase 1B (PTP1B) or peroxisome proliferator-activated receptor gamma (PPARγ), using molecular docking or simulations. Only English-language research papers published after 2015 were considered for this analysis. Articles were excluded due to the following factors: those published before 2015, inaccessible because of paywalls or lack of author response, involving non-diabetic models or unrelated conditions, or review articles or editorials. All retrieved full-text articles were independently screened by two reviewers, and any disagreements were resolved through discussion to ensure uniform application of the eligibility requirements.
Data extraction was guided by predefined variables, including plant source, study type, diabetes model, molecular target, and key outcomes (Table 1). Across the 177 included studies, 92 (52%) were in vitro, 66 (37.3%) in vivo, 15 (8.5%) in silico, and 4 (2.3%) others [hybrid: human trial, DIA proteomics, high-content screening (HCS)]. The majority of the investigated phytoconstituents were derived from medicinal plants traditionally associated with antidiabetic activity, notably polyphenols, alkaloids, and flavonoids, which together accounted for over half of all reported compounds. This distribution indicates a prevailing emphasis on in vivo validation and molecular mechanisms involving antioxidant and insulin-sensitizing pathways.
We extracted biomarker data associated with each pathway to contextualize mechanistic evidence (e.g., IRS-1, GLUT4 for PI3K/AKT; ACC phosphorylation for AMPK).
| Variable | Description | Source example (Entry #) |
|---|---|---|
| Plant/Phytoconstituent | Ficus deltoidea, Curcumin | #1, #101 |
| Study type | In vitro, in vivo, in silico, or combined | #6 (in vitro), #54 (in vivo) |
| Diabetes model | STZ rats, HFD mice, computational targets | #1 (STZ-NA rats), #2 (HFD) |
| Molecular target | PI3K/AKT, PTP1B, PPARγ, α-glucosidase | #12 (IRS-1/AKT), #102 (α-amylase) |
| Key outcomes | ↓ Glucose, ↑ insulin sensitivity, and ↓ inflammation | #3 (↓ glucose), #46 (↑ insulin sensitivity) |
ACC: acetyl-CoA carboxylase; AMPK: AMP-activated protein kinase; GLUT4: glucose transporter 4; HFD: high-fat diet; IRS-1: insulin receptor substrate 1; PI3K/AKT: phosphoinositide 3-kinase/protein kinase B; PPARγ: peroxisome proliferator-activated receptor gamma; PTP1B: protein tyrosine phosphatase 1B; STZ: streptozotocin.
A thematic synthesis approach was used to categorize extracted data by pathway and model type, as shown in Table 1. Data synthesis was conducted qualitatively using a thematic framework approach. The extracted data were first coded into key themes, including pathway targeted, study model, biomarker outcomes, and therapeutic effects. These themes were then compared across studies to identify recurring mechanistic patterns, convergence of therapeutic outcomes, and cross-validation between in vitro, in vivo, and in silico designs. This process enabled an integrative narrative synthesis, which was chosen over meta-analysis due to the methodological and outcome heterogeneity across included studies.
A total of 177 studies were incorporated into the qualitative synthesis.
SYRCLE’s risk of bias (ROB) tool for in vivo investigations is used for quality assessment of in vivo investigations. In vitro investigations were assessed using the ToxRTool, which evaluates study quality based on test substance characterization, test system description, study design, and data reporting.
The combined results were arranged according to the molecular pathway (e.g., PI3K/AKT: 44.6%, GLUTs: 19.8%, AMPK: 14.1%, others: 21.5%).
Four reviewers participated in this systematic review, each with a clearly defined role throughout the process. The study selection process was conducted collaboratively by two reviewers (Reviewers A and B) who jointly performed the systematic search across all four databases (each person working on two databases), downloaded articles, removed duplicates, and conducted title and abstract screening. All screening decisions during this phase were made by consensus among these two reviewers to ensure consistent application of inclusion and exclusion criteria. Following the completion of study selection, data extraction was performed independently by the remaining two reviewers (Reviewers C and D) using the standardized extraction form to ensure consistency in captured variables. Reviewer C extracted data from 78 studies while Reviewer D extracted data from 99 studies, totaling the 177 included studies. Each reviewer was responsible for removing all relevant variables from their assigned studies, including plant/phytoconstituent information, study type, diabetes model used, molecular targets, and key outcomes.
To ensure methodological rigor and compliance with the PRISMA 2020 guidelines, this review was prospectively registered in PROSPERO (CRD420251073083) before the screening process began. The search strategy, inclusion and exclusion criteria, and data extraction framework were defined before registration, while data analysis and synthesis were conducted afterward. The studies were carefully examined and chosen by four reviewers. To verify the reproducibility of the screening process, a sensitivity check was performed by randomly selecting 20% of the full-text articles for independent screening by two reviewers. Inter-rater reliability was then quantified using Cohen’s kappa (κ) to measure the level of agreement between reviewers. Agreement was substantial across key domains, with κ = 0.81 for study type classification, 0.78 for molecular target identification, and 0.85 for outcome classification. Disagreements were resolved by consensus through discussion between the two reviewers, and a third senior reviewer adjudicated unresolved cases. This approach ensured consistent and reliable data capture while maintaining the efficiency of the collaborative process. Table S1 contains the complete search strategy for replication, and a tabular summary of exclusion grounds is provided for transparency. Furthermore, 85% of the included studies used mammalian models, enhancing the clinical relevance and translational validity of the findings. Consistent dosage reporting (in mg/kg) across in vivo studies enabled insightful comparisons across different experimental setups.
For data management and reference handling, we used Publish or Perish, Microsoft Excel, and EndNote. Publish or Perish was used for bibliometric retrieval and citation analysis; EndNote for structured referencing and citation management; and Excel for organizing extracted variables, tabulating study characteristics, and generating descriptive statistics.
The studies reviewed targeted key molecular mechanisms involved in diabetes pathogenesis collectively; the most molecular pathways and targets are PI3K/AKT signaling (44.6%) of the studies, GLUTs (19.8%), AMPK activation (14.1%), and other pathways (α-glucosidase inhibition, PPAR modulation, antioxidant/ROS regulation, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), dipeptidyl peptidase (DPP)-4 inhibition, insulin receptor modulation) were reported across 38 studies, which cover about 21.5% of the studies.
Table 2 presents representative biomarkers modulated within each pathway. PI3K/AKT had the highest count (79 studies), followed by GLUT transporters (35 studies) and AMPK (25 studies). Biomarker-level findings highlight mechanistic plausibility, including insulin receptor substrate 1 (IRS-1) and GLUT4 for PI3K/AKT, acetyl-CoA carboxylase/PPARγ coactivator 1-alpha (ACC/PGC-1α) for AMPK, and GLUT2/GLUT4/SGLT2 for GLUT transporters.
Molecular pathways and representative biomarkers.
| Molecular pathway | Key biomarkers/targets (examples) | Study count (n) |
|---|---|---|
| PI3K/AKT | IRS-1, AKT, p-AKT, GSK3β, IGF-1, GLUT4 | 79 |
| GLUT transporters | GLUT2, GLUT4, SGLT2 | 35 |
| AMPK | ACC, SIRT1, PGC-1α, CPT1, LKB1 | 25 |
| Other pathways | PPARγ, adiponectin, FABP4, NF-κB, TNF-α, IL-6, NLRP3, STAT3, MAPK, ER stress, oxidative stress markers, apoptosis | 38 |
ACC: acetyl-CoA carboxylase; AMPK: AMP-activated protein kinase; CPT1: carnitine palmitoyltransferase 1; ER: endoplasmic reticulum; FABP4: fatty acid-binding protein 4; GLUT: glucose transporter; GSK3β: glycogen synthase kinase 3 beta; IGF-1: insulin-like growth factor-1; IL-6: interleukin-6; IRS-1: insulin receptor substrate 1; LKB1: liver kinase B1; MAPK: mitogen-activated protein kinase; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; PGC-1α: peroxisome proliferator-activated receptor gamma coactivator 1-alpha; PI3K/AKT: phosphoinositide 3-kinase/protein kinase B; PPARγ: peroxisome proliferator-activated receptor gamma; SGLT2: sodium-glucose cotransporter 2; SIRT1: sirtuin 1; STAT3: signal transducer and activator of transcription 3; TNF-α: tumor necrosis factor-alpha.
The summarized molecular targets and biomarkers (Table 2) highlight the predominance of PI3K/AKT, GLUT, and AMPK signaling in phytoconstituent research on diabetes. These pathways (AMPK, PI3K/AKT, and GLUT) were prioritized because they represent critical molecular nodes in glucose homeostasis and insulin signaling. AMPK is a master regulator of cellular energy metabolism, enhancing glucose uptake and fatty acid oxidation. The PI3K/AKT pathway is the canonical insulin signaling cascade, essential for GLUT4 translocation and pancreatic β-cell survival. GLUT transporters, particularly GLUT2 and GLUT4, directly mediate cellular glucose uptake. Dysregulation of these three mechanisms is central to the pathophysiology of diabetes, making them highly relevant therapeutic targets. Their combined modulation offers strong mechanistic plausibility for phytoconstituents as multi-target antidiabetic agents.
The overall distribution of ROB ratings (low, moderate, high) is presented in Table 3 and visualized in Figure 2.
Row labels and ROB.
| Row labels | Count of ROB |
|---|---|
| High | 1.13% |
| Low | 29.94% |
| Moderate | 68.93% |
| Grand total | 100.00% |
ROB: risk of bias.
Figure 2 presents the distribution of ROB ratings (low, moderate, high), showing that 68.93% of the studies carried a moderate risk, 29.94% a low risk, and 1.13% a high risk.
The studies that met the inclusion criteria were analyzed based on available experimental and computational evidence, excluding meta-analytic synthesis. A formal bias risk scoring was performed for the qualitative synthesis; however, the quality of the study and its consistency/replicability will be addressed in the Discussion.
From this research, we observed a progressive increase in publications on plant-based therapies for diabetes over 11 years. The statistics are as follows: 2015–2019 (47 studies), 2020–2022 (53 studies), 2023–2025 (77 studies). However, 2024 had the highest number of publications (40 studies), indicating a recent increase in interest in plant-based therapies for diabetes (Table 4).
Trends in publication year.
| Year | Count of publications |
|---|---|
| 2015 | 6 |
| 2016 | 9 |
| 2017 | 11 |
| 2018 | 13 |
| 2019 | 8 |
| 2020 | 18 |
| 2021 | 19 |
| 2022 | 16 |
| 2023 | 17 |
| 2024 | 40 |
| 2025 | 20 |
| Total | 177 |
Articles published from 2015 to 2025 were used for the research, of which a total of 177 articles met the inclusion criteria for the systematic review. These articles were chosen for the systematic review using systematic screening procedures during the study identification and selection process. The included studies have different types of research designs and approaches (experimental methodology), the study types include in vivo found in about 66 studies covering 37.3% of the studies, in vitro (n = 92, 52%), in silico (n = 15, 8.5%), and other hybrid methods, which include human trial, computational, DIA proteomics, HCS (n = 4, 2.3%). Due to rounding, the sum of various percentages may not equal 100% (Table 5). These studies have their origins in multiple research groups that use various models and analytical techniques to investigate and analyze the effects of phytoconstituents on diabetes (Figure 1). Table S1 lists the studies’ authors, year, title, plant/phytochemical, study type, diabetes model used, molecular target pathway, main findings, outcome, notes, diabetes model category, ROB, and tools used.
Study type distribution.
| Study type | Number of studies (n) | Percentage of studies (%) |
|---|---|---|
| In vivo | 66 | 37.3% |
| In vitro | 92 | 52.0% |
| In silico | 15 | 8.5% |
| Other hybrid methods: human trial, computational, DIA proteomics, HCS | 4 | 2.3% |
| Total | 177 | 100.1% |
HCS: high-content screening. Due to rounding, the sum of various percentages may not equal 100%.
Cross-tabulation suggests that, focusing on the relationship between study type and molecular pathway, the PI3K/AKT and AMPK pathways were most investigated using combined in vitro and in vivo designs. In contrast, modulation of GLUT activity was more evenly distributed across in vitro and in vivo studies. Additionally, enzymatic inhibition (e.g., α-glucosidase, DPP-4) was predominantly explored in vitro or in silico. In contrast, computational models were primarily used for molecular docking, absorption, distribution, metabolism, excretion, and toxicity (ADMET) profiling, and virtual screening of phytoconstituents. This relationship complements the molecular evidence summarized in Table 2, confirming that hybrid models primarily investigated PI3K/AKT and AMPK mechanisms.
Various study models were used in the studies to investigate the phytoconstituents activity, the study models used includes; rodent models only (n = 85, 48.02%), cell line models only (n = 26, 14.69%), combined rodent and cell line models (n = 28, 15.82%), in silico computational models (n = 14, 7.91%), and others (e.g., zebrafish, organ-specific ex vivo system) (n = 23, 13.00%) (Table 6). This distribution demonstrates a firm reliance on whole-animal testing, which is supported by in vitro and in silico mechanistic studies.
Diabetes model category.
| Diabetes model | Count of diabetes model | Percentage (%) |
|---|---|---|
| Cell line models | 26 | 14.69% |
| Cell line models and rodent models | 28 | 15.82% |
| Human models | 1 | 0.56% |
| In silico models | 14 | 7.91% |
| Rodent models | 85 | 48.02% |
| Others | 23 | 13.00% |
| Total | 177 | 100.00% |
From the articles reviewed, therapeutic outcomes were observed. The outcomes shown were heterogeneous. The most frequently observed therapeutic effects involve reduced glucose levels (n = 49), anti-inflammatory effects (n = 20), antioxidant activity (n = 10), improved insulin sensitivity (n = 7), improved lipid profile (n = 5), and other outcomes (renal function, pancreatic protection, cognitive improvements, hormonal regulation, etc.) were observed in 86 studies.
An integrative review of the data revealed a strong alignment between pathway targeting and therapeutic outcomes, consistent improvements in glucose homeostasis across multiple models and compounds, and a high rate of insulin signaling modulation, suggesting a potential for mechanistic synergy and an emerging preference for hybrid study designs that support deeper validation of bioactivity.
The core study findings from the studies include that the most common study design found from the studies was in vitro having found in 52% of the studies, the most targeted pathway was the PI3K/AKT found in 44.6% of the studies, the top therapeutic outcome was glucose reduction in about 27.7% of the studies, the leading study year was 2024 having a total number of 40 articles written in the year. The most used model type was the rodent model (e.g., rats, mice), accounting for 48.02% of the studies.
This systematic review qualitatively synthesizes data from 177 experimental trials (see Tables 1–3 and Figures 1 and 2) to evaluate the therapeutic potential and mechanistic basis of phytoconstituents in the management of diabetes. Preclinical research regularly and effectively targets a core group of dysregulated pathways, with the AMPK, PI3K/AKT, and GLUT signaling networks being the most frequently targeted. According to Taniguchi et al. [191] and Vargas et al. [3], the PI3K/AKT pathway is the canonical pathway for insulin-mediated glucose uptake and β-cell survival. At the same time, AMPK serves as a crucial master regulator of cellular energy homeostasis and a key sensor for insulin-sensitizing agents. The prevalence of these pathways is highly consistent with the known pathophysiology of diabetes. The noteworthy modification of these pathways by various phytoconstituents, such as beta-sitosterol, luteolin, and curcumin, highlights their potential as a rich source for targeted antidiabetic drug discovery [37, 180]. However, these mechanistic interpretations remain largely correlative; most included studies demonstrated pathway modulation through marker expression rather than direct causal validation using inhibitors or knockout models.
According to our review, one of the main advantages of the existing body of data is the move toward integrative, multi-model validation. A more comprehensive and physiologically plausible validation of bioactivity is provided by hybrid study designs, such as combining in vitro and in vivo techniques, rather than single-model investigations. The extensive use of insulin-resistant cell lines to discover basic processes, which are then confirmed in HFD/STZ rodent models, is one example of how cellular efficacy and whole-organism physiology can be effectively linked. In silico studies are also included (7.91% of included research), reflecting a modern approach to drug discovery. These computational methods enable the prediction of ADMET characteristics [192], the determination of binding affinities to diabetes targets (e.g., PTP1B, PPARγ), and the ranking of lead compounds for costly and time-consuming experimental work.
The reported therapeutic effects, which primarily include enhanced insulin sensitivity, improved glucose homeostasis, and reduced oxidative stress and inflammation, show patterns that are mechanistically consistent with the targeted pathways, although definitive causal validation is still limited. The observed antioxidant and anti-inflammatory benefits are particularly relevant, as oxidative stress and chronic low-grade inflammation have been shown to contribute to the pathogenesis of insulin resistance and diabetic complications [193]. This implies that one of the main advantages of phytoconstituents is their polypharmacological activity. Ficus deltoidea and Syzygium cumini are two examples of complex botanical extracts that can simultaneously modulate multiple pathological nodes, including insulin signaling, inflammation, and oxidative stress. This enables a comprehensive therapeutic profile that is well-suited to the multifaceted nature of diabetes [14, 136]. This contrasts with many synthetic medications that target only one specific site. However, this very complexity poses significant challenges for standardization, regulatory approval, and the precise identification of active principles.
Despite this promising preclinical outcome, our study reveals a significant translational gap. 78.53% of studies focus on rodent and cell-based models, which is not matched by a matching body of clinical evidence. The discrepancy between the bench and the bedside can be attributed to several key factors identified by our analysis. The first notable variation is in methodology. Directly comparing studies and extrapolating to human dose is extremely difficult due to the inconsistent phytoconstituent extraction methods, extract standardization, dosages, and treatment durations. Second, our risk assessment revealed that a significant majority of studies (≈ 69%) had a moderate ROB. Common issues were a lack of knowledge regarding randomization, allocation concealment, and blinding methods in in vivo experiments, which could inflate reported efficacy. Third, despite its potential, mechanistic evidence sometimes relies on correlational data rather than causal data. For example, without loss-of-function experiments (e.g., using pathway-specific inhibitors), improved glucose homeostasis and increased p-AKT expression are suggestive but do not prove causation.
Despite using a comprehensive search approach and adhering to PRISMA principles, this review has limitations. The restriction on English-language publications may have led to language prejudice. The process and scope of article inclusion are visually summarized in Figure 1 to ensure transparency. Narrative synthesis is inherently more susceptible to interpretive bias than meta-analysis, due to significant variation in experimental paradigms, outcomes, and substances. Furthermore, although we assessed the ROB in the included studies, selection bias may still be present because, due to resource constraints, our own screening and data extraction process did not employ full, dual-independent screening at every stage, despite being conducted with cross-checking and consensus.
It is necessary to close the indicated translational gap to utilize phytoconstituents for the treatment of diabetes. Future research should concentrate on:
The implementation of proven protocols for the extraction, characterization, and standardization of plant extracts is necessary to ensure repeatability and precise dosing. Finding isolated active principles or producing standardized extracts (with known flag molecules) is a significant scientific and regulatory conundrum.
Mechanistic rigour: Examining causal linkages using specific pharmacological inhibitors or genetic knockout models to confirm the involvement of proposed pathways, going beyond correlational observations.
Clinical translation is the process of developing closely watched early-stage clinical studies that verify pre-clinical findings in humans using mechanistic biomarkers (e.g., assessing pathway activation in patient samples). The successes and failures of earlier clinical studies of better-known diabetic herbs should serve as guidance for these investigations.
Solutions for bioavailability: New delivery strategies (such as nanoparticles and phospholipid complexes) are being researched in an effort to solve the limited bioavailability that plagues many otherwise promising phytoconstituents.
Integrated methods: In vitro and in vivo models are being utilized in conjunction with in silico predictions to efficiently find and evaluate the most promising lead drugs with good ADMET profiles.
This comprehensive review, which focuses on AMPK, PI3K/AKT, and GLUT as the primary mechanisms of action, concludes by combining compelling preclinical evidence demonstrating that phytoconstituents significantly modify key pathways associated with diabetes. Their pleiotropic effects align with the complex nature of diabetes. However, the transition from promising pre-clinical data to clinical application is hampered by methodological errors, bioavailability issues, and a lack of human studies. Addressing these problems through systematic, rigorous, and translational research is necessary to fully realize the medicinal potential of the plant kingdom in the global fight against diabetes.
In conclusion, this systematic review integrates a wealth of pre-clinical evidence demonstrating that phytoconstituents effectively ameliorate diabetes symptoms by targeted modification of key biochemical pathways, including PI3K/AKT, GLUT, and AMPK signaling. Mechanistically coherent and consistently documented are improvements in insulin sensitivity and glucose homeostasis, and reductions in oxidative stress and inflammation across various experimental paradigms. Unfortunately, the translation of this high pre-clinical promise into clinical practice is significantly limited by a severe shortage of human trials, significant methodological heterogeneity, and a modest ROB in current investigations. We are now at a pivotal moment in the field. Future studies should focus on standardizing phytoconstituent extraction and characterization, developing mechanistic evidence, and conducting meticulously organized clinical trials to validate these preclinical mechanisms in human subjects. These problems can be addressed by carefully evaluating the substantial therapeutic potential of plant-derived chemicals and applying them to develop novel, multi-targeted strategies for the worldwide management of diabetes mellitus. This conclusion is based on integrated results across experimental models and pathways (Tables 1–3, Figures 1 and 2).
ADMET: absorption, distribution, metabolism, excretion, and toxicity
AMPK: AMP-activated protein kinase
DPP: dipeptidyl peptidase
GLUT4: glucose transporter 4
HCS: high-content screening
HFD: high-fat diet
IDDM: insulin-dependent diabetes mellitus
IRS-1: insulin receptor substrate 1
NIDDM: non-insulin-dependent diabetes mellitus
PI3K/AKT: phosphoinositide 3-kinase/protein kinase B
PPARγ: peroxisome proliferator-activated receptor gamma
PTP1B: protein tyrosine phosphatase 1B
ROB: risk of bias
STZ: streptozotocin
The supplementary table for this article is available at: https://www.explorationpub.com/uploads/Article/file/1008139_sup_1.xlsx.
This review benefited from insightful feedback provided by Dr. Kusum Dua, Prof. Asma Wasim, and Timothy G. Leaks (MSc), at the Department of Health Science at the University of the People, whose critical comments helped improve the clarity and depth of our analysis.
CPI: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing. ESMT: Conceptualization, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing. EFE: Conceptualization, Investigation, Validation, Resources, Software, Writing—original draft. MRI: Conceptualization, Investigation, Resources, Software, Validation, Writing—original draft. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
The primary data for this review were sourced online from databases listed in the methods. Referenced articles are accessible on PubMed, Scopus, Google Scholar, and Europe PMC. Additional supporting data are available from the corresponding author upon request.
Not applicable.
© The Author(s) 2026.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2026. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1171
Download: 41
Times Cited: 0
