Affiliation:
1Department of Pathophysiology and Transplantation, Università degli Studi Di Milano, 20122 Milan, Italy
†These authors contributed equally to this article and share the first authorship.
ORCID: https://orcid.org/0009-0000-8824-3717
Affiliation:
1Department of Pathophysiology and Transplantation, Università degli Studi Di Milano, 20122 Milan, Italy
†These authors contributed equally to this article and share the first authorship.
Email: alessandra.chiei@unimi.it
ORCID: https://orcid.org/0000-0001-8028-5453
Affiliation:
2Dermatology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 20122 Milan, Italy
ORCID: https://orcid.org/0000-0002-7188-7310
Affiliation:
1Department of Pathophysiology and Transplantation, Università degli Studi Di Milano, 20122 Milan, Italy
2Dermatology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 20122 Milan, Italy
ORCID: https://orcid.org/0000-0001-7690-7221
Affiliation:
1Department of Pathophysiology and Transplantation, Università degli Studi Di Milano, 20122 Milan, Italy
2Dermatology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 20122 Milan, Italy
ORCID: https://orcid.org/0000-0002-8160-4169
Affiliation:
2Dermatology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 20122 Milan, Italy
ORCID: https://orcid.org/0000-0002-6734-1366
Explor Asthma Allergy. 2025;3:100999 DOI: https://doi.org/10.37349/eaa.2025.100999
Received: September 24, 2025 Accepted: October 31, 2025 Published: November 13, 2025
Academic Editor: Hae-Sim Park, Ajou University Medical Center, Korea (the Republic of)
The article belongs to the special issue The Era of Biologics in Allergy
Schnitzler syndrome is a rare acquired autoinflammatory disorder defined by a chronic urticarial rash, monoclonal IgM (or IgG) gammopathy, and systemic features including fever, arthralgia, and elevated inflammatory markers. Interleukin-1 blockade with anakinra is the current treatment of choice. We report the case of a 75-year-old woman who, after seven years of misdiagnosis as chronic spontaneous urticaria, fulfilled the Strasbourg Criteria for Schnitzler syndrome. Treatment with anakinra induced rapid clinical improvement but was complicated by the onset of recall urticaria (RU), characterized by delayed giant wheals at both current and previous injection sites. Laboratory findings suggested an inflammatory response, and the reaction was managed with corticosteroids and antihistamines, followed by colchicine, which achieved stable disease control. RU is a rare hypersensitivity phenomenon previously described with immunotherapy, heparin, NSAIDs, and other biologics, but to our knowledge, this is the first report associated with anakinra. This case broadens the spectrum of anakinra-related adverse effects and highlights the need for further investigation into the immunopathogenesis of RU.
Schnitzler syndrome is an extremely rare acquired autoinflammatory systemic syndrome. It is characterized by the presence of a chronic urticarial-like rash and immunoglobulin-M (IgM) monoclonal gammopathy (or, less commonly, IgG), along with recurrent fever, arthralgia, fatigue, lymphadenopathy, bone morphological changes, leukocytosis, and elevated inflammatory markers [1]. The exact prevalence of this syndrome remains undefined, and diagnosis is frequently delayed. The diagnosis is based on the Strasbourg Criteria, which require two obligate criteria—chronic urticarial rash and monoclonal IgM or IgG gammopathy—and at least two minor criteria. Minor criteria include recurrent fever, abnormal bone remodeling, a neutrophilic dermal infiltrate on skin biopsy, leukocytosis, and/or elevated C-reactive protein (CRP). Complications may include lymphoproliferative disorders and, in untreated cases, rare secondary amyloidosis [2]. The pathophysiology of Schnitzler syndrome is not fully understood, but research suggests it involves an abnormal autoinflammatory response driven by the activation of inflammatory cytokines, particularly interleukin-1 (IL-1), IL-6, and tumor necrosis factor-alpha (TNF-α). Accordingly, IL-1 blockade with anakinra is considered the treatment of choice. Tocilizumab, corticosteroids, colchicine, and dapsone represent second-line options [3]. This is, to our knowledge, the first case of anakinra-related recall urticaria (RU) in a patient with Schnitzler syndrome.
We present the case of a 75-year-old Caucasian woman, referred to our clinic seven years after symptom onset. The patient had previously been treated, based on the diagnostic hypothesis of chronic spontaneous urticaria, with high-dose antihistamines (four administrations/day), and subsequently with subcutaneous omalizumab 300 mg every 4 weeks for over a year, without significant improvement. Histopathological examination of lesional skin revealed acanthosis, spongiosis, papillary dermal edema, and an interstitial and perivascular inflammatory infiltrate composed of eosinophils and rare neutrophils—findings consistent with urticaria. The diagnosis of Schnitzler syndrome was subsequently established, fulfilling both obligate criteria (urticarial rash and monoclonal IgM gammopathy) and three minor criteria (recurrent fever, leukocytosis, elevated inflammatory markers). Laboratory findings at the time of diagnosis are described in Table 1. Subcutaneous anakinra (100 mg daily) was initiated, leading to rapid improvement of cutaneous and systemic symptoms. However, by the fourteenth administration of anakinra, the patient developed a delayed-onset giant wheal at the injection site and previous injection areas, arising more than 12 hours post-administration (Figure 2). Laboratory tests during the episode revealed leucocytosis, neutrophilia, elevated CRP, and an elevated erythrocyte sedimentation rate (Table 2). Anakinra was discontinued, and the patient was started on a tapering course of oral steroids and antihistamines, resulting in the resolution of pruritus but persistence of hyperpigmented macules for two weeks. The clinical pattern suggested a dual mechanism: an immediate hypersensitivity reaction (pruritic wheals) and a delayed response (persistent lesions). At the patient’s request, no further investigations were conducted. Colchicine (1.5 mg/day) was initiated, leading to sustained control of systemic manifestations.
Laboratory findings at the time of diagnosis of Schnitzler syndrome.
| Test name | Result | Normal range | Interpretation |
|---|---|---|---|
| White blood cells | 17.83 × 109/L | 3.6–10.5 × 109/L | Increased |
| Neutrophils | 12.48 × 109/L | 1.5–7.7 × 109/L | Increased |
| C-reactive protein | 12 mg/L | < 5 mg/L | Increased |
| Erythrocyte sedimentation rate | 15 mm/h | < 6 mm/h | Increased |
| Creatinine | 0.8 mg/dL | 0.50–0.95 mg/dL | Normal |
| Aspartate aminotransferase | 32 U/L | 11–34 U/L | Normal |
| Alanine aminotransferase | 23 U/L | < 33 U/L | Normal |
| Thyroid-stimulating hormone | 0.77 mU/L | 0.35–4.94 mU/L | Normal |
| Anti-thyroid peroxidase antibodies | 8 kU/L | < 34 kU/L | Negative |
| Anti-thyroglobulin antibodies | 27 kU/L | < 115 kU/L | Negative |
| Immunoglobulin E | 3 kU/L | < 100 kU/L | Normal |
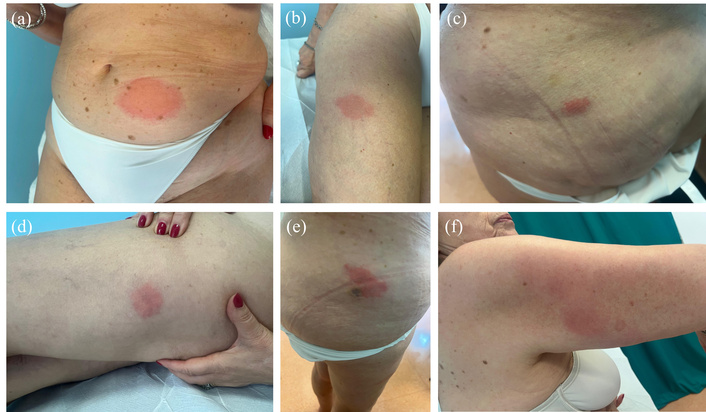
75-year-old female with Schnitzler syndrome presenting with a giant wheal at the injection site at the fourteenth administration of anakinra. (a–f) Giant wheals at the anakinra’s previous injection site.
Laboratory findings at the time of the reaction.
| Test name | Result | Normal range | Interpretation |
|---|---|---|---|
| White blood cells | 11.86 × 109/L | 3.6–10.5 × 109/L | Increased |
| Neutrophils | 8.68 × 109/L | 1.5–7.7 × 109/L | Increased |
| C-reactive protein | 9.04 mg/L | < 5 mg/L | Increased |
| Erythrocyte sedimentation rate | 13 mm/h | < 6 mm/h | Increased |
| Creatinine | 0.65 mg/dL | 0.50–0.95 mg/dL | Normal |
| Aspartate aminotransferase | 33 U/L | 11–34 U/L | Normal |
| Alanine aminotransferase | 31 U/L | < 33 U/L | Normal |
| Immunoglobulin E | 7.5 kU/L | < 100 kU/L | Normal |
At the time of diagnosis, a comprehensive laboratory assessment was performed, and the main findings consistent with Schnitzler syndrome are summarized in Table 1. Laboratory parameters obtained during the acute reaction are presented in Table 2, highlighting the changes observed compared with baseline values.
“I was relieved to finally receive a clear diagnosis after years of uncertainty and unsuccessful treatments. When the reaction to anakinra occurred, I was frightened and chose not to undergo further allergy testing, but I felt reassured by my doctors’ care and am now satisfied with the improvement achieved with the new therapy.”
Although local reactions at the injection site occur in up to 70% of patients treated with anakinra [4], the presentation in this case was consistent with RU—a rare phenomenon characterized by the reappearance of wheals at previous injection sites after re-exposure to the same drug. RU has been described with subcutaneous allergen immunotherapy, heparin, nonsteroidal anti-inflammatory drugs [5], and other biological agents. The underlying mechanisms of RU remain debated, with hypotheses including IgE-mediated pathways, T-cell involvement, and cyclooxygenase type I inhibition [4]. In our case, the latency and persistence of lesions point toward a type IV hypersensitivity reaction mediated by intraepidermal CD8+ T cells.
To our knowledge, this is the first documented case of RU triggered by anakinra in a patient with Schnitzler syndrome. This finding broadens the spectrum of anakinra-associated adverse events and highlights the importance of awareness and further research into the immunopathogenesis of RU.
CRP: C-reactive protein
IgG: immunoglobulin-G
IgM: immunoglobulin-M
IL-1: interleukin-1
RU: recall urticaria
CPR: Conceptualization, Investigation, Writing—original draft. ACG: Conceptualization, Investigation, Writing—original draft. FB: Investigation, Validation, Writing—review & editing. PC: Investigation, Validation. AVM: Validation, Writing—review & editing. SMF: Validation, Writing—review & editing. All authors read and approved the submitted version.
S. M. Ferrucci is a principal investigator in clinical trials to Amgen, Sanofi, Novartis, Lilly, Leo Pharma, AbbVie and she is an advisory board or speaker to Novartis, Menarini, Sanofi, AbbVie and Leo Pharma. A. V. Marzano is on consultancy/advisory boards and received disease-relevant honoraria from AbbVie, Boehringer Ingelheim, Novartis, Pfizer, Sanofi, and UCB. F. Barei has received honoraria from Leo Pharma and Almirall. P. Calzari has received honoraria from Almirall. The other authors declare that there is no conflict of interest.
The study complies with the Declaration of Helsinki. Ethical approval is not required for a case report study according to the local ethics committee.
Informed consent to participate in the study was obtained from the participant.
Informed consent to publication was obtained from the participant.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
This work was supported by Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico and the Italian Ministry of Health, grant number: [RC 2024]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 839
Download: 19
Times Cited: 0
Alejandra Carrón-Herrero ... Giovanni Paoletti
Francesca Losa, Arianna Cingolani
Palma Carlucci ... Danilo Di Bona
Carlo Alberto Vignoli, Riccardo G. Borroni
Karl-Christian Bergmann ... Torsten Zuberbier
Giulia Costanzo, Andrea Giovanni Ledda
Diego Bagnasco ... Fulvio Braido
Shuichiro Matsumoto ... Hisatoshi Sugiura
Alexandru Corlateanu, Cristina Toma
