Affiliation:
Clinical Department of Internal Diseases, Allergology and Clinical Immunology, Medical University of Silesia, 40-752 Katowice, Poland
Email: edytajura@interia.pl
ORCID: https://orcid.org/0000-0001-8160-1948
Affiliation:
Clinical Department of Internal Diseases, Allergology and Clinical Immunology, Medical University of Silesia, 40-752 Katowice, Poland
ORCID: https://orcid.org/0000-0001-6339-9053
Affiliation:
Clinical Department of Internal Diseases, Allergology and Clinical Immunology, Medical University of Silesia, 40-752 Katowice, Poland
ORCID: https://orcid.org/0000-0002-8537-9165
Explor Asthma Allergy. 2025;3:1009100 DOI: https://doi.org/10.37349/eaa.2025.1009100
Received: September 27, 2025 Accepted: November 13, 2025 Published: November 21, 2025
Academic Editor: Chae-Seo Rhee, Seoul National University, South Korea
The article belongs to the special issue Update on Chronic Rhinosinusitis
In recent years, biological therapy based on endotyping has become a therapeutic option for patients with type 2 inflammatory process with chronic rhinosinusitis with nasal polyps with or without bronchial asthma. It is also used in the course of a particularly aggressive chronic rhinosinusitis with nasal polyps in patients with bronchial asthma and hypersensitivity to non-steroidal anti-inflammatory drug (NSAID-exacerbated respiratory disease, N-ERD). Identification of the patient’s inflammatory endotype enables targeted biological treatment and optimal results from biological therapy. The search for biomarkers that identify patients who will benefit from biological treatment remains a current topic of investigation. A particularly difficult therapeutic decision concerns patients with a mixed endotype (eosinophilic/allergic), which affects patients with N-ERD. The authors present a case of multidimensional beneficial results in a 39-year-old female patient with N-ERD syndrome during biological treatment with omalizumab. The parameters of inflammation, the results of computed tomography, and the results of questionnaires assessing the patient’s quality of life before, after 4 months and 9 years of biological treatment are presented. Excellent results were found in all criteria of efficacy of biological treatment: reduced nasal polyp size (complete remission of polyps in the paranasal sinuses), reduced need for systemic corticosteroids (no systemic steroids have been used since the start of therapy), improved sense of smell, reduced impact of comorbidities (complete control of bronchial asthma) and in the patient’s opinion, an extremely significant improvement in quality of life. Thus, the patient can be considered as a super-responder. Analysis of the parameters of patients who achieved optimal therapeutic results may also be helpful in selecting type of biological treatment for future patients.
The current approach to managing chronic diseases does not concentrate only on the effort to treat symptoms, but also (and sometimes primarily) on improving the patient’s quality of life. The World Health Organization (WHO) defines quality of life as: “An individual’s perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards, and concerns.” [1]. According to current knowledge, personalized medicine based on endotyping enables optimal therapeutic effects in terms of disease symptoms and improvement in patients’ quality of life [2]. Personalization of treatment is also recommended in cases of chronic rhinosinusitis with nasal polyps (CRSwNP), which includes a heterogeneous group of diseases to affect 1–4% of the general population and creates a health, social, and economic problem [3]. CRSwNP is usually a T2-predominant eosinophilic and/or allergic-driven disease [4]. Existing treatment options for patients with CRSwNP remain limited. According to guidelines topical and general corticosteroids, antibiotics, nasal saline irrigations are recommended as medical therapies. Patients with serious disease or who have failed medical management may be eligible for sinus surgery [5]. Due to the incomplete understanding of the aetiology of CRSwNP, its management remains ineffective in some patients [4]. Recent advances in our understanding of the pathogenetic mechanisms of CRSwNP endotypes have led to the introduction of effective biologic agents for its management [6]. A particularly aggressive course of chronic sinusitis with nasal polyps is seen in patients with bronchial asthma and hypersensitivity to non-steroidal anti-inflammatory drug (NSAID-exacerbated respiratory disease; N-ERD) [7]. Biological therapy has also been approved for patients with N-ERD, leading to a reduction in the severity of rhinological symptoms and the need for systemic corticosteroid use or endoscopic sinus surgery, improvement of smell, control of bronchial asthma, and quality of life [8]. Because increasing evidence suggests that N-ERD is heterogeneous in terms of both endotypes-eosinophilic and allergic, the decision on the type of biological treatment requires a particular analysis of a patient’s endotype for optimal therapeutic effect [9].
The authors present a case of multidimensional beneficial results of biologic therapy with omalizumab in a 39-year-old female patient with N-ERD. The markers of inflammation, results of computed tomography (CT), and questionnaires assessing the patient’s quality of life before therapy and after 4 months and 9 years of therapy were assessed. In the authors’ opinion, analyzing the parameters of patients who have achieved optimal therapeutic results may also be helpful in selecting biological treatment for further patients.
A 39-year-old non-smoker female patient with N-ERD was admitted to the department for qualification for biological treatment (Figure 1).
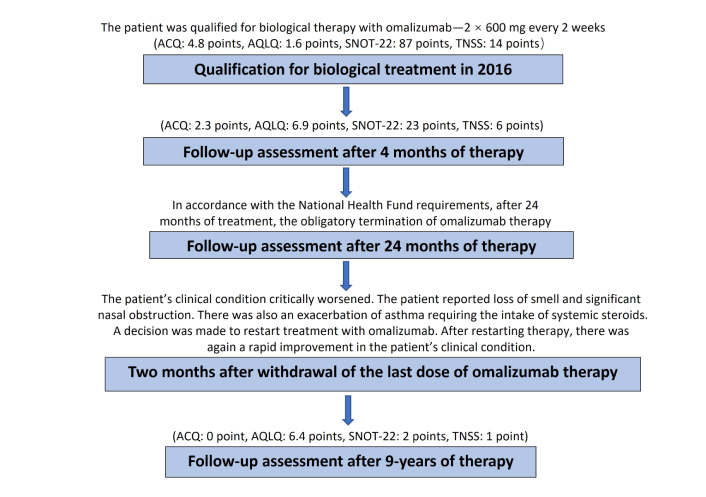
Flowchart. Abbreviations: ACQ: Asthma Control Questionnaire; AQLQ: Asthma Quality of Life Questionnaire; SNOT: Sino-Nasal Outcome Test; TNSS: Total Nasal Symptom Score.
At the age of 25 patient was diagnosed with CRSwNP. She was also diagnosed with an allergy to house dust mites. Due to the aggressive course of the disease, the patient was surgically treated twice, at the age of 30 and 32 with orbital complications. Bronchial asthma was diagnosed a year later. From the beginning, a severe clinical presentation was observed, with frequent asthma exacerbations during infections and higher exposure to house dust mites. At the age of 35, the patient was diagnosed with hypersensitivity to non-selective nonsteroidal anti-inflammatory drugs.
Despite intensive treatment the patient had severe breathing problems and the symptoms significantly impaired the patient’s quality of life. Due to the severe rhinological symptoms, a third sinus surgery was planned. After an insightful differential diagnosis, in accordance with the regulations of the National Health Fund (Appendix B.44), she was qualified for a therapeutic program of biological treatment of severe IgE-dependent allergic asthma using the anti-IgE antibody omalizumab, which at that time was the only available biological drug recommended for the treatment of severe asthma in Poland [10]. A dose of omalizumab 600 mg every 2 weeks was adjusted based on total IgE level and body mass [11]. Surprisingly, already two hours after receiving the first dose of omalizumab, the patient reported improvement in nasal congestion and sense of smell. Before qualification for biological treatment, extensive inflammatory changes were found in the CT scan of the paranasal sinuses (Figure 2). The patient reported a complete loss of smell and a significant increase in rhinological symptoms (Total Nasal Symptom Score [TNSS], 14 points; Sino-Nasal Outcome Test (SNOT)-22, 87 points). In endoscopic examination massive polyposis causing complete obstruction of the nasal cavity was revealed. Nasal polyp score (NPS) was 8 points. Spirometry using the Ganshorn Medizin Electronic spirometer (SpiroScout) revealed severe obturation (FEV1 66%). Our patient’s total IgE was 1,007 IU/mL, and his body mass was 58 kg.
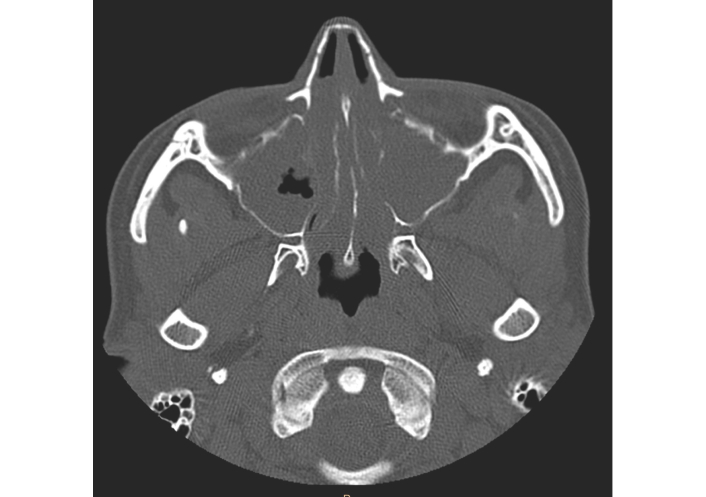
Computed tomography scan of the patient’s paranasal sinuses before biological treatment.
The Asthma Control Questionnaire (ACQ) scored 4.8 points and the Asthma Quality of Life Questionnaire (AQLQ) 1.6 points. During the first assessment of the effectiveness of the therapy after 4 months, a very good response to treatment was observed.
The return of her sense of smell and a significant improvement in the severity of nasal symptoms (TNSS, 6 points; SNOT-22, 23 points), quality of life (AQLQ, 6.9 points) and asthma control (ACQ, 2.3 points) were observed. The endoscopic examination did not reveal any nasal polyps (NPS, 0 point). The total IgE level was 590 UI/mL. In accordance with the National Health Fund requirements, after two years of biological treatment, we were obliged to withdraw biological treatment. The patient’s clinical condition critically worsened two months after withdrawal of the last dose of omalizumab. The patient reported loss of smell and significant nasal obstruction. There was also an exacerbation of asthma requiring intake of systemic steroids. A decision was made to restart treatment with omalizumab, followed by a rapid general clinical improvement. In the 5th year of therapy, a CT scan performed by a neurologist due to headaches showed complete remission of inflammatory changes in the paranasal sinuses (Figure 3).
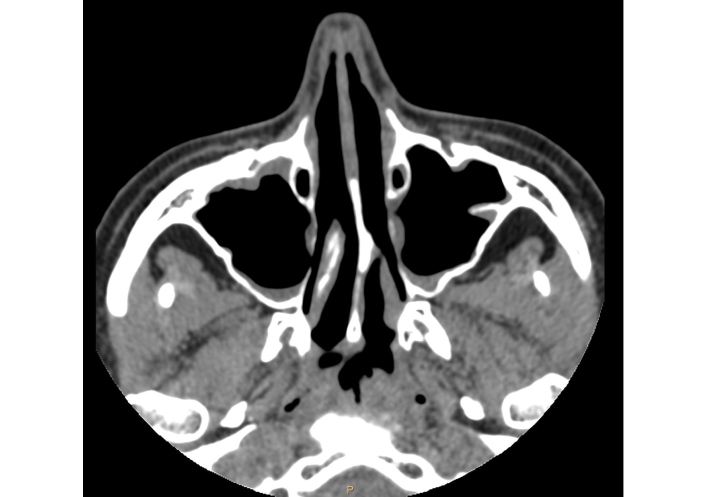
Computed tomography scan of the patient’s paranasal sinuses after 5 years of biological treatment.
The patient is currently in the ninth year of therapy. We have not observed any adverse reactions for nine years. Currently, no polyps are observed in the endoscopic examination of the nasal cavity and patient has a regular sense of smell (NPS—0 point, TNSS—1 point, SNOT-22—2 points). The patient has not been using nasal and inhaled steroids for 3 years. We confirm complete control of bronchial asthma (ACQ—0 point, AQLQ—6.4 points). Despite the excellent improvement in the patient’s clinical condition, only a very small improvement was observed in spirometry parameters: FEV1 from 66% to 69%.
In addition, a multidimensional evaluation of the benefits of omalizumab therapy across various life domains was conducted using the Wheel of Life, a widely recognized personal development tool. It ranges from 1 to 10 points in the analyzed area of life, where 1 point means very low satisfaction and 10 points means excellent satisfaction. The maximum score is 80 points [12]. Before treatment, the patient assessed her satisfaction with important aspects of daily life at 11 points; after 4 months of therapy, the score was 52 points. Currently, in the 9th year of therapy, the patient estimates her satisfaction in daily life at 77 points (Figure 4).
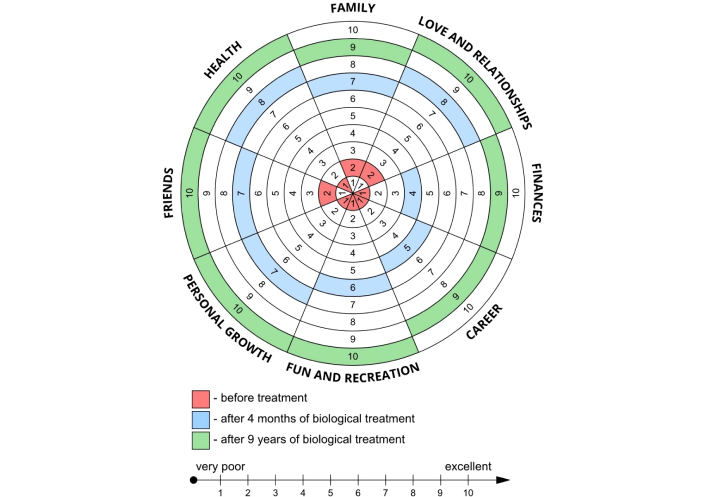
Wheel of Life—assessment of changes in satisfaction across important areas of the patient’s life during 9 years of omalizumab therapy.
Allergy was diagnosed on the basis of allergen-specific IgE antibody determinations in the serum of patients using the Polycheck method. A concentration > 0.35 kU/L was considered a positive result.
The hypersensitivity to nonsteroidal anti-inflammatory drugs was confirmed by an unambiguous clinical medical history, a history of respiratory reactions (nasal congestion, rhinorrhea, sneezing, pruritus, bronchospasm) triggered by three different COX-1 inhibitors: aspirin, ibuprofen, and diclofenac.
The parameters analyzed during the 9-year observation period are presented in Table 1.
Parameters of the analyzed case before treatment, 4 months and 9 years of treatment with omalizumab.
| Variables | Before treatment | After 4-month-treatment | After 9-year-treatment |
|---|---|---|---|
| BMI (kg/m2) | 20.5 | 20.1 | 20.7 |
| Eosinophils (%) (normal range: 1.00–5.00) | 3.4 | 4.6 | 6.3 |
| Eosinophils (1.0 × 103/μL) (normal range: 0.0–0.5 × 103/μL) | 0.22 | 0.32 | 0.48 |
| Total IgE (IU/mL) | 1,007 | 590 | 230 |
| FEV1/FVC (%) | 66 | 62 | 70 |
| FVC (%) | 86 | 89 | 93 |
| FEV1 (%) | 66 | 67 | 69 |
| ACQ (points) | 4.8 | 2.3 | 0 |
| AQLQ (points) | 1.6 | 6.9 | 6.4 |
| SNOT-22 (points) | 87 | 23 | 2 |
| TNSS (points) | 14 | 6 | 1 |
| NPS (points) | 8 | 0 | 0 |
| Sense of smell | Absent | Present | Present |
| Intranasal fluticasone (µg daily) | 400 | 400 | 0 |
| Inhaled fluticasone (µg inhaled daily) | 1,000 | 500 | 0 |
| Inhaled salmeterol (µg inhaled daily) | 100 | 50 | 0 |
| Wheel-of-Life (points) | 11 | 52 | 77 |
Abbreviations: ACQ: Asthma Control Questionnaire; AQLQ: Asthma Quality of Life Questionnaire; NPS: nasal polyp score; SNOT: Sino-Nasal Outcome Test; TNSS: Total Nasal Symptom Score; FEV1: forced expiratory volume in 1st second; FVC: forced vital capacity.
After 9 years of treatment with omalizumab, the patient considers her life to be a new reality. What was particularly amazing was the remarkable and rapid improvement in her quality of life. Complete control of bronchial asthma and chronic sinusitis has enabled the patient to return to her family and social life. Improvement in the patient’s health has enabled her to expand her professional qualifications and take up gainful employment. The patient has impressive physical fitness. She regularly participates in strength training classes, engages in high-altitude hiking, and runs marathons. The multidimensional benefits beyond the therapeutic effect of omalizumab in our patient with N-ERD are well illustrated by the Wheel of Life, which measures satisfaction in the most important areas of personal life.
In the context of complete remission of symptoms, multidimensional improvement in quality of life, and a rapid recurrence of symptoms after an attempt to discontinue the medication after two years of treatment, the question remains: should a new attempt to discontinue the medication be made after 9 years of omalizumab therapy?
In the described case, the beneficial therapeutic effects of omalizumab in a patient with N-ERD were observed. The treatment resulted in a complete control of bronchial asthma with discontinuation of inhaled steroids was achieved. In addition, long-term steroid requirement in patients was reduced and eliminated with biological treatment. An interesting aspect of the discussed case was the excellent therapeutic effect of omalizumab on CRSwNP, which is particularly aggressive in patients with N-ERD and significantly impaired the quality of life [7]. In order to control rhinological symptoms in this group of patients, pharmacological and surgical treatment is recommended. Patients with N-ERD are in a higher risk group of sinus reoperations and are significantly younger at the time of their first surgery than patients without N-ERD [13]. Frequent revision surgeries of the paranasal sinuses are associated with an elevated risk of orbital and intracranial complications, which may be life-threatening factor to the patient [14]. Novel biologic therapy currently offers a new therapeutic option for these patients [8, 15]. Since omalizumab is not currently recommended for the treatment of CRSwNP, its therapeutic effects can be observed in patients with concomitant allergic asthma [16]. It is in accordance with the concept of united airways. Notably, one study demonstrated greater efficacy of anti-IgE therapy in the treatment of CRSwNP in the group of patients with N-ERD compared to those receiving anti-IL-5 therapy [17]. The presented case of the excellent therapeutic effect of omalizumab in allergic bronchial asthma and CRSwNP confirms its positive impact on the IgE-dependent component of the N-ERD disease. During therapy, a significant decrease in total IgE concentration was observed (from 1,007 UI/mL to 230 UI/mL). However, it indicates the need for further research on the effect of omalizumab on non-IgE-dependent components of the disease. The advent of biologics has even disrupted the established place of aspirin desensitization therapy in NERD treatment. Studies on the induction of tolerance to acetylsalicylic acid in of patients with N-ERD seem particularly interesting. In the study conducted by Quint et al. [18] 56% patients with N-ERD treated with omalizumab developed complete aspirin tolerance independent of atopic sensitization.
In conclusion, during the 9-year follow-up, omalizumab treatment in our patient with N-ERD resulted in remission of CRSwNP and full control of bronchial asthma symptoms. Exacerbations and the use of oral glucocorticosteroids were completely eliminated, and an impressive multidimensional improvement in quality of life was achieved. According to international criteria, our patient can be classified as a super-responder [19]. The presented case of complete remission of bronchial asthma symptoms, but especially the excellent therapeutic effect in chronic sinusitis with polyps in the case of N-ERD syndrome, may provide additional suggestions when qualifying for biological treatment in this group of patients.
ACQ: Asthma Control Questionnaire
AQLQ: Asthma Quality of Life Questionnaire
FEV1: forced expiratory volume in 1st second
CRSwNP: chronic rhinosinusitis with nasal polyps
CT: computed tomography
FVC: forced vital capacity
N-ERD: non-steroidal anti-inflammatory drug-exacerbated respiratory disease
NPS: nasal polyp score
SNOT: Sino-Nasal Outcome Test
TNSS: Total Nasal Symptom Score
EJS: Conceptualization, Investigation, Writing—original draft. JG: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. RG: Investigation, Writing—review & editing, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The study complies with the Declaration of Helsinki. Ethical approval from the Bioethics Committee is not required at our university for case reports.
Informed consent to participate in the study was obtained from the patient.
Informed consent to publication was obtained from the patient.
The datasets for this study are available from the corresponding author upon reasonable request.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 573
Download: 13
Times Cited: 0
Ludger Klimek ... Claus Bachert
Ayşegül Verim ... Tuğba Çelik
Martin Wagenmann ... Juby A. Jacob-Nara
Sepideh Darougar ... Pantea Bozorg Savoji
Mariye Doğru ... Abdulkadir Basturk
