Affiliation:
1Department of Precision and Regenerative Medicine and Ionian Area, University of Bari Aldo Moro, 70100 Bari, Italy
†These authors contributed equally to this work.
Email: palma.carlucci@uniba.it
ORCID: https://orcid.org/0000-0001-7661-0866
Affiliation:
1Department of Precision and Regenerative Medicine and Ionian Area, University of Bari Aldo Moro, 70100 Bari, Italy
†These authors contributed equally to this work.
Affiliation:
1Department of Precision and Regenerative Medicine and Ionian Area, University of Bari Aldo Moro, 70100 Bari, Italy
†These authors contributed equally to this work.
Affiliation:
2Personalized Medicine Asthma and Allergy Unit-IRCCS Humanitas Research Hospital, 20122 Milan, Italy
3Department of Biomedical Sciences, Humanitas University, 20089 Milan, Italy
ORCID: https://orcid.org/0000-0003-3953-9225
Affiliation:
1Department of Precision and Regenerative Medicine and Ionian Area, University of Bari Aldo Moro, 70100 Bari, Italy
4Department of Medical and Surgical Sciences, Medical Area Facilities c/o A.O.U. Ospedali Riuniti Medical Complex, 71122 Foggia, Italy
ORCID: https://orcid.org/0000-0002-9728-3806
Explor Asthma Allergy. 2023;1:126–141 DOI: https://doi.org/10.37349/eaa.2023.00014
Received: April 14, 2023 Accepted: September 26, 2023 Published: October 20, 2023
Academic Editor: Lawrence DuBuske, Physician George Washington University Hospital, Immunology Research Institute of New England, United States
The article belongs to the special issue The Era of Biologics in Allergy
Allergen-specific immunotherapy (AIT) is a proven efficacy treatment for allergic rhinitis (AR), asthma, and Hymenoptera venom allergy, but its use in food allergy (FA) is still under investigation. Because some efficacy and safety concerns still remain, biologic drugs, including omalizumab and dupilumab, have been studied as an adjunctive therapy to AIT for these conditions. In this article, the evidence supporting the use of monoclonal antibodies (mAbs) as an add-on therapy to AIT for FA, AR, asthma, and Hymenoptera venom allergy has been reviewed. The review will delve into the mechanisms of action of different mAbs, their efficacy, and how they can be integrated into personalized medicine approaches to treat allergic diseases. Furthermore, future research areas will be considered. Evidence suggests that omalizumab in combination with AIT may be a beneficial option for respiratory allergies or food desensitisation, especially during the escalation or build-up phase, when adverse events are more frequent. Currently, there is a small number of well-structured clinical trials in Hymenoptera venom allergy, and the available data consist mainly of single-case reports that provide information of limited value. Dupilumab has been studied as adjunctive therapy in patients with respiratory and FAs. Clinical trials are ongoing to evaluate the efficacy of dupilumab as monotherapy or as an adjunct to oral immunotherapy (OIT) in peanut allergy. Other studies are investigating the use of dupilumab in patients with multiple FAs and as an adjunct to milk OIT. Overall, mAbs have the potential to improve outcomes in various allergic conditions when used as an add-on to AIT, especially during the build-up phase. Further research is needed to fully understand their optimal dosing and duration of treatment, as well as to identify which patients may benefit the most from these therapies.
Allergen-specific immunotherapy (AIT) represents an effective treatment for allergic disorders. AIT consists of the administration of the allergen responsible for the allergic reaction at increasing doses in order to modify the immune system’s response to the allergen, resulting in reduced severity and frequency of allergic symptoms and long-term immune tolerance.
Although AIT is generally safe and well tolerated, some patients still experience persistent symptoms or are unable to complete the treatment due to side effects. This is particularly true for food AIT, which, despite being able to increase the dose of tolerated food and reduce the risk of accidental reactions, is associated with a risk of allergic reactions, requires prolonged treatment, and, therefore, should be performed in a specialized setting during the initial dose escalation and the first dose of each updosing level. Same considerations are valid for Hymenoptera venom immunotherapy (VIT), which was associated with severe anaphylactic reactions especially during the build-up phase.
To address the need for treatment in these patients, omalizumab and other monoclonal antibodies (mAbs) targeting specific molecules involved in the allergic response [such as immunoglobulin E (IgE), interleukin 4 (IL-4) and IL-13, and IL-5] might be considered. These mAbs have been extensively studied in asthma and urticaria and they have been evaluated as an adjunct to AIT, as they can reduce the risk of adverse reactions and potentially enhance the efficacy.
In this paper, a comprehensive examination was conducted on the current evidence regarding the use of mAbs as an additional therapy for AIT for food allergy (FA), Hymenoptera venom allergy, allergic rhinitis (AR), and asthma. We will discuss the mechanisms of action of various mAbs, their efficacy, and their potential role in personalised medicine approaches to allergic disease management. We will also highlight areas for future research and clinical practice guidelines for the use of mAbs as an adjunct to AIT.
AIT can be administered by the subcutaneous, sublingual, or oral routes. There is some evidence suggesting that the immune system can develop tolerance thanks to the action of regulatory T cells (Tregs). These T cells release ILs such as transforming growth factor β (TGF-β) and IL-10, leading to an immune deviation in favour of T-helper 1 (Th1) secreting interferon γ (IFN-γ) [1]. IL-10, TGF-β and IFN-γ exert inhibitory effects on Th2 cytokines, reducing the levels of IL-4 and IL-5 production with the result of suppressing Th2 and innate lymphoid cells type 2 (ILC-2s), as well as mast cells (MCs), eosinophils (Eos), and basophils (Bas), which are key players in allergic inflammation. In addition, IL-10, TGF-β, and IFN-γ induce a switch in the Ig class and promote the production of blocking antibodies, particularly IgG4, which compete with specific IgE for allergen binding [2].
The inhibition of allergen-specific IgE interactions by IgG4 limits cross-linking of high-affinity IgE receptors Fcε receptor I (FcεRI) on both MCs and Bas, lowering degranulation and avoiding anaphylaxis. Furthermore, IgG4 can also block low-affinity receptors (FcγRIIb) on B cells, thereby preventing IgE from facilitating allergen presentation to T cells.
The binding of allergen-specific IgE to IgE receptors (FcεRI) on Bas and MCs can lead to the release of inflammatory mediators and cause anaphylaxis. However, IgG4 can inhibit these interactions, thus preventing the cross-linking of high-affinity IgE receptors and reducing degranulation [2].
Another possible mechanism of action of AIT could be the inhibition of ILC-2s, which contribute to allergic inflammation by type 2 cytokine production after their activation by the epithelium-derived cytokines thymic stromal lymphopoietin (TSLP), IL-25, and IL-33 [3].
mAbs effectively interfere with signaling pathways occurring subsequent to the activation of ILC-2, thereby contributing to mitigating inflammatory processes. A visual representation of the complex cellular and cytokine networks involved in allergic responses is provided in Figure 1, showing how mAbs can modulate these networks during allergen immunotherapy.
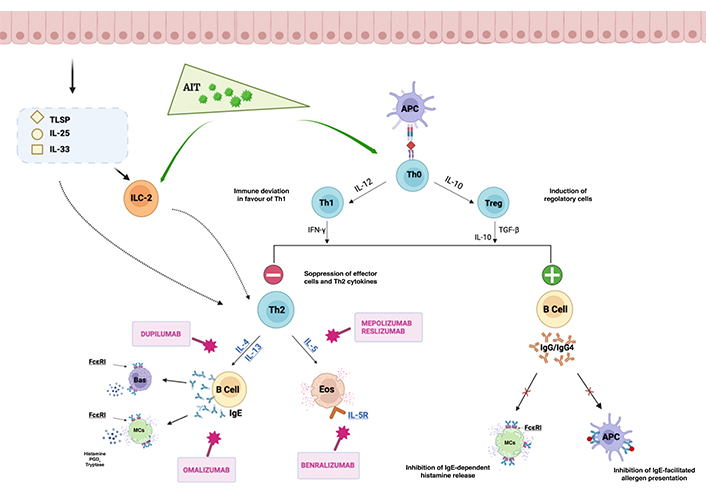
Cellular and cytokine networks as a consequence of AIT, with possible mAb interactions. Key cell types involved in the process include MCs, Bas, Eos, T cells, B cells, and dendritic cells. These cells communicate through a network of cytokines, including ILs. AIT induces allergen-specific tolerance through the action of Tregs, releasing TGF-β and IL-10. These cytokines determine immune deviation towards Th1 responses characterized by IFN-γ secretion. The inhibitory effects of IL-10, TGF-β, and IFN-γ on Th2 cytokines (IL-4 and IL-5) suppress the activity of Th2 cells, ILC-2s, MCs, Eos, and Bas, key mediators in allergic inflammation. Additionally, IL-10, TGF-β, and IFN-γ promote the production of IgG4 antibodies, which compete with allergen-specific IgE for binding, impeding IgE-dependent histamine release from MCs. Consequently, degranulation is reduced, anaphylaxis is prevented, and the interaction of IgE with low-affinity receptors (FcγRIIb) on B cells, involved in allergen presentation to T cells, is hindered. Additionally, AIT may exert its effects by inhibiting ILC-2, which contribute to allergic inflammation through the production of type 2 cytokines upon activation by epithelium-derived cytokines TSLP, IL-25, and IL-33. Specific mAbs can interact with cellular and cytokine networks. Dupilumab targets the IL-4 receptor (IL-4R) and disrupts IL-4 and IL-13 signaling. Omalizumab binds to free IgE and inhibits its interaction with FcεRI on MCs and Bas. Benralizumab, reslizumab, and mepolizumab target IL-5 or its receptor and interfere with Eo activation and survival. APC: antigen presenting cell; PGD2: prostaglandin D2. The figure was created with https://www.biorender.com/
Anti-IgE mAbs, including omalizumab [4], ligelizumab [5], and quilizumab [6], have been developed as potential therapeutic agents. Currently, only omalizumab has received approval for clinical use. Omalizumab exerts its action by specifically targeting the heavy chain of IgE, preventing its interaction with the high-affinity IgE receptor FcεRI. This binding inhibition leads to a reduction in circulating free IgE levels and a down-regulation of FcεRI expression on Bas and MCs [7]. Consequently, the diminished activation of Bas and MCs results in the decreased release of histamine and other inflammatory mediators involved in allergic responses.
Omalizumab is proved to be effective in the treatment of severe allergic asthma and AR. The primary goals of using omalizumab during AIT are:
To permit AIT-mediated tolerance in patients who would not be able to increase allergen dosage during early build-up phase due to their significant immunoreactivity.
To minimise the risk of allergic side effects related to AIT, especially in patients with bronchial asthma which represents a risk factor for adverse events.
There is some evidence based on the findings of double-blind, placebo-controlled (DBPC) studies (see Table 1) [8–13] showing the effect of omalizumab used before and during AIT as an adjunctive treatment in patients with respiratory allergies (AR and bronchial asthma).
Use of omalizumab as adjuvant therapy in AIT for respiratory allergies
| Reference | Study type | Allergen | Patients | Main results/aims |
|---|---|---|---|---|
| Kuehr et al. [8], 2002 | RCT, DBPC | Grass, birch | 221 | Combined therapy outperforms either component alone in terms of the symptom score and the rescue medication score. |
| Casale et al. [9], 2006 | RCT, DBPC | Ragweed | 159 | Omalizumab before treatment showed a five-fold reduction in the risk of rush anaphylaxis associated with SCIT. The use of combination therapy was associated with a decrease in the intensity of symptoms compared to SCIT alone. |
| Kopp et al. [10], 2009 | RCT, DBPC | Grass | 140 | Combination therapy led to a decrease in daily symptom scores, an improvement in QoL and in the management of rhinoconjunctivitis and asthma. |
| Massanari et al. [11], 2010 | RCT, DBPC | Mite, cat, dog | 225 | Omalizumab before treatment correlated with a decreased occurrence of systemic reactions and an increased chance of reaching the maintenance SCIT dose. |
| Stelmach et al. [12], 2015 | RCT, DBPC | Mite, moulds | 7 | Consecutive combination therapy resulted in the reduction of exacerbation frequency and hospitalizations. Significant decrease in the utilization of steroids was observed. |
| Valdesoiro-Navarrete et al. [13], 2022 | Retrospective study | Mite, Alternaria, and pollens | 29 | Combination therapy resulted in a significant improvement of asthma control score (CAN questionnaire) and forced expiratory volume in one second (FEV1), following a year of treatment. |
RCT: randomized controlled trial; SCIT: subcutaneous immunotherapy; QoL: quality of life
In 2009, Kopp et al. [10] evaluated the effects of the combination of omalizumab and AIT compared to AIT alone in 140 patients with AR and co-morbid seasonal allergic asthma. Results showed a reduction of AIT side effects during the build-up and/or maintenance phase, reduction of daily symptoms, improved control of rhinoconjunctivitis and asthma, and improvement in QoL. Other authors suggested that this combined therapy promotes a better effect on respiratory symptoms and a long-lasting tolerance to specific allergens [14].
Severe asthma currently represents a contraindication to AIT. However, a recent study by Bożek et al. [15] revealed that a combination of AIT targeting house dust mites (HDM) and omalizumab is more effective in reducing symptoms and decreasing the daily dose of inhaled corticosteroids (ICS) than omalizumab alone or AIT alone in patients with HDM-driven asthma. Another study conducted in a paediatric allergy unit by Valdesoiro-Navarrete et al. [13] showed that omalizumab was administered successfully and safely before the initiation of AIT to achieve asthma control and during the AIT build-up-maintenance phase. This combination therapy was effective in achieving asthma control and improving lung function and QoL compared to baseline values [13]. Thus, when AIT is contraindicated, omalizumab may allow patients to be treated safely with AIT. On the other hand, allergic reactions occurred in some other patients in whom this combined approach was used [16].
Since follow-up data are lacking in the majority of studies, it is unclear if omalizumab treatment is related to quicker and longer-lasting tolerance development [17].
A recent review by Pfützner and Schuppe [16] suggested the use of recombinant IgG antibodies directed against specific epitopes of an allergen: similar to the AIT-induced IgG antibodies, they can prevent allergens from binding to IgE antibodies.
The build-up and maintenance phases of Hymenoptera VIT are known to be characterised by recurrent adverse reactions that encompass local reactions at the injection site, as well as systemic allergic manifestations including generalized urticaria, angioedema, bronchospasm, and, in severe instances, anaphylaxis [18]. Antihistamines proved effective in preventing mild hypersensitivity reactions, but omalizumab pre-treatment has been proven effective in cases of recurrent severe adverse events that prevent reaching the full maintenance dose, particularly in those with MCs disorders (such as mastocytosis or MCs activation syndrome) [19].
A case report by Soriano Gomis et al. [20] showed that omalizumab pretreatment before starting bee-VIT was not effective in preventing recurrent anaphylactic events during the build-up phase.
However, in most studies, omalizumab showed efficacy in protecting high-risk patients from severe reactions during VIT and also in improving adherence to treatment. To our knowledge, 19 studies were published so far (Table 2) [20–38]. Most of them are case reports, from limited patient subsets, with different timings of administration and dosage; large randomised studies are currently lacking.
Use of omalizumab as adjuvant therapy in VIT
| Reference | Study type | Allergen | VIT protocol | Omalizumab dose (mg) | Timing of omalizumab administration | Patients | Main results/aims | Adverse events during omalizumab + VIT |
|---|---|---|---|---|---|---|---|---|
| Wedi et al. [21], 2007 | Case report | Honeybee | Ultra-rush | 150 | One-time dose | 1 | VIT tolerance achieved after a single dose, even after the end of treatment | Absent |
| Schulze et al. [22], 2007 | Case report | Honeybee | Ultra-rush | 300 | One-time dose | 1 | VIT tolerance achieved after a single dose, even after the end of treatment | N.A. |
| Soriano Gomis et al. [20], 2008 | Case report | Honeybee | Ultra-rush | 300 | Every 28 days | 1 | Failure to achieve VIT tolerance | Patients showed anaphylaxis during the build-up phase |
| Averbeck et al. [23], 2008 | Case report | Vespula | Ultra-rush | 300 | Every 28 days | 1 | VIT tolerance achieved after a single dose, even after the end of treatment | Absent |
| Rerinck et al. [24], 2008 | Case report | Honeybee | N.A. | 150 | 2 doses at 14-days-interval before ultra-rush, then every 28–42 days | 1 | VIT tolerance achieved after a single dose, even after the end of treatment | Absent |
| Galera et al. [25], 2009 | Case report | Honeybee | Rush | 150 | Every 14 days | 1 | After lowering to 75 mg, anaphylaxis occurred, but 150 mg showed no more adverse reactions | Absent |
| Kontou-Fili and Filis [26], 2009 | Case report | Honeybee | Modified rush | 300 | Every 28 days | 1 | VIT tolerance achieved; anaphylaxis following decreased attempt | Absent |
| González-Pérez et al. [27], 2010 | Case report | Honeybee | N.A. | 300 | N.A. | 1 | VIT tolerance achieved; anaphylactic reaction followed omalizumab discontinuation | Absent |
| Palgan et al. [28], 2014 | Case report | Vespula | Rush | 150 | Every 28 days | 1 | VIT tolerance achieved | Absent |
| da Silva et al. [29], 2013 | Case report | Honeybee | Ultra-rush | 300 | Every 28 days | 1 | VIT tolerance achieved, even after the end of treatment | Absent |
| Boni et al. [30], 2016 | Case report | Honeybee | Rush | 450 | Every 28 days | 1 | VIT tolerance achieved | Absent |
| Stretz et al. [31], 2017 | Case series | Honeybee and Vespula | Rush and ultra-rush | According to total IgE and body weight | Every 28 days | 10 | VIT tolerance achieved, even after the end of treatment | Absent |
| Toldrá et al. [32], 2017 | Case report | Honeybee | Rush | 300 | Every 14 days | 1 | VIT tolerance achieved; anaphylaxis after discontinuation attempt | Absent |
| Lourenço et al. [33], 2017 | Case report | Honeybee | Ultra-rush | 300 | Every 14 days | 1 | VIT tolerance achieved | Absent |
| Lopes et al. [34], 2017 | Case report | Honeybee | N.A. | 450 or 300 | Every 28 days | 3 | VIT tolerance achieved | Absent |
| Yilmaz et al. [35], 2018 | Case report | Honeybee | Conventional | 150 | Every 14 days | 1 | VIT discontinuation | Facial erythema |
| Droitcourt et al. [36], 2019 | Case report | Honeybee or Vespula | Rush | According to total IgE and body weight | Every 14 days | 3 | VIT tolerance achieved, even after the end of treatment | Absent |
| Gülsen [37], 2021 | Case report | Honeybee | Rush | 150 | 5 weeks, 3 weeks, and 1 week prior to re-start of immunotherapy and for 2 months in parallel to VIT | 1 | Omalizumab is useful as a premedication in patients with mastocytosis who do not tolerate VIT | Absent |
| Çetin et al. [38], 2022 | Retrospective study | Honeybee and Vespula | Rush and ultra-rush | 150 | Every 14 days | 72 (total) 2 (VIT + omalizumab) | Omalizumab use as an add-on in OIT results in a strong premedication effect but not an immunomodulatory effect on VIT | Absent |
N.A.: not applicable; OIT: oral immunotherapy
In 2017 Stretz et al. [31] published a retrospective case series on 10 patients, assessing the effectiveness of anti-IgE antibodies in combination with a high-maintenance dose of VIT in preventing severe adverse reactions among patients with a record of systemic allergic reactions to insect bites. The combination therapy was effective in reducing the frequency and severity of adverse reactions to immunotherapy. The study also reported a favourable safety profile; evidence suggests that omalizumab should be stopped when complete tolerance is reached for both VIT and stings and that VIT should eventually be continued for an adequate duration [39].
FA is characterised by an adverse immune-mediated response to a dietary protein. Unlike allergies to aeroallergens and Hymenoptera venoms, foods AIT (known as OIT) are still an experimental treatment and only a formulation for peanut allergy was approved in children and adolescents [40]. Hypersensitivity reactions occur significantly more frequently than in respiratory and Hymenoptera venom allergies, leading to AIT discontinuation [41].
In a recent meta-analysis by Chu et al. [42], the efficacy and safety of OIT as a treatment for peanut allergy were examined. The findings revealed that, although OIT effectively induces desensitization, it considerably increases the risk of allergic and anaphylactic reactions compared to avoidance or placebo [42]. Consequently, the authors emphasized the need for safer treatment approaches [42].
In a study conducted by Nadeau et al. [43] in 2011, representing the first phase I study, 11 children with cow’s milk allergy were treated with a combination of omalizumab and rapid oral milk desensitisation. Omalizumab was administered at a dose ranging from 150 mg to 300 mg for nine weeks prior to the start of rapid oral desensitisation. The build-up phase included weekly up-dosing over the next 7–11 weeks, while omalizumab was continued until the 16th week to reach a maximum milk dose of 2,000 mg. Nine patients were able to tolerate the maximum dose, and desensitization was maintained in all patients with continuous milk administration after the discontinuation of omalizumab. Since then, several clinical trials have been conducted on the use of anti-IgE mAbs in combination with OIT for peanut, egg, milk, and multiple allergens, resulting in safe and effective rapid desensitisation with high maintenance rates, as reported in Table 3 [43–54].
Use of omalizumab as adjuvant therapy in AIT in FA
| Reference | Study type | Allergen | Patients | Main results/aims |
|---|---|---|---|---|
| Nadeau et al. [43], 2011 | RCT, DBPC | Milk | 11 | Omalizumab helped to shorten the escalation phase of milk OIT, which decreased the number of dose-related reactions requiring intervention, including those needing epinephrine injections |
| Bégin [44], 2014 | Phase 1, SC, DBPC | Multiple foods | 25 | Phase 1 rush OIT protocol with omalizumab pre-treatment was conducted in an open-label manner to evaluate its dose tolerability and safety. Results indicated that the treatment was generally well-tolerated |
| Martorell-Calatayud et al. [45], 2016 | Case series | Milk | 14 | Omalizumab as an add-on therapy to cow’s milk OIT resulted to be effective in enhancing the safety of milk desensitization protocols. |
| Wood [46], 2016 | RCT, DBPC | Milk | 57 | In the omalizumab arm, the increase in dose was shorter in length. Regarding the success of the oral challenge, no significant differences were found. Omalizumab had a higher percentage of doses without symptoms, compared to placebo |
| MacGinnitie et al. [47], 2017 | Phase 2, RCT, DBPC | Peanuts | 37 | Addition of omalizumab increased the speed of the desensitization process. Fewer adverse reactions were recorded during the desensitization process |
| Takahashi et al. [48], 2017 | Phase 2, RCT, DBPC | Cow’s milk | 16 | Throughout the escalation phase, each patient in the omalizumab group was able to consume 200 mL of milk without experiencing any severe adverse reactions. All patients treated with omalizumab passed the DBPCFC at week 32, while none of the untreated patients did |
| Andorf [49], 2018 | Phase 2, RCT, MC, DBPC | Multiallergen | 48 | Significant improvement in tolerance of various food allergens. Treatment also led to a decrease in the frequency and severity of allergic reactions, as well as an increase in the participants’ QoL |
| Andorf [50], 2019 | Phase 2, RCT, DBPC | Multiallergen | 60 | Percentage of patients who passed the DPBCFC while consuming two or more of their prohibited food at the end of the 16-week period. The study found that omalizumab-facilitated OIT was safe and well-tolerated and that a high proportion of participants remained desensitized to all foods at the end of the 16-week period. However, a significantly higher proportion of participants in the continued omalizumab group remained desensitized compared to the discontinued omalizumab group |
| Sindher [51], 2022 | Phase 2, RCT, DBPC | Multiallergen | 60 | Data suggest that changes in IgG4/IgE ratio are induced early when OIT is combined with fixed-dose omalizumab |
| NCT03881696 [52] | Phase 3, RCT, MC, DBPC | Multiallergen | 225 | Number of patients who are able to consume a single dose of > 600 mg of peanut protein approximately 16 to 20 weeks after the initiation of stage 1 treatment |
| NCT04984876 [53] | Phase 3, RCT, MC, DBPC | Peanuts | 486 | Number of patients who tolerate a single dose of > 600 mg of peanut protein at week 12 |
| NCT01781637 [54] | Phase 2, RCT, MC, DBPC | Peanuts | 36 | Number of patients who tolerate 2,000 mg 6 weeks after their last dose of either omalizumab or placebo |
DBPCFC: DBPC food challenge; MC: multicentre; SC: single centre
The data consistently demonstrates effectiveness in accelerating the desensitization process [43–47]. One notable study by Sindher et al. [51] has provided substantial evidence supporting the benefits of combining omalizumab with AIT. Their research indicates that the administration of fixed-dose omalizumab alongside OIT induces early changes in the ratio of IgG4/IgE [51]. This alteration is a crucial marker for immune tolerance and serves as an indicator of the desensitization process.
Recent studies have investigated the feasibility of sustained long-term desensitisation thanks to omalizumab in patients with FAs. Andorf et al. [50] conducted a 5-year follow-up observational study on 34 patients, using rapid desensitisation facilitated by omalizumab. A number of participants in the study had their long-term maintenance dosage lowered after achieving the maintenance dose of 2 g of protein for their respective allergens. At the end of the follow-up, each patient passed the 2 g oral food challenge (OFC), demonstrating the persistence of desensitization.
Omalizumab has been observed to prevent adverse events as well as facilitate high-dose desensitisation when used in combination with OIT. In fact, in a recent meta-analysis of randomised and non-randomised observational studies, the addition of omalizumab to different OITs showed a faster dose increase, high-dose desensitisation, higher maintenance doses of immunotherapy, and a positive impact on the QoL of patients and caregivers [55].
The omalizumab as Monotherapy and as Adjunct Therapy to Multi-Allergen OIT in Food Allergic Participants study (OUtMATCH; NCT03881696), focuses on patients who have multiple FAs and aims to evaluate the efficacy of omalizumab as both monotherapy and as an adjunct to OIT [52]. Participants aged from 1 year to 55 years with an allergy to peanuts and at least two of the following foods will be included: cashew, hazelnut, walnut, milk, egg, and wheat. The study consists of three stages and the primary outcome is tolerance of a single dose of > 600 mg peanut (cumulative > 1,044 mg). Secondary outcomes include tolerance to other allergens and QoL.
Omalizumab is the only mAb approved to date, but the optimal dosage remains unclear. Recent studies suggest that doses should be adjusted based on body weight alone, irrespective of total IgE levels. However, increased local reactions cannot be usually prevented, and AIT-related anaphylaxis cannot be completely ruled out by omalizumab treatment.
In this context, the potential of newer developments and preparations like the anti-IgE antibodies ligelizumab and quilizumab (which are capable of depleting IgE-bearing plasmablasts and B cells) may be of particular interest [56–58].
A clinical trial (NCT04984876) is underway to investigate the efficacy and safety of ligelizumab as monotherapy for patients with peanut allergy [53]. This trial is open to participants aged from 6 years to 55 years, who have a peanut allergy. Participants will receive either ligelizumab or a placebo for 52 weeks, with the primary outcome of determining the ability to tolerate a single dose > 600 mg (cumulative > 1,044 mg) of peanut protein by week 12. Key secondary outcomes will include the ability to tolerate higher doses of peanut (> 1,000 mg or > 3,000 mg) at weeks 12 and 52, as well as measuring the QoL of participants.
Dupilumab is a humanised mAb that interferes with the signaling pathways of IL-4 and IL-13, two pivotal cytokines involved in the type-2 response. It is currently authorised for the treatment of atopic dermatitis (eczema), asthma, and chronic rhinosinusitis with nasal polyps [59].
In a study conducted on 103 patients suffering from grass pollen-induced seasonal AR, the patients were assigned to four groups namely SCIT, dupilumab, SCIT + dupilumab, or placebo in a randomised 1:1:1:1 manner [60]. The study was a phase 2a multicenter, DBPC, parallel-group study; investigators noted that administering SCIT together with dupilumab for 16 weeks increased SCIT tolerability but did not result in any significant improvement in nasal symptom scores when compared to SCIT alone [60]. It is worth noting, however, that this study was of short duration, and further research is required to determine the long-term safety and effectiveness of using dupilumab in combination with SCIT for the treatment of AR.
Dupilumab’s efficacy and safety as an OIT adjuvant therapy and as a monotherapy in FA are being examined in different ongoing studies.
In the first case study reported by Rial et al. [61], a 30-year-old woman affected by a severe form of atopic dermatitis and corn and nuts allergy was treated with dupilumab, leading to desensitization after three months of treatment. A confirmatory OFC was conducted to assess the patient’s reaction to corn and nuts.
The efficacy of dupilumab as a monotherapy in children between the age of 6 and 17 is the subject of a study that started in 2019 [62]. The aim of this study is to estimate the percentage of children who can tolerate peanuts in a food challenge after 24 weeks of treatment with dupilumab [62].
Another study is assessing whether dupilumab might improve the proportion of children who tolerate an oral peanut challenge after completing peanut OIT [63]. The study additionally evaluates whether dupilumab has the potential to improve the safety and tolerability of peanut OIT and whether long-term use of dupilumab after achieving maintenance gives further benefits in comparison with short-term use until the maintenance dose is reached [63].
In addition, there is an RCT in phase 2 examining the efficacy of dupilumab in enabling immunotherapy for patients with a history of multiple FAs, including peanuts [64].
Another phase 2 RCT is investigating dupilumab as a supplement to milk OIT. Patients in the treatment arm will receive dupilumab before and during the milk OIT updosing phase, followed by eight weeks of milk OIT without dupilumab. The proportion of patients who can tolerate at least 2,040 mg of cumulative cow’s milk protein will be evaluated at week 18 [65].
For a complete list of ongoing studies investigating the use of dupilumab in combination with allergen immunotherapy, refer to Table 4.
Use of dupilumab as adjuvant therapy in AIT
| Reference | Study type | Allergen | Patients | Main results/aims |
|---|---|---|---|---|
| Corren et al. [60], 2021 | Phase 2, RCT, MC, DBPC | Grass | 103 | Dupilumab may enhance the tolerability of SCIT, but it does not appear to reduce nasal symptoms after challenge when compared to SCIT alone. |
| NCT03682770 [63] | Phase 2, RCT, MC, DBPC | Peanuts | 149 | Number of patients able to complete an exit food challenge with 2,044 mg of cumulative peanut protein. |
| NCT03679676 [64] | Phase 2, RCT, SC, DBPC | Multiallergen OIT | 110 | Number of patients who successfully passed a food challenge for peanut and two other FAs. |
| NCT04148352 [65] | Phase 2, RCT, MC, DBPC | Milk | 40 | Number of patients who received dupilumab plus milk protein OIT vs. placebo plus milk protein OIT, and were able to tolerate a minimum of 2,040 mg (cumulative) of cow’s milk protein during food challenge at week 18. |
IL-5 is produced and secreted by different types of cells, including Eos, Th2 cells, MCs, natural killer T cells, and ILC-2s [66]. ILC-2s may also contribute to FA development by producing IL-5 and IL-13 in response to IL-25 and activating alarmins [67, 68]. Additionally, a recent study using a mouse model of FA has shown that IgE-activated MCs may be capable of inducing intestinal ILC-2s, which might increase responsiveness to anaphylactic mediators released by MCs [69].
Currently, there are three biologics targeting the pathogenic IL-5/IL-5R pro-eosinophilic axis: mepolizumab, reslizumab, and benralizumab.
While anti-IL-5 agents are typically used as a treatment for Eo-related diseases, their effectiveness in treating eosinophilic gastrointestinal disorders suggests they may have a potential application in the management of FAs as well. In fact, eosinophilic gastrointestinal disorders, particularly eosinophilic esophagitis, have been suggested as a type of chronic FA with genetic and environmental links to IgE-mediated FA. Some researchers believe that eosinophilic gastrointestinal illnesses and IgE-mediated FA should be regarded as two distinct kinds of FA that exist in a constant state of balance with each other, presenting challenges but also opportunities in treatment [70].
The potential application of these biologics as adjuncts to FA immunotherapy is an area of interest for future research.
The use of biologics targeting upstream alarmins, such as IL-25, IL-33, and TSLP, is an additional approach to the treatment of allergic diseases.
Increased levels of IL-4, IL-5, and IL-13 can be observed as a consequence of alarmins’ combined actions, which leads to a downstream transition from a tolerant Th1-dominant state to a pro-allergic Th2 response. Alarmins are crucial for triggering and maintaining FA since they are a key factor in the Th2 response. According to a mouse model, blocking TSLP, IL-25, and IL-33 with mAbs has the potential to prevent the development of FA [71].
A humanised IgG1/kappa mAb named etokimab was developed specifically to bind IL-33 and neutralize its biological impact [72]. IL-33 is a versatile cytokine that contributes significantly to the onset and progression of several medical conditions. Its actions are dual, as it behaves both as an alarmin in response to signs of tissue damage while also prompting the production of IL-5 and IL-13 by ILC-2, thus stimulating Th2 immune responses. In turn, these two cytokines inhibit the growth of Tregs in favour of Th2 differentiation and MCs activation [73, 74].
In a DBPC phase 2a trial for adults with peanut allergy, a single administration of etokimab significantly increased desensitization to peanut protein in the active group, with reduced skin prick test (SPT) wheal size and peanut-specific IgE after 14 days [75]. Compared with the placebo, the etokimab group experienced fewer adverse events. The clinical result correlated with a reduction of IL-4, IL-5, IL-9, and IL-13 in CD4+ T cells.
No clinical trials on tezepelumab, a TSLP antagonist, in FA are being conducted at the moment [76].
Currently, the use of biological agents against IL-25 in humans has not been investigated.
Overall, the combination of mAbs and immunotherapy has emerged as a promising approach to the treatment of allergic diseases in patients with severe side effects to AIT leading to treatment discontinuation.
The combination of mAbs and immunotherapy could be effective in reducing allergic symptoms by addressing both symptoms and the underlying cause of the allergic reaction.
Furthermore, it may also have a long-lasting effect on the immune system, providing a sustained reduction in allergic symptoms even after the treatment has ended. This is because immunotherapy can lead to changes in the immune system that can persist over time, resulting in reduced sensitivity to the allergen. This approach has the potential to provide long-lasting relief from allergic symptoms and improve the QoL for millions of people worldwide.
Despite these promising findings, many aspects require a better understanding:
Optimal dosing: the optimal dosing of mAbs as an add-on therapy in AIT is still unclear. More studies are needed to determine the most effective dose and duration of treatment.
Patient selection: mAbs may not be suitable for all patients with allergies. Further research is needed to identify the patients who are most likely to benefit from this treatment.
Long-term safety: the long-term safety of using mAbs as an add-on therapy in AIT is not yet fully understood. More studies are needed to assess the potential risks associated with prolonged treatment.
Cost-effectiveness: mAbs are expensive, and the cost-effectiveness of using them needs to be evaluated.
Ongoing research in this field is needed to fully understand the benefits and risks of this combination therapy and to further improve the efficacy and safety of this treatment approach.
AIT: allergen-specific immunotherapy
AR: allergic rhinitis
Bas: basophils
DBPC: double-blind placebo-controlled
Eos: eosinophils
FA: food allergy
FcεRI: Fcε receptor I
IFN-γ: interferon γ
IgE: immunoglobulin E
IL-4: interleukin 4
IL-4R: interleukin 4 receptor
ILC-2s: innate lymphoid cells type 2
mAbs: monoclonal antibodies
MCs: mast cells
OIT: oral immunotherapy
QoL: quality of life
RCT: randomized control trial
SCIT: subcutaneous immunotherapy
TGF-β: transforming growth factor β
Th1: T-helper 1
Tregs: regulatory T cells
TSLP: thymic stromal lymphopoietin
VIT: venom immunotherapy
PC, FS, and MFD equally contributed to: Conceptualization, Writing—original draft, Investigation. DDB and GP: Writing—original draft, Writing—review & editing.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Alejandra Carrón-Herrero ... Giovanni Paoletti
Francesca Losa, Arianna Cingolani
Carlo Alberto Vignoli, Riccardo G. Borroni
Karl-Christian Bergmann ... Torsten Zuberbier
Giulia Costanzo, Andrea Giovanni Ledda
Diego Bagnasco ... Fulvio Braido
Shuichiro Matsumoto ... Hisatoshi Sugiura
Christian Paolo Ratti ... Silvia Mariel Ferrucci
Alexandru Corlateanu, Cristina Toma
