Affiliation:
Department of Gastroenterology, Cleveland Clinic Florida, Weston, FL 33331, USA
ORCID: https://orcid.org/0009-0003-4043-791X
Affiliation:
Department of Gastroenterology, Cleveland Clinic Florida, Weston, FL 33331, USA
Email: uklejaa@ccf.org
ORCID: https://orcid.org/0000-0002-3718-5331
Explor Asthma Allergy. 2025;3:100998 DOI: https://doi.org/10.37349/eaa.2025.100998
Received: September 11, 2025 Accepted: October 16, 2025 Published: November 12, 2025
Academic Editor: Eleonora Nucera, Catholic University of the Sacred Heart, Italy
The article belongs to the special issue Beyond Eosinophilic Gastrointestinal Diseases: Pathogenetic Mechanisms and Therapeutic Strategies
Eosinophilic esophagitis (EoE) is an adaptive immune T-cell-mediated type 2 inflammatory disease involving the esophagus. Major advances were made in diagnosis and therapy for EoE in the last decade. A correct diagnosis is important to guide therapy to achieve optimal treatment goals. The diagnostic algorithm eliminated the need for a proton pump inhibitor (PPI) trial, allowing for direct proceeding to disease-specific anti-inflammatory drugs if indicated. PPIs are the first line of therapy in EoE. Two topical steroids and one biologic drug, dupilumab, have been used for the treatment of EoE, with a number of novel therapeutic agents in the pipeline. Because of the chronicity of the disease and nonresponders to PPIs and topical steroids, a subgroup of patients may require long-term therapy with dupilumab. More data also became available on dietary interventions, elimination diets in adults with EoE. A least restrictive diet should be trialed first with milk or milk and wheat elimination. Importantly, EoE patients with fibrostenotic disease often do not respond well to drug therapy and require esophageal dilations. Medical therapy may need to be tailored for each patient depending on the patient’s specific comorbidities, patient preferences, and health status. This review will summarize a current approach to the treatment of adult patients with EoE.
Eosinophilic esophagitis (EoE) is a chronic immune, antigen-mediated disease of the esophagus and clinically presents with a range of upper gastrointestinal (GI) symptoms, including dysphagia, heartburn, epigastric, chest pain, or food impaction. Diagnosis of EoE is based on histologic findings of eosinophil predominant inflammation with at least 15 eosinophils per high-power field (eos/hpf) on esophageal biopsies in conjunction with symptoms of esophageal dysfunction [1, 2]. Recently, the EoE American College of Gastroenterology (ACG) clinical guidelines have been published to address the key points in diagnosis and management of EoE and provide an updated treatment algorithm with the advancements in the drug therapies [3]. Pathogenesis of EoE is still poorly understood and multifactorial, including genetic, host immune system, and environmental factors. Adaptive T-cell immunity involving T-helper type II cells and interleukin (IL)-13, IL-5, and IL-20 appears to play a pivotal role in the pathogenesis of EoE [4, 5]. It is believed that the mast cells may play a role in esophageal remodeling, leading to muscle cell hyperplasia and hypertrophy [6]. Therefore, mast cell depletion is one of the targets for therapy in EoE. In addition, high levels of systemic and local immunoglobulin IgG4 have been reported in EoE patients [7]. There is an overlap with IgG4 and T-cell responses, with IgE playing a less significant or ancillary role in the pathogenesis of EoE [8]. A strong association of EoE with allergic conditions has been observed, with a high number of patients (up to 75%) having atopy and food allergies [9]. It is recognized that EoE can be reversed by the use of an allergen-free diet.
The diagnosis of EoE required previous presence of eosinophilia in the esophagus, which was resistant to proton pump inhibitor (PPI) therapy, but currently this criterion is not required for diagnosis [1, 3]. Establishing the correct diagnosis of EoE is important to select proper therapy and response to it. The primary goal of treatment in EoE is not only symptom control and improvement in quality of life, but also histologic improvement in esophageal eosinophilia [10]. In addition, prevention of food impaction, esophageal stenosis, and perforation is a target of therapy. The treatment for EoE includes dietary modifications, pharmacologic therapy, and endoscopic interventions for strictures. The treatment response should be assessed not only by symptom evaluation, but also by endoscopic and histologic disease activity, requiring a follow-up endoscopy. The approach to therapy of EoE has been outlined in multiple guidelines from professional societies as well as expert recommendations [1, 3, 10–13]. This article will focus on the diet and pharmacologic therapy for EoE in adults.
Dietary therapy serves as a non-pharmacologic approach and has been shown to be particularly effective as a first-line intervention in pediatric patients with EoE. Concurrently, in adults, adherence may be more challenging due to the restrictive nature and complexity of elimination diets. Given the high prevalence of food allergies in EoE, dietary triggers are thought to markedly contribute to disease pathogenesis. Dietary avoidance has been associated with symptom improvement, histologic remission of esophageal eosinophilia, and, in some cases, reversal of subepithelial fibrosis [14, 15]. However, discontinuation of the diet can lead to a relapse of symptoms. Three types of dietary modifications have been proposed, including an empiric elimination diet, an elemental diet, or a test-directed elimination diet.
The empiric elimination diet is the most widely used and effective dietary intervention for patients with EoE. Cow’s milk has been deemed the most common food trigger causing immediate hypersensitivity and is therefore frequently eliminated from the diet. The six-food elimination diet (6FED, see Table 1), which excludes cow’s milk, hen’s egg, soy, wheat, peanuts/tree nuts, and fish/shellfish, has demonstrated higher rates of histologic remission, as these food groups account for the majority of IgE-mediated food reactions [16]. In a prospective study of 50 patients following a 6FED, 70% achieved histologic remission (< 10 eos/hpf), with 94% showing symptomatic improvement [17]. Milk and wheat were the most frequently implicated food triggers upon reintroduction. A 4FED, targeting cow’s milk, eggs, soy, and wheat, is also commonly used. Notably, recent evidence suggests that elimination of cow’s milk alone may achieve remission rates comparable to 4FED or 6FED [18]. Supporting this, a multicenter randomized trial of 129 adults comparing single-food elimination (milk) versus 6FED reported similar improvements in histologic and peak eosinophil outcomes (34% vs. 40% remission) [18]. Data from the meta-analysis of 14 observational studies indicate that 6FED, 4FED, and 1FED achieve similar efficacy in adults and children with EoE, with failure rates of 33%, 43%, and 46%, respectively, compared with 88% in untreated controls [16]. These data support a step-up elimination strategy, initiated with the least restrictive diet. Cow’s milk elimination (1FED) is recommended as first-line due to its simplicity, tolerability, and reasonable remission rates. Patients unresponsive to 1FED may escalate sequentially to 2FED, 4FED, and ultimately 6FED if prior regimens are unsuccessful [19]. Based on the available data, an initial elimination of cow’s milk in all forms (1FED) should be trialed first before consideration of multi-FEDs. If the patient has no response to 1FED, then 2FED/4FED should be considered. In cases of elimination diet, dietitians can be very helpful with advice regarding substitutes for favorite foods, a nutrition-balanced diet, and suggestions for recipes. The diet can be started almost immediately following the diagnosis of EoE. Patients typically will need a follow-up endoscopy to assess a histologic response to the elimination diet. If on follow-up endoscopy no improvement in histologic or endoscopic findings is observed, pharmacologic therapy may be initiated, or more strict food elimination or elemental diets may be considered. Possible reasons for elimination diet failure include lack of all food triggers elimination, poor adherence to the diet, or presence of allergies to inhalant allergens. For those patients who had symptomatic and histologic responses, food reintroduction can be initiated. A common approach is reintroduction of food and food groups for 8 to 12 weeks, followed by repeat endoscopy to reassess histologic response. Recurrence of esophageal eosinophilia can occur rapidly within 3 to 7 days after food reintroduction [20]. If the patient becomes symptomatic with food reintroduction and no histologic improvement is found, the food is removed from the diet again. The decision regarding food reintroduction and diet continuation will often depend on the severity of endoscopic and histologic findings. It has been suggested that if reintroduction of a particular food failed, this particular food should be avoided for at least 2 years before another attempt at reintroduction. Once food triggers are identified, the patient can be maintained long-term on an elimination diet. From a clinical standpoint, the most important aspect of diet selection is patient adherence to the diet.
Overview of the food elimination dietary approach.
| Diet | Eliminated food |
|---|---|
| 1FED | Animal milk |
| 2FED | Milk and wheat |
| 4FED | Dairy, wheat, egg, and soy |
| 6FED | Dairy, wheat, egg, soy, nuts, and seafood |
| Elemental formula | Amino acid-based hypoallergenic formula |
FED: food elimination diet.
Elemental diet composed of amino acid-based formulas eliminates all potential dietary allergens and is generally administered for 6–8 weeks [21, 22]. While highly effective, they are rarely employed as first-line therapy due to significant adherence challenges, and histologic relapse is common within two weeks of discontinuation. In a recent randomized trial, adjunctive elemental formula to 4FED for six weeks in 41 adults improved quality of life without a statistically significant enhancement in histologic remission compared with 4FED alone [23].
Testing-directed elimination diets based on skin prick or atopy patch testing have limited clinical utility due to cost and complexity [20]. Current ACG guidelines do not recommend routine allergy testing to guide elimination diets in EoE.
Regarding pharmacologic treatment options for EoE, the recommended regimen includes PPIs, topical steroids (budesonide, fluticasone), and a biologic, dupilumab.
PPIs are the first-line treatment option for patients with EoE and may be combined with elimination diets and topical steroids. The mechanisms of PPI efficacy extend beyond gastric acid suppression to include anti-inflammatory effects, such as downregulation of eotaxin-3, a chemokine central to eosinophil recruitment, enhancement of esophageal barrier integrity, and maintenance of epithelial homeostasis [3]. Interestingly, acid reflux condition may coexist or can contribute to EoE, while EoE may contribute to reflux symptoms by impairing clearance of the acid from the esophagus. Gastroesophageal reflux disease (GERD) may coexist with or exacerbate EoE, whereas EoE can impair esophageal acid clearance, contributing to reflux symptoms. Standard initial therapy consists of daily or twice-daily PPI administration before meals for a minimum of eight weeks, followed by reassessment of clinical symptoms and upper endoscopy to evaluate both endoscopic and histologic response [3]. In case of good response to therapy, PPIs should be continued at the lowest effective dose to control symptoms, whereas non-responders should be considered for topical corticosteroids. Comparative data between PPIs, topical corticosteroids, and elimination diets remain limited. In a randomized trial of 30 adults, esomeprazole 40 mg daily for eight weeks achieved reductions in esophageal eosinophil counts comparable to fluticasone 440 mcg twice daily, without significant improvement in dysphagia [24]. Another study of 42 adults demonstrated that esomeprazole 40 mg daily did not significantly reduce eosinophil counts, though 33% of patients achieved fewer than seven eos/hpf [25]. A meta-analysis encompassing 33 studies and 619 pediatric and adult patients reported clinical and histologic response rates of 61% and 51%, respectively [26]. Similarly, a multicenter observational cohort of 630 patients demonstrated clinical response and histologic remission rates of 71% and 49%, with significantly lower efficacy in patients with esophageal strictures [27]. The presence of GERD does not reliably predict response to PPI therapy, although patients with documented pathologic acid exposure may exhibit enhanced benefit. Across studies, variations in PPI type, dose, frequency, and duration preclude recommendations regarding a preferred agent or regimen. Patient education emphasizing the anti-inflammatory benefits of PPIs and strict adherence is critical to optimize therapeutic outcomes.
Potassium-competitive acid blockers (PCABs), such as vonoprazan, approved for erosive esophagitis and GERD, have been investigated as potential therapies for EoE. In a retrospective cohort of 118 patients, vonoprazan demonstrated efficacy comparable to rabeprazole (10–20 mg) and esomeprazole (20 mg), achieving complete symptomatic response in 75.7% versus 54–72% for PPIs, a 2-point reduction in eosinophilic esophagitis endoscopic reference score (EREFS), and complete histologic remission (0–1 eos/hpf) in 39.4% of patients [28]. A subsequent study of 236 histologically confirmed EoE patients reported no statistically significant differences between PCAB and PPI therapy in symptomatic response (84% vs. 80%), endoscopic response (90% vs. 86%), or histologic response (79% vs. 74%) after eight weeks of treatment [29]. Complete normalization of symptoms, endoscopic findings, and histology was achieved with vonoprazan in 25%, 50%, and 36% of patients, respectively. While preliminary data are promising, further prospective studies are required to confirm the efficacy and safety of PCABs in EoE.
Budesonide and fluticasone represent the mainstay of pharmacologic therapy for EoE. The majority of patients demonstrate both symptomatic improvement and histologic reduction in eosinophil counts with these agents [30]. However, symptom and histologic recurrence are common following treatment discontinuation. Oral budesonide suspension is generally preferred over fluticasone due to its more consistent esophageal drug delivery [31]. Fluticasone, while effective, is used off-label and has limitations related to variable esophageal deposition, higher cost, and availability [32]. Randomized controlled trials have consistently revealed that topical glucocorticoids were effective in achieving clinical and histological improvement in patients with EoE. Most studies excluded patients who responded well to PPI therapy. A large meta-analysis of 6 trials comprising 583 adults and pediatric patients with EoE revealed that topical therapy was associated with significantly greater symptomatic improvement compared to placebo after 2 to 12 weeks of treatment (RR 1.74) [33]. In a sub-analysis of 12 trials encompassing 978 patients, topical glucocorticoid therapy was associated with significant histologic remission (RR 11.94). Reported histologic response rates for both budesonide and fluticasone generally range between 60–70%. Predictors of treatment response to topical therapy remain poorly understood. Individuals presenting with abdominal pain had a good response to topical steroids, while those with esophageal stenosis had a much lower response to the therapy. Current recommendations suggest reassessment with upper endoscopy and biopsy within 8–12 weeks of initiating steroid therapy to evaluate for endoscopic and histologic improvement.
Budesonide has been approved in the United States by the FDA as an oral suspension, consumed 2 mg twice daily for a total of 12 weeks [34]. Medication should be taken over 5 to 10 minutes, with the avoidance of food or liquid intake for 30 minutes. The oral suspension provides consistent drug delivery. In Europe, budesonide is also available as a 1-mg orodispersible tablet administered twice daily [35]. Treatment with budesonide is typically well-tolerated, and symptomatic improvement can be seen within several days. Adverse effects of budesonide include adrenal suppression, headache, candidiasis, throat irritation, and respiratory tract infection. In a randomized controlled trial comprising 318 patients with EoE, oral suspension of budesonide at a dose of 2 mg twice daily was associated with higher rates of symptomatic improvement (52% vs. 39%), and higher rates of histologic remission (53% vs. 1%) after 12 weeks of therapy compared to placebo [36]. Another randomized, placebo-controlled trial of 93 adolescents and young adults with EoE and dysphagia demonstrated significant improvements in dysphagia symptom scores (15 vs. 21.5), endoscopic severity scores (–3.8 vs. 0.4), and histologic response rates (39% vs. 3%) relative to placebo [37]. In the randomized, double-blind, double-dummy clinical trial of 129 adults, comparison was made between oral budesonide suspension (1 mg twice daily) versus swallowed fluticasone provided by metered-dose inhaler (MDI) (880 mcg twice daily) given for 8 weeks. Both agents produced comparable improvements in dysphagia symptom scores (5 vs. 4), endoscopic severity scores (EREFS 2 vs. 3), and histologic remission rates (71% vs. 64%), indicating no clear superiority of one topical steroid over the other [38]. Budesonide suspension preparation off-label can be formulated by compounding pharmacies by prescription in the USA. However, the concentration of budesonide may vary in the suspension, which may affect the effectiveness of the medication [39].
Fluticasone propionate is administered via MDI without a spacer, with the spray swallowed. For adults ≥ 18 years, the recommended induction dose is 220 mcg twice daily for 4–8 weeks. An orally disintegrating tablet formulation is in phase 3 trials. In the phase 2b FLUTE trial of 106 adults, fluticasone tablets significantly reduced dysphagia at 12 weeks versus placebo, with sustained benefit at 52 weeks [40]. Histologic response rates were 86% with 1.5 mg BID and 67% with 3 mg daily dosing [40]. Symptomatic improvement may occur within days to 1 week, but relapse is frequent (14–90%) after discontinuation [41]. In a study of 21 adults, all reported symptom improvement with 220 mcg BID, though this did not correlate with eosinophil counts [42]. Reported adverse effects include esophageal candidiasis, herpes esophagitis, and dry mouth [43]. Although adrenal insufficiency is rare with induction therapy, a meta-analysis reported a 15.8% rate across steroid trials [44]. Patients with a narrow caliber esophagus or requiring dilations demonstrate lower response rates [45]. There are no established guidelines for maintenance therapy, though it is often considered in patients with severe dysphagia, food impactions, high-grade stenosis, or rapid relapse. A lower maintenance dose (often half of induction) is suggested, though higher doses may prolong remission [46]. In a randomized trial of 204 adults in remission, budesonide oral dispersible tablets (0.5–1 mg BID for 48 weeks) maintained remission in 75% compared with 4% on placebo [47]. A smaller study of 28 adults showed budesonide 0.25 mg BID maintained remission in 64% versus 36% with placebo at 50 weeks, with improvement in esophageal remodeling [48]. Meta-analyses report pooled histologic responses of 77% for budesonide and 68% for fluticasone [49, 50]. Both agents are considered reasonable first-line options. Other topical glucocorticoids evaluated in EoE, mainly in pediatrics, include ciclesonide and mometasone furoate [51, 52]. In a retrospective series of 34 children, mometasone induced histologic improvement in 76% and remission in 68% after 1 month [52]. A novel mometasone delivery system (impregnated membrane) is under investigation (NCT04849390).
Systemic glucocorticoids have a limited role in EoE and should be reserved for patients with severe disease when dietary and topical steroid therapies are not feasible or have failed. Due to frequent relapse and chronic disease course requiring repeat therapy, oral steroids should generally be avoided. Prednisone is typically administered at 1–2 mg/kg/day (maximum 60 mg/day) in divided doses. While oral prednisone has demonstrated greater efficacy than topical fluticasone, it carries a significantly higher risk of adverse effects [53]. For refractory cases, dupilumab is now preferred over systemic corticosteroids.
Dupilumab, an IL-4 receptor alpha antagonist, blocks signaling of IL-4 and IL-13 and reduces Th2-mediated inflammation [54]. Initially approved for moderate-to-severe asthma and atopic dermatitis, dupilumab is now approved for both pediatric and adult EoE in the U.S., administered as 300 mg subcutaneously once weekly. In a phase 2 randomized trial of adults with active EoE, 12 weeks of dupilumab therapy led to reductions in dysphagia, histologic activity (68.3% response), improved endoscopic appearance (EREFS), and increased esophageal distensibility [55]. In a phase 3, three-part trial involving 321 PPI-nonresponsive patients (≥ 12 years), dupilumab 300 mg weekly or every 2 weeks for 24 weeks achieved histologic remission (< 6 eos/hpf) in 60% vs. 6% for placebo (P < 0.001) [54]. Weekly dosing produced significant improvement in dysphagia symptom questionnaire (DSQ) score and endoscopic severity (EREFS). The 52-week extension confirmed sustained histologic, symptomatic, and endoscopic benefits, particularly with weekly dosing [56]. In a pediatric trial of 102 patients with PPI-refractory EoE, both lower and higher dupilumab doses for 16 weeks induced histologic remission in 58–68% vs. 3% for placebo [57], with benefits maintained through 36 weeks. A retrospective study of 46 patients with fibrostenotic, refractory EoE showed histologic remission (< 15 eos/hpf) in 80%, < 6 eos/hpf in 57%, and symptomatic improvement in 91% after a median of 6 months [58]. Common adverse effects include injection site reactions, upper respiratory infections, arthralgia, and herpes viral infections; rare cytokine-mediated complications such as seronegative arthritis, iridocyclitis, and psoriasis have been reported. While long-term safety beyond one year remains to be established in EoE, safety data from other atopic conditions are reassuring [59]. A systematic review of five retrospective studies (209 subjects) demonstrated symptom improvement in 89.2% and significant reductions in endoscopic and histologic scores after an average of 5.6 months of therapy [60]. According to ACG guidelines, dupilumab is recommended for PPI-nonresponders and may be best suited for refractory EoE, contraindications to steroid therapy, or patients with concomitant atopic diseases such as asthma or eczema. Although dupilumab is costly, future studies are needed to define its long-term efficacy in preventing fibrosis or strictures and to assess cost-effectiveness as a potential first-line therapy. Table 2 provides an overview of medications currently utilized in the management of EoE in adults.
Medications used for the treatment of EoE in adults.
| Medication | Dosing | Duration |
|---|---|---|
| PPIs | Double dose of omeprazole 20 mg twice daily or 40 mg daily, or another PPI equivalent | 8 weeks |
| Budesonide | 2–4 mg/day divided into twice-a-day dosing | 8 weeks |
| Fluticasone | 1,760 mcg/day in divided doses; typically, twice a day | 8 weeks |
| Dupilumab | 300 mg subcutaneously every week (for a weight of more than 40 kg) | Long-term |
EoE: eosinophilic esophagitis; PPIs: proton pump inhibitors.
A number of newer generation biologics have been under investigation for the treatment of EoE by targeting IL-13 and IL-5 (type II cytokines), interfering with thymic stromal lymphopoietin (TSLP), and the depletion of mast cells [61].
Cendakimab (RPC-4046) is a humanized monoclonal antibody directed against soluble IL-13, thereby attenuating IL-13 receptor-mediated signaling. In a phase 2 randomized controlled trial including 99 adults with EoE, weekly subcutaneous administration of cendakimab at 180 mg or 360 mg demonstrated significant reductions in mean eosinophil counts, histologic remission (< 6 eos/hpf), and improved endoscopic features (EREFS) compared with placebo at week 16. The 360 mg dose additionally improved dysphagia in steroid-refractory patients. The most common adverse events were headache and upper respiratory tract infections [62, 63]. A phase 3 study is currently in progress.
Dectrekumab (QAX576) is a human monoclonal antibody targeting IL-13. In a randomized trial of 23 adults with EoE receiving monthly intravenous infusions of 6 mg/kg, the predefined primary endpoint (> 75% reduction in peak eosinophil count) was not met. However, treatment was associated with a mean 60% reduction in esophageal eosinophil counts compared with a 23% increase in the placebo group, as well as a non-significant improvement in dysphagia severity and frequency [64].
IL-5 is a key eosinophil-specific cytokine produced by Th2 lymphocytes, mast cells, and eosinophils, regulating proliferation, differentiation, and apoptosis. Benralizumab, an anti-IL-5 receptor monoclonal antibody that induces near-complete eosinophil depletion through antibody-dependent cellular cytotoxicity, has been evaluated in phase 2 and 3 clinical studies. In a cohort of 211 adults and children with EoE receiving benralizumab 30 mg subcutaneously every 4 weeks for 24 weeks, histologic remission was achieved in 87.4% compared with 6.5% in the placebo group, although improvements in DSQ score and endoscopic (EREFS) scores were not statistically significant [65, 66].
Mepolizumab and reslizumab are monoclonal antibodies targeting soluble IL-5, thereby inhibiting eosinophil maturation and activation. In adults with chronic EoE and fibrostenotic disease, monthly intravenous mepolizumab (750 mg) led to significant reductions in eosinophil counts but did not achieve complete histologic remission (< 5 eos/hpf) [67, 68]. Subcutaneous mepolizumab similarly reduced tissue eosinophilia without corresponding improvement in symptom scores [69]. Across studies, anti-IL-5 agents have consistently demonstrated histologic responses superior to placebo but with limited symptomatic benefit [66, 70, 71].
TSLP is an epithelial-derived cytokine that initiates and amplifies type 2 inflammation via dendritic and mast cell activation. Tezepelumab, a human monoclonal antibody inhibiting TSLP, is being evaluated in the phase 3 randomized, double-blind, placebo-controlled CROSSING trial assessing efficacy and safety in adults and adolescents with EoE over 52 weeks [72]. Tezepelumab is currently approved for severe asthma. Solrikitug, a next-generation anti-TSLP antibody that prevents TSLP-receptor binding with higher potency than tezepelumab, is being studied in the phase 2 ALAMARE trial—a 24-week study with a 28-week extension evaluating histologic and symptomatic responses across three dosing regimens [73].
Barzolvolimab is a mast cell–depleting anti-tyrosine protein kinase (KIT) monoclonal antibody under investigation in the phase 2 “EvolvE” trial. In this randomized, double-blind, placebo-controlled study, 151 adults with EoE received barzolvolimab 300 mg every 4 weeks for 8 weeks. The primary endpoint—a reduction in peak esophageal mast cell count—was achieved, with concomitant decreases in eosinophil infiltration and improvement in DSQ score, suggesting potential therapeutic efficacy [74].
CALY-002, an anti-IL-15 monoclonal antibody, is being investigated in patients with EoE and celiac disease. IL-15, expressed by dendritic cells, macrophages, and fibroblasts, is upregulated in basal epithelial layers in EoE. The ongoing trial (ClinicalTrials.gov Identifier: NCT04593251) aims to evaluate safety, tolerability, pharmacokinetics, and pharmacodynamics in patients with EoE [75].
Lirentelimab (AK002) is a humanized IgG1 monoclonal antibody targeting sialic acid-binding Ig-like lectin 8 (SIGLEC-8), a receptor selectively expressed on mast cells and eosinophils. The antibody inhibits mast cell activation and induces eosinophil apoptosis through antibody-dependent cytotoxicity. In the KRYPTOS trial, both high- and low-dose lirentelimab achieved complete histologic remission in 88% and 92% of patients, respectively, compared with 11% in the placebo group; however, the primary endpoint of symptomatic improvement (by DSQ) was not met [76]. A meta-analysis of 17 randomized trials, including topical steroids, PPIs, dupilumab, and lirentelimab, demonstrated that monthly lirentelimab (1 mg/kg) achieved the highest rates of histologic remission, though without consistent endoscopic or symptomatic improvement [77].
The prostaglandin D2 receptor antagonist OC000459, evaluated in a randomized controlled trial of 26 adults with steroid-dependent or steroid-refractory EoE, resulted in significant reductions in eosinophil counts and symptomatic improvement over 8 weeks compared with baseline [78]. A meta-analysis of six randomized controlled trials (533 patients) demonstrated that monoclonal antibodies significantly reduced both peak and mean esophageal eosinophil counts compared with placebo. Agents targeting IL-13 (dupilumab, dectrekumab, cendakimab) were associated with the most consistent improvements in histologic (EoE-HSS), endoscopic (EREFS), and DSQ score. The incidence of serious adverse events was comparable to placebo across studies [79]. Several investigational therapies targeting key inflammatory pathways are under evaluation in phase 2 and 3 clinical trials, as outlined in Table 3.
Investigation of drugs in phase 2 or 3 for the treatment of EoE.
| Drug | Targets |
|---|---|
| Cendakimab (RPC-4046) | IL-13 |
| Dectrekumab (QAX576) | IL-13 |
| Benralizumab | IL-5 |
| Reslizumab | IL-5 |
| Mepolizumab | IL-5 |
| CALY-002 | IL-15 |
| Lirentelimab | SIGLEC-8 |
| Tezepelumab | TSLP |
| Solrikitug | TSLP |
| Barzolvolimab | KIT |
KIT: tyrosine protein kinase; TSLP: thymic stromal lymphopoietin; SIGLEC-8: sialic acid-binding Ig-like lectin 8.
Montelukast, a leukotriene receptor antagonist, has been evaluated in EoE with inconsistent outcomes, and its therapeutic role remains uncertain [80, 81]. Omalizumab, an anti-IgE monoclonal antibody, did not demonstrate meaningful histologic or clinical improvement, with a response observed in only 33% of patients, further supporting that EoE is not primarily an IgE-mediated disorder [82, 83]. Likewise, infliximab, an anti-TNFα IgG1 antibody, failed to show a significant benefit in EoE [84]. Accordingly, recent ACG guidelines do not recommend the use of these agents in EoE management [3].
There are no standardized recommendations for long-term monitoring in EoE. Follow-up endoscopic evaluation should be individualized based on symptom trajectory, esophageal pathology, need for therapeutic adjustment, and patient preference. Endoscopy is particularly warranted in patients with worsening dysphagia, therapeutic modification, or when dilation is anticipated. The reliance on serial endoscopic assessments remains a major limitation in EoE care. Although several noninvasive biomarkers have been investigated for diagnostic and monitoring purposes, heterogeneity among candidate markers and methodologies limits their clinical utility [85]. Endoscopy with biopsy remains the gold standard for both diagnosis and surveillance. Nonetheless, the development of validated noninvasive biomarkers could substantially reduce procedural burden and improve cost-effectiveness in the future.
Disease progression may lead to a fibrostenotic phenotype, characterized by persistent dysphagia and the need for repeated dilations. Esophageal dilation is typically reserved for patients refractory to medical therapy or those with high-grade strictures. Given the increased risk of deep mucosal tears or perforation, dilation should be performed gradually, with luminal expansion not exceeding 3 mm per session [86, 87]. Earlier reports suggested a perforation rate of 5–7%, whereas more recent evidence demonstrates a markedly lower risk. In a meta-analysis of 37 studies encompassing 977 patients and 2,034 dilations, the perforation rate was only 0.03%, with hospitalization required in 0.7% of cases [88, 89].
A stepwise approach to the management of EoE in adults is outlined in Figure 1.
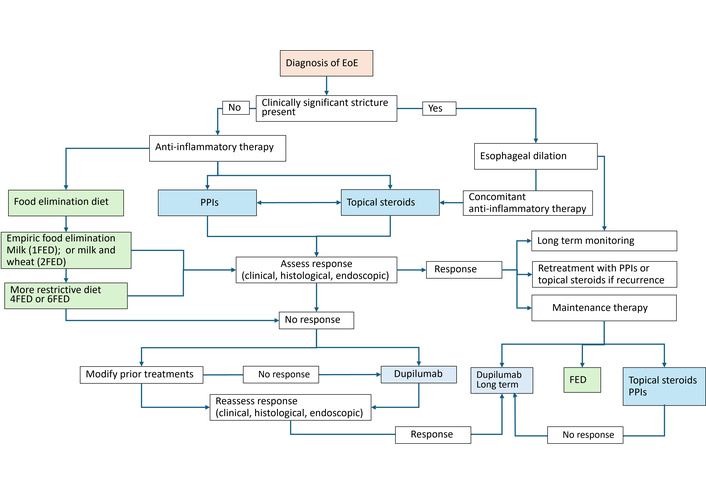
Proposed management algorithm for EoE in adults. PPIs: proton pump inhibitors; FED: food elimination diet.
EoE is a chronic immune-mediated esophageal disorder that often requires long-term maintenance therapy to prevent relapse and complications. Dietary therapy remains a key therapeutic option, though adherence in adults is limited by complexity and social constraints. A step-up elimination approach is recommended, starting with the least restrictive 1FED or 2FED, progressing to 4FED or 6FED if necessary. Treatment response should be assessed by endoscopic and histologic improvement, rather than symptoms alone, given the poor correlation between symptom severity and histologic activity. Collaboration with a dietitian is strongly advised to enhance adherence and ensure nutritional adequacy. High-dose PPI therapy is recommended as initial management, with appropriate counseling to clarify its anti-inflammatory mechanism in EoE rather than solely acid suppression. For patients with persistent disease, topical corticosteroids are considered first-line pharmacologic therapy, either as monotherapy or in combination with PPIs. Oral viscous budesonide is generally preferred over fluticasone due to more consistent mucosal contact, although both agents are comparably effective. Some patients may benefit from as-needed use during flares, while those with severe or recurrent disease often require long-term maintenance therapy to prevent relapse. Therapeutic decisions should be individualized based on disease severity, symptom burden, histologic findings, and patient preference. For patients who are refractory or intolerant to PPIs and topical glucocorticoids, dupilumab represents an effective next-line therapy. Current data primarily involve patients with longstanding or treatment-resistant EoE, and its role in newly diagnosed disease remains undefined. Identifying clinical or biomarker predictors of dupilumab response could enable more personalized treatment and reduce reliance on invasive monitoring. Patients with concomitant atopic diseases such as severe asthma or eczema may particularly benefit from dupilumab due to its dual therapeutic effects. For those with fibrostenotic disease, esophageal dilation remains an important adjunctive therapy. Dilation may be performed concurrently with or following anti-inflammatory treatment, depending on the severity of symptoms and degree of luminal narrowing. In patients with critical strictures, dilation should precede anti-inflammatory therapy to restore luminal patency. The approach should always be individualized, balancing efficacy, safety, and patient tolerance. Given the chronic relapsing nature of EoE, recurrence of histologic and symptomatic disease is common upon discontinuation of therapy, underscoring the need for maintenance treatment and periodic reassessment. Follow-up should include clinical, endoscopic, and histologic evaluation, as symptom improvement alone does not reliably indicate disease remission. Even asymptomatic patients warrant long-term follow-up for potential recurrence and therapy adjustment. The economic burden of EoE is substantial, with estimated annual healthcare costs exceeding $1 billion in the United States [90]. Biologic therapies, while effective, significantly increase treatment costs. Therefore, adopting guideline-based, stepwise management algorithms and optimizing the use of existing therapies remain critical for cost-effective care. Future studies are needed to define the optimal duration of maintenance therapy, the role of biologics in earlier disease stages, and the cost-effectiveness of emerging treatment strategies.
Substantial progress has been achieved in recent years in elucidating the pathogenesis and optimizing the therapeutic approach to EoE. The recent approval of the first biologic agent represents a significant milestone, providing new therapeutic opportunities and prompting the refinement of contemporary management algorithms. Evidence-based clinical guidelines now support a systematic, stepwise treatment strategy favoring initiation with a single therapeutic modality rather than combination therapy, followed by objective assessment of treatment response through endoscopy with biopsy. Therapeutic escalation should be pursued in patients demonstrating persistent histologic or clinical activity despite initial intervention. The role of biologic therapy in the long-term management of EoE remains to be fully defined. Currently, dupilumab is recommended for patients refractory to PPIs and topical corticosteroids, as well as those with concomitant atopic disease. While highly efficacious, its substantial cost underscores the need for careful patient selection and cost-effectiveness analyses. Future research should focus on delineating predictors of treatment response, establishing noninvasive biomarkers for disease monitoring, and defining the optimal duration and sequencing of biologic therapy. Despite these ongoing challenges, the therapeutic landscape of EoE is rapidly evolving. Emerging targeted therapies hold considerable promise to improve disease control, reduce treatment burden, and ultimately transform the long-term management of EoE.
ACG: American College of Gastroenterology
DSQ: dysphagia symptom questionnaire
EoE: eosinophilic esophagitis
eos/hpf: eosinophils per high-power field
EREFS: eosinophilic esophagitis endoscopic reference score
FED: food elimination diet
GERD: gastroesophageal reflux disease
KIT: tyrosine protein kinase
MDI: metered-dose inhaler
PCABs: potassium-competitive acid blockers
PPI: proton pump inhibitor
SIGLEC-8: sialic acid-binding Ig-like lectin 8
TSLP: thymic stromal lymphopoietin
AW: Conceptualization, Writing—original draft, Writing—review & editing. AU: Conceptualization, Writing—original draft, Writing—review & editing, Supervision. Both authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 4121
Download: 33
Times Cited: 0
