Affiliation:
1Greenberg Division of Cardiology, Weill Cornell Medicine, New York, NY 10021, USA
2Department of Cardiology, Frederiksberg and Bispebjerg Hospital, 2200 Copenhagen, Denmark
Affiliation:
3Department of Cardiology, Ullevaal Hospital, University of Oslo, 0407 Oslo, Norway
4Division of Cardiovascular Medicine, University of Michigan, Ann Arbor, MI 48109, USA
ORCID: https://orcid.org/0000-0003-2389-0272
Affiliation:
1Greenberg Division of Cardiology, Weill Cornell Medicine, New York, NY 10021, USA
ORCID: https://orcid.org/0000-0001-5861-1478
Affiliation:
1Greenberg Division of Cardiology, Weill Cornell Medicine, New York, NY 10021, USA
Email: pokin@med.cornell.edu
Explor Med. 2022;3:149–159 DOI: https://doi.org/10.37349/emed.2022.00081
Received: December 16, 2021 Accepted: February 17, 2022 Published: March 29, 2022
Academic Editor: Kathleen G. Morgan, Boston University School of Medicine, USA
The article belongs to the special issue Angiotensins—A Century of Progress
Aim: Whether incident left bundle branch block (LBBB) is associated with increased cardiovascular (CV) morbidity and mortality in treated hypertensive patients with left ventricular hypertrophy (LVH) is unknown. Thus, the present study aimed to examine CV outcomes of incident LBBB in treated hypertensive patients with LVH.
Methods: In the Losartan Intervention For Endpoint reduction in hypertension (LIFE) study, 9,193 hypertensive patients with LVH on screening electrocardiogram (ECG) were randomized to losartan or atenolol based treatment. Participants (n = 8,567) did not have LBBB (Minnesota code 7.1) on baseline ECG. Cox regression models controlling for significant covariates assessed independent associations of incident LBBB with CV events and all-cause mortality during 4.8 years mean follow-up.
Results: Annual follow-up ECGs identified 295 patients (3.4%) with incident LBBB associated with male gender (P < 0.05), older age, higher Cornell voltage (both P < 0.005) and history of diabetes, isolated systolic hypertension and prevalent CV disease. When adjusted for the history of previous CV disease, diabetes, isolated systolic hypertension, the Framingham risk score, ECG-LVH and randomized study treatment, Cox regression models showed that incident LBBB predicted higher risk of the composite endpoint CV death, myocardial infarction and stroke [hazard ratio (HR) 1.9, 95% confidence intervals (CIs) 1.3–2.9, P < 0.001], CV death (HR 3.0, 95% CIs 1.84–5.0, P < 0.001), heart failure (HR 3.6, 95% CIs 1.9–6.6, P < 0.001) and all-cause mortality (HR 3.0, 95% CIs 2.0–4.3, P < 0.001).
Conclusions: These data suggest that among hypertensive patients with ECG-LVH receiving aggressive antihypertensive therapy, incident LBBB independently predicts increased risk of subsequent CV events including congestive heart failure and CV and all-cause mortality (ClinicalTrials.gov identifier: NCT00338260).
Left bundle branch block (LBBB) documented by electrocardiography (ECG) is associated with abnormal left ventricular geometry and left ventricular hypertrophy (LVH), left ventricular dysfunction in otherwise normal individuals, coronary artery disease and ECG-LVH [1–5]. Prevalent LBBB predicts higher risk of cardiovascular (CV) events, increased CV mortality and morbidity in patients with congestive heart failure [6], dilated cardiomyopathy [7], and in hypertensive patients with ECG-LVH [8]. Whether incident LBBB will predict higher CV morbidity and mortality in patients with hypertension and ECG-LVH during aggressive antihypertensive therapy has, however, never to date been investigated.
The Losartan Intervention For Endpoint reduction in hypertension (LIFE) study was a double-blind, prospective parallel group study designed to compare the effects of losartan vs. atenolol based antihypertensive treatment in reducing the rate of CV morbidity and mortality in 9,193 hypertensive patients with ECG-LVH [9]. The current study aimed to investigate the risk of CV mortality and morbidity in LIFE study participants who developed incident LBBB detected on yearly protocol ECGs during the course of the LIFE study.
The LIFE study design, inclusion and exclusion criteria, baseline characteristics, and the main results of LIFE study have been previously published [9–11]. Participants in the LIFE study were men and women between 55 and 80 years of age at baseline with previously untreated or treated essential hypertension and ECG-LVH. All patients had initial sitting diastolic blood pressure 95 to 115 mmHg and/or systolic blood pressure 160 to 200 mmHg after 1–2 weeks of single-blind placebo treatment. Blood pressure and heart rate were measured with standardized techniques at trough with subjects quietly seated for 5 minutes. All participants were asked about alcohol intake, smoking habits, exercise level and employment history. Weight and height were measured. Past medical history was taken and a physical examination was performed to detect concomitant disease. Laboratory tests including hemoglobin, serum sodium, potassium, creatinine, uric acid, total high-density lipoprotein (HDL) cholesterol, and glucose levels were performed in central laboratories [10].
Participants in LIFE were randomly assigned to losartan or atenolol based regimens and were followed for a mean of 4.8 years for the occurrence of a primary composite endpoint of CV death, stroke or myocardial infarction [11].
The current analyses included 8,567 LIFE participants with no evidence of LBBB on baseline ECG, of whom 295 subsequently developed LBBB on yearly follow-up ECGs. These 295 LIFE participants are the case population and those without LBBB on baseline or follow-up ECGs are the control population of the current analyses.
In LIFE, all screening and in-study ECGs had a paper speed of 50 mm/s and were read at a central laboratory at Sahlgrenska University Hospital, Ōstra, Gothenburg, Sweden for LVH criteria and Minnesota coding. Studies ECGs were performed at baseline, 6 months and then yearly throughout. ECG diagnosis of LVH was defined by Cornell voltage-duration product (+ 6 mm in women) > 2,440 mm × ms or Sokolow–Lyon voltage > 38 mm criteria [11–13]. LBBB was defined by ECG criteria (Minnesota code 7.1) as follows: QRS duration of at least 0.12 s in the presence of sinus or supraventricular rhythm, QS or rS complex in lead V1 and R-wave peak time of at least 0.06 s in leads I, V5 or V6 associated with the absence of a Q-wave in the same lead [8]. Incident LBBB was diagnosed by these criteria in LIFE study follow-up ECGs.
The primary CV mortality and morbidity endpoint of the LIFE study consisted of the first occurrence of CV death, stroke or myocardial infarction. Other pre-specified outcomes included components of the primary endpoint (CV death, stroke or myocardial infarction), all-cause mortality, angina pectoris and heart failure requiring hospitalization [14]. An expert endpoint committee blinded to ECG results and to treatment allocation adjudicated the endpoints [9, 11].
Analyses of CV endpoints were based on the intention-to-treat principle, consistent with other analyses in the LIFE study. Participants who experienced more than one endpoint were counted as having had an event in all relevant endpoint analyses; however, only the first event in a specific category was counted in individual analyses [11]. Dichotomized groups of patients with and without new-onset LBBB detected by follow-up ECG examinations were created. Data are presented as mean ± standard deviation (SD) for continuous variables or as proportions for categorical variables. Mean values were compared between groups by independent sample t-tests and multivariate general linear models adjusted for significant covariates. Proportions were compared by x2-tests. Logistic regression was used to calculate odds ratios after adjustment for significant covariates. To investigate incident LBBB as an independent predictor of outcome, we performed univariate and multivariate Cox proportional hazard models with LBBB treated as a delayed entry covariate and adjusted for significant covariates including history of diabetes, isolated systolic hypertension and CV disease on baseline evaluation, randomized treatment, baseline Framingham risk score (gender, total and high-density lipoprotein cholesterol, smoking status, presence of diabetes and LVH, and systolic blood pressure [13]), and degree of LVH on follow-up ECGs and systolic and diastolic blood pressures obtained at annual study visits entered as time-varying variables. Including both Framingham risk score and presence of diabetes may possibly have led to minor over-adjustment although not with visible differences in outcomes [11, 14]. To test if the results were sensitive to differences in all-cause mortality, endpoints independently associated with LBBB were tested in competing risk regression as suggested by Fine and Gray with death as a competing event [15]. Differences in risk of endpoints in patients with and without LBBB, respectively, are shown by plots of cumulative incidence of the endpoints with death as a competing event (with the use of the Fine and Gray method). The effects of new-onset LBBB were measured by hazard ratios (HRs) and 95% confidence intervals (CIs) from these Cox regression models.
Finally, we made a propensity score-matched Cox proportional-hazard analysis. We quantified a propensity score for the likelihood of having LBBB by multivariate Cox regression analysis conditional on all former variables inserted in the multivariable Cox analysis. Using the Greedy matching macro (https://support.sas.com/resources/papers/proceedings/proceedings/sugi26/p214-26.pdf), we matched each case to one control on the basis of the propensity score.
For all tests, two-tailed P < 0.05 was considered statistically significant. Data were analyzed with SAS statistical software package version 9.2 for PC (SAS Institute Inc., Cary, NC, USA).
The current analyses of 8,567 hypertensive LIFE study participants with ECG-LVH without LBBB on baseline ECG identified incident LBBB in 295 patients [3.4%, including 143 (48%) women] during 4.8 years mean follow-up. The cases of incident LBBB appeared linearly with time, or in other words, there was a smooth rise in risk of both cases with LBBB and CV events with time as illustrated in Figure 1. Of patients who developed LBBB, 25.8% and 53.7% had prolonged QRS > 0.12 s or > 0.11 s, respectively, but no other block features in their ECG at baseline. This was opposed to 2.3% who had normal QRS and developed a full LBBB block picture out of a previously normal QRS picture.
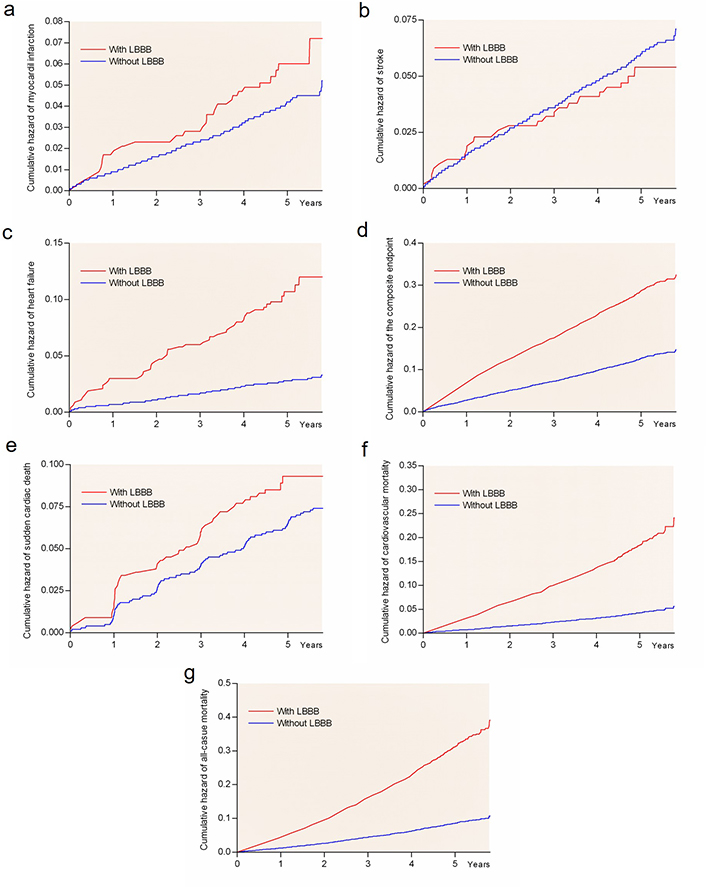
Kaplan–Meier plots of CV complications in patients. Rate of myocardial infarction (panel a), stroke (panel b), heart failure (panel c), the composite endpoint (panel d), sudden cardiac death (panel e), CV death (panel f), and all-cause mortality (panel g) by LBBB group
Demographic and clinical characteristic data of study participants with and without new-onset LBBB are included in Table 1. Participants who developed new-onset LBBB were older (P < 0.001) and more likely to be male (P < 0.05). After adjustment for age and gender, patients with new-onset LBBB had higher baseline body weight and body mass index and lower systolic and diastolic blood pressures (all P < 0.05), but did not differ significantly from those without incident LBBB in height, pulse pressure, heart rate, serum creatinine, glucose concentration, or Framingham risk score (all P > 0.05). After adjustment for age and gender, patients with incident LBBB had greater baseline LVH measured by sex-adjusted Cornell product and Cornell voltage (both P < 0.001) but not by Sokolow–Lyon voltage.
Baseline demographics, clinical characteristics, ECG findings and randomized antihypertensive treatment in patients without and with incident LBBB
| Clinical characteristic | No new LBBB (n = 8,271) | Incident LBBB (n = 295) | P | Adjusted P |
|---|---|---|---|---|
| Female gender (%) | 53.6 | 46.8 | 0.025 | n/a |
| Age, years | 67 ± 7 | 69 ± 6 | < 0.001 | n/a |
| Race, % white | 92.2 | 93.9 | 0.32 | 0.63 |
| Height (cm) | 167.6 ± 9.4 | 168.5 ± 9.7 | 0.152 | 0.152 |
| Weight (kg) | 78.9 ± 14.9 | 80.9 ± 15.3 | 0.071 | < 0.005 |
| Body mass index, kg/m2 | 28.0 ± 4.8 | 28.4 ± 4.9 | 0.12 | < 0.05 |
| Obesity (> 35 kg/m2), % | 0.50 | 0.64 | 0.18 | 0.14 |
| Systolic BP, mmHg | 175 ± 14 | 173 ± 14 | 0.205 | < 0.005 |
| Diastolic BP, mmHg | 98 ± 9 | 96 ± 9 | < 0.001 | < 0.01 |
| Pulse pressure, mmHg | 77 ± 16 | 78 ± 15 | 0.237 | 0.190 |
| Heart rate, beats/min | 70 ± 13 | 71 ± 13 | 0.043 | 0.164 |
| Serum creatinine, μmol/L | 86.7 ± 20.2 | 89.4 ± 19.1 | < 0.05 | 0.519 |
| Serum glucose, mmol/L | 6.01 ± 2.18 | 6.27 ± 2.22 | 0.053 | 0.106 |
| Framingham risk score | 22.3 ± 9.4 | 25.1 ± 9.6 | < 0.001 | 0.150 |
| Sokolow–Lyon voltage, mm | 30.0 ± 10.5 | 30.8 ± 10.7 | 0.17 | 0.717 |
| **Cornell product, mm × ms | 2,654 ± 764 | 3,530 ± 1,225 | < 0.001 | < 0.001 |
| Cornell voltage, mm | 26.5 ± 7.5 | 34.7 ± 12.2 | < 0.001 | < 0.001 |
| QRS duration, ms | 97.2 | 113.7 | < 0.001 | < 0.001 |
| Randomized to losartan, (%) | 50 | 50 | 0.26 | 0.26 |
* Adjusted for sex and age; ** sex-adjusted; BP: blood pressure; n/a: not applicable
In medical history taken at baseline, participants with incident LBBB had higher prevalence of isolated systolic hypertension (P < 0.05), pre-existing CV disease (P < 0.005) and diabetes (P < 0.05) after adjustment for age and gender (Table 2), but did not differ from those without incident LBBB in prevalence of smoking, atrial fibrillation or peripheral arterial disease (all P > 0.05).
Medical history of patients without and with incident LBBB
| Medical history item | No new LBBB (n = 8,271) | Incident LBBB (n = 295) | P | Adjusted P |
|---|---|---|---|---|
| Current smokers, % | 16.3 | 18.6 | 0.30 | 0.115 |
| Isolated systolic hypertension, n (%) | 1,161 (14.1) | 53 (20.1) | < 0.01 | < 0.05 |
| **CV disease, n (%) | 1,985 (24.2) | 106 (35.6) | < 0.001 | < 0.005 |
| Coronary disease, n (%) | 1,323 (16) | 74 (25) | - | - |
| Cerebrovascular disease, n (%) | 662 (8) | 32 (11) | - | - |
| Atrial fibrillation, n (%) | 295 (3.6) | 13 (4.4) | 0.425 | 0.814 |
| Diabetes, n (%) | 1,071 (12.9) | 53 (18.0) | < 0.05 | < 0.05 |
| Peripheral vascular disease, n (%) | 466 (5.6) | 22 (7.5) | 0.199 | 0.45 |
* Adjusted for sex and age; ** Self-reported; -: P-values not calculated for components of self-reported CV diseases at baseline
Univariate Cox regression models showed that incident LBBB as a delayed-entry covariate was associated with 47% higher risk rate of developing the primary composite endpoint, and with 68 to > 395% higher risks of myocardial infarction, heart failure requiring hospitalization, CV mortality and all-cause mortality during subsequent follow-up (Table 3). These findings were confirmed in univariate competing risk calculations (all P < 0.001). Differences in risk of all endpoints in groups of LBBB are included in Figure 1, with all-cause mortality as the competing event for all endpoints. However, the univariate Cox models did not detect associations of new-onset LBBB with higher risk of developing stroke or sudden cardiac death (Table 3 and Figure 1, panels b and e).
Association of incident LBBB with clinical endpoints in univariate Cox regression models
| Endpoint | No new LBBB, n (%) | Incident LBBB, n (%) | HR (95% CIs) | P |
|---|---|---|---|---|
| Primary composite endpoint | 988 (25.8) | 28 (19.7) | 2.68 (1.83–3.92) | < 0.001 |
| Stroke | 502 (12.9) | 6 (4.0) | 1.17 (0.52–2.64) | 0.700 |
| Myocardial infarction | 360 (9.2) | 5 (3.3) | 1.25 (0.51–3.04) | 0.624 |
| CV mortality | 364 (9.0) | 21 (13.2) | 4.33 (2.76–6.80) | < 0.001 |
| Sudden cardiac death | 564 (14.6) | 7 (4.8) | 3.07 (0.96–2.08) | 0.080 |
| Hospitalization for heart failure | 264 (6.7) | 12 (8.4) | 4.95 (2.74–8.94) | < 0.001 |
| All-cause mortality | 705 (17.7) | 34 (21.6) | 3.40 (2.40–4.80) | < 0.001 |
Because of the differences between patients with and without incident LBBB with respect to demographic and clinical variables that could affect outcomes (Tables 1 and 2), multivariate Cox regression models were performed considering history of diabetes, isolated systolic hypertension, CV disease, Framingham risk score and randomized treatment as fixed covariates and LVH defined by Cornell ECG voltage and systolic and diastolic blood pressures as time-varying covariates. Cornell product was not included as a covariate because it includes QRS duration in its calculation. Incident LBBB was in these adjusted Cox regression models associated with a 1.9-fold (95% CIs 1.3–2.9) increased risk of the composite endpoint of the first occurrence of CV death, myocardial infarction or stroke, with a 3.0-fold (95% CIs 1.8–5.0) increased risk rate of CV death, with a 3.6-fold higher risk (95% CIs 1.9–6.6) of developing heart failure that required hospitalization, and with a 3.0-fold (95% CIs 2.0–4.3, all P < 0.001) increased risk of all-cause mortality (Table 4). In contrast with these associations with hospitalized heart failure and end-points that included death, incident LBBB was not independently related to the subsequent occurrence of non-fatal myocardial infarction, stroke or sudden cardiac death (adjusted P > 0.80). No interaction was found between randomized allocation and any of the endpoints (all P > 0.447).
Association of incident LBBB with clinical endpoints in multivariate Cox regression models
| Endpoint | No new LBBB, n (%) | Incident LBBB, n (%) | HR (95% CIs) | P |
|---|---|---|---|---|
| Primary composite endpoint | 988 (25.8) | 28 (19.7) | 1.91 (1.26–2.88) | < 0.001 |
| Stroke | 502 (12.9) | 6 (4.0) | 0. 66 (0.36–1.20) | 0.655 |
| Myocardial infarction | 360 (9.2) | 5 (3.3) | 1.06 (0.43–2.62) | 0.893 |
| CV mortality | 364 (9.0) | 21 (13.2) | 3.04 (1.83–5.05) | < 0.001 |
| Sudden cardiac death | 564 (14.6) | 7 (4.8) | 1.26 (0.82–1.93) | 0.294 |
| Hospitalization for heart failure | 264 (6.7) | 12 (8.4) | 3.55 (1.90–6.64) | < 0.001 |
| All-cause mortality | 705 (17.7) | 34 (21.6) | 2.97 (2.04–4.33) | < 0.001 |
Adjusted for diabetes, isolated systolic hypertension, prevalent CV disease, Framingham risk score, randomized treatment assignments and degree of ECG-LVH at baseline as fixed covariates, and incident LBBB, ECG-LVH by Cornell voltage and diastolic and systolic blood pressures obtained on LIFE study follow-up examinations as time-varying covariates. All-cause mortality was considered as a competing end-point for outcomes including non-fatal events
Finally, we performed propensity score Cox analysis matched for the same variables used for the multivariable models. The matching resulted in a well-matched cohort of 850 patients, 425 in the LBBB group and 425 in the control group. LBBB was still independently associated with a 1.59-fold (95% CIs 1.0–2.4, P = 0.035) increased risk of all-cause mortality during follow-up.
Our study is the first to demonstrate that incident LBBB in patients with hypertension and ECG-LVH receiving aggressive antihypertensive therapy increases the risk of heart failure, CV and all-cause mortality.
The ECG sign of LBBB has been reported to be associated with different clinical features and prognosis, depending on characteristics of various study populations. Studies in cardiac outpatient clinics and hospital populations have indicated that LBBB is frequently associated with hypertension and clinically overt ischemic heart disease [16–23]. In a series of 555 consecutive hospitalized patients with LBBB, 77% had clinical coronary heart disease, hypertension or both [16]. Cardiac enlargement seen on chest X-ray or at autopsy was frequently associated with LBBB in hospital populations [22, 23]. In clinic and hospital settings, newly acquired LBBB was associated with either progressive congestive heart failure or an acute ischemic event in 80% [20]. However, in a study of young, asymptomatic and relatively healthy U.S. Air Force cadets, LBBB was not associated with demonstrable evidence of CV abnormalities [24]. In young military populations incident LBBB was frequently found in young people without clinical or angiographic evidence of CV disease [25].
These widely divergent findings may reflect differing characteristics of the various populations from which the LBBB cases were drawn. Populations from hospitals and clinics are older, with symptomatic diseases such as hypertension, diabetes and coronary artery disease; thus, the associations of LBBB with CV diseases are amplified in these study populations. Military populations are relatively young, healthier than general population and without clinically evident systemic disease or predisposing factors such as hypertension or diabetes, which both have shown to be associated with LBBB. Therefore, separate mechanisms are responsible for LBBB in different study populations; and conclusions of studies in highly selected populations should not be used to make assumptions concerning the clinical significance of LBBB in broader populations [26]. Our study indicates that, in patients with hypertension and ECG-LVH receiving aggressive antihypertensive treatments, those developing incident LBBB are at significantly higher risks of CV events including heart failure requiring hospitalization as well as of CV and all-cause mortality. These findings are consistent with findings from other studies. In hospital and clinic populations, newly acquired LBBB was associated with progressive congestive heart failure and acute ischemic events [19]. In the Framingham study, new-onset LBBB was associated with coronary heart disease (P < 0.05), congestive heart failure (P < 0.001), cardiac enlargement by chest roentgenogram (P < 0.005) and diabetes (P < 0.005) diagnosed coincident with or after the onset of LBBB [26]. In a random-sampled population of 855 men aged 50 years at initial screening, Eriksson et al. [27] found that left or right bundle branch block was associated with significantly higher risk of developing heart failure in those surviving to age 67 years (36% vs. 14%, P < 0.01) and to age 80 years (17% vs. 5%, P < 0.05).
However, in the present study LBBB was not associated with myocardial infarction, stroke or sudden cardiac death. The patients in the LIFE study were a selected group of patients with hypertension and LVH at inclusion, and the incident LBBB may merely reflect LVH instead of primary degenerative disease of the conduction system, which could explain the lack of association with sudden cardiac death. Another explanation could be that too few myocardial infarctions, strokes and sudden cardiac deaths occurred during follow-up.
A higher percentage incidence of primary outcomes in patients without LBBB compared to those who develop LBBB may look like a contradiction; however, the primary endpoint was a composite of myocardial infarction, stroke and CV death. The number of patients in the incident LBBB groups with myocardial infarction and stroke was very small and thus the primary study endpoint was lower in the LBBB group. Overall this may be a chance finding or possibly a true finding if incidences of the two endpoints truly are unrelated to incident LBBB.
Our study included adjustment for randomized treatment, losartan vs. atenolol, though in fact there was no imbalance in randomized study drug in the two groups (50%). Regarding add-on medication, in particular hydrochlorothiazide and calcium-antagonists, hydrochlorothiazide was given to about 90% of patients and about 80% were on thiazide at any time during follow-up with no difference between the study arms. In as much as randomized study medication showed no differences between the two groups in the present study, we assume the similar for add-on medication and that mg dosage follows the same pattern as in the main LIFE study [11] and averaged 82 and 79 mg in the arms, respectively.
In a separate study of the LIFE population, Okin et al. [28] found that persistence or development of a prolonged QRS during antihypertensive therapy (≥ 110 ms) was associated with an increased risk of developing new-onset heart failure, independent of treatment modality, BP lowering, incident myocardial infarction, the predictive value of other heart failure risk factors and regression of ECG-LVH. This finding is consistent with that in the current study because hypertensive patients with incident LBBB all have new-onset prolongation of QRS duration to at least 0.12 s during follow-up.
Our analyses also indicate that, with prognostic covariates taken into account, incident LBBB is associated with higher risks of CV and all-cause mortality, but not of sudden CV death or stroke during follow-up in hypertensive participants in the LIFE study. The present demonstration of adverse prognostic implications of incident LBBB is consistent with previous analyses showing that the 564 LIFE participants with LBBB detected by baseline ECGs had significantly higher risks of developing CV mortality, sudden CV death and hospitalization for heart failure during mean follow-up of 4.8 years in the LIFE study [8].
However, differences in BP between the two study arms did not explain our findings in as much as statistical multivariate adjustment included BP as a continuous variable. Thus, all BP measurements at study visits were included and these adjustments then include changes in BP and achieved BP in the participating patients. However, we cannot from our data conclude that more aggressive treatment of BP would have further lowered incident LBBB and the CV complications.
The present data may suggest that incident LBBB comes with higher risk than baseline LBBB [8]. The data then suggest that a more dynamic complication such as incident LBBB carries more risk than a more stationary prevalent condition. A similar situation was present in another hypertension outcome trial when some of us compared 4,634 patients with type-2 diabetes and 7,874 patients who never developed diabetes with a group of patients with incident type-2 diabetes (n = 1,252) in a pre-specified protocol [29]. Patients with incident type-2 diabetes developed more incident and consistent atrial fibrillation compared to patients with baseline type-2 diabetes and hypertensive patients without diabetes during the follow-up time, which had about the same length as in the LIFE study [29].
In conclusion, the present study demonstrates that, among hypertensive patients with ECG defined LVH and receiving aggressive antihypertensive therapy, new-onset LBBB independently predicts increased risk of developing CV events including heart failure as well as of suffering CV and all-cause death. Further evaluation will be necessary to determine whether incident LBBB is a marker of changes in LV structure and function that might warrant further investigation and possibly treatment in this population.
The main limitation of our study is the rather small number of patients who developed incident LBBB (n = 295) and thus the subsequent limited numbers of patients with the various CV events and death. As pointed out above, the statistical analyses for some endpoints like myocardial infarction and stroke were therefore underpowered. Further, that the use of heart failure required hospitalization to define heart failure almost certainly underestimates the true incidence of heart failure, potentially reducing precision of estimates of the relation between new-onset LBBB and heart failure incidence [28]. In addition, the LIFE study enrolled patients selected for the combination of moderately severe hypertension and ECG-LVH and thereby may not be directly applicable to other populations. However, the number of adults who would meet entry criteria for the LIFE study has been estimated to be 7.8 million in the first 15 member states of the European Union [30], with nearly as many in either the United States or Eastern Europe. The randomized allocation in the current study could have affected our results. One could imagine that the angiotensin receptor blocker increased conduction velocities [31] thereby preventing the incidence of LBBB, whereas beta-blockers prolong conduction, and facilitate the occurrence of LBBB. However, no interactions with the randomized treatment and endpoints were found in our analyses.
We also want to remind the reader that an approach based on atenolol and losartan may not be standard practice in 2022 where many guidelines recommend against the primary use of beta-blockers, and angiotensin receptor blockers that are acting longer than losartan or have a higher affinity to the angiotensin type-1 receptor are often preferred. More importantly, however, is that only about 50% of LIFE participants achieved a BP of < 140/90 mmHg, leaving 50% sub-optimally treated according to 2002 standards and probably even more according to some more recent guidelines. Thus, while 20 years ago the LIFE study represented optimal study design and performance [9–11], the clinical findings may not directly translate into today’s clinical practice.
BP: blood pressure
CIs: confidence intervals
CV: cardiovascular
ECG: electrocardiogram/electrocardiography
HR: hazard ratio
LBBB: left bundle branch block
LIFE: Losartan Intervention For Endpoint reduction in hypertension
LVH: left ventricular hypertrophy
The authors thank the 945 clinical investigators who participated in the LIFE study.
CNB, ZL and PMO contributed to the conception and design of this LIFE sub-study. CNB, ZL and PMO performed the statistical analyses. ZL wrote the first draft of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Sverre E. Kjeldsen has received lecture honoraria within the past 3 years from Getz Pharma, Merck Healthcare KGaA, Sanofi-Aventis and Vector-Intas. The other authors declare that they have no conflicts of interest.
Ethical committees for all participating clinical centers approved the LIFE study. The study was performed in accordance with the Declaration of Helsinki. The study was chaired by an academic steering committee, and it was overseen by an independent data and safety monitoring board.
Written informed consent to participate in the study was obtained from all participants.
Not applicable.
The data that support the findings of this study are available from the corresponding author (PMO) upon dire need.
Merck & Co., Inc. supported the LIFE study with an unrestricted grant from 1993. Merck had a non-voting member of the Steering Committee, which designed the study and wrote the protocol. Merck did monitoring, and accumulated data in a Merck database until study end in 2002. Merck provided the study steering committee with free access to blinded data until 2002 and then un-blinded data after the database was cleared for queries and frozen. The steering committee including a committee statistician validated independently the main outcomes. The steering committee has always been free to analyze and interpret the data, make decisions to publish and write the papers. Merck has not had any formal role after 2002 except for sporadically providing expert statistical assistance.
© The Author(s) 2022.
Copyright: © The Author(s) 2022. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Rafael Luzes ... Adalberto Vieyra
Natalia L. Rukavina Mikusic ... Mariela M. Gironacci
Gian Paolo Rossi ... Teresa Maria Seccia
Sukhwinder K. Bhullar ... Naranjan S. Dhalla
Kristin E. Reeve ... Babbette LaMarca
Eran S. Zacks ... Richard B. Devereux
Casper N. Bang ... Peter M. Okin
Marcello Chinali ... Richard B. Devereux
