Affiliation:
Emergency Medicine Unit and European Society of Hypertension Specialized Center of Excellence for Hypertension, Department of Medicine-DIMED, University of Padua, Italy
Email: gianpaolo.rossi@unipd.it
ORCID: https://orcid.org/0000-0002-7963-0931
Affiliation:
Emergency Medicine Unit and European Society of Hypertension Specialized Center of Excellence for Hypertension, Department of Medicine-DIMED, University of Padua, Italy
ORCID: https://orcid.org/0000-0001-6146-3015
Affiliation:
Emergency Medicine Unit and European Society of Hypertension Specialized Center of Excellence for Hypertension, Department of Medicine-DIMED, University of Padua, Italy
ORCID: https://orcid.org/0000-0002-3775-0011
Affiliation:
Emergency Medicine Unit and European Society of Hypertension Specialized Center of Excellence for Hypertension, Department of Medicine-DIMED, University of Padua, Italy
ORCID: https://orcid.org/0000-0001-9023-0835
Affiliation:
Emergency Medicine Unit and European Society of Hypertension Specialized Center of Excellence for Hypertension, Department of Medicine-DIMED, University of Padua, Italy
ORCID: https://orcid.org/0000-0002-3639-4086
Explor Med. 2021;2:294–304 DOI: https://doi.org/10.37349/emed.2021.00047
Received: March 12, 2021 Accepted: May 21, 2021 Published: June 30, 2021
Academic Editor: Carlos Ferrario, Wake Forest School of Medicine, USA
The article belongs to the special issue Angiotensins—A Century of Progress
The adrenal cortex plays a key role in the regulation of metabolism, salt and water homeostasis and sex differentiation by synthesizing glucocorticoid, mineralocorticoid and androgen hormones. Evidence exists that angiotensin II regulates adrenocortical function and it has been contended that angiotensin peptides of the non-canonical branch of the renin angiotensin system (RAS) might also modulate steroidogenesis in adrenals. Thus, the aim of this review is to examine the role of the RAS, and particularly of the angiotensin peptides and their receptors, in the regulation of adrenocortical hormones with particular focus on aldosterone production.
The adrenal cortex plays a key role in the regulation of metabolism, salt and water homeostasis and sex differentiation by synthesizing glucocorticoid, mineralocorticoid and androgen hormones. Hence, an altered regulation in the production of these hormones is involved in the pathophysiology of multiple diseases including high blood pressure, Cushing’s and Addison’s syndromes, and virilization. A full appreciation of the pathophysiology of these disorders, therefore, requires a deep understanding of the regulation of adrenocortical function.
Excess production of the main mineralocorticoid hormone aldosterone is involved in several human diseases where it plays a substantial role in causing hypertension-mediated organ damage, cardiovascular events, and deaths [1, 2]. For example, a secretion of aldosterone in excess of the physiological need is a feature of primary (essential) hypertension associated with overweight and obesity, which involves a multitude of patients affected by the so called “metabolic syndrome” [3]. Secretion of aldosterone occurring in a fashion apparently autonomous from angiotensin (Ang) II and in excess of what needed to preserve body fluids volume and maintain blood pressure, is a key feature of primary aldosteronism, the most common curable cause of human arterial hypertension [4]. Furthermore, there are numerous other conditions, as congestive heart failure, liver cirrhosis, nephrotic syndrome, primary reninism, Bartter’s and Gitelman’s syndromes and renovascular hypertension, which are usually referred to as secondary aldosteronism, where hyperaldosteronism is driven by activation of the renin angiotensin system (RAS) [5]. Therefore, the regulation of aldosterone secretion by angiotensin peptides plays a role that needs to be appreciated in order to fully understand the pathophysiology of hyperaldosteronism. The contention has also been made that angiotensin peptides of the non-canonical branch of the RAS might regulate adrenocortical function and evidence exists that Ang II can also regulate the production of glucocorticoids [6–8].
Thus, this review was conceived to provide information on the regulation of adrenocortical hormones mediated by the RAS and related peptides.
For several years it was contended that a local RAS existed in tissues, including the adrenal cortex. However, conclusive demonstration of this came in the nineties of the last century with the application of molecular biology techniques, as Northern blotting and ribonuclease (RNase) protection assays. This allowed several laboratories to detect renin mRNA in the adrenal cortex of experimental animals and humans [9, 10]. This suggested the possibility of local generation of Ang I in the adrenal cortex. Further stronger support for this hypothesis was recently provided with use of state-of-the-art techniques, such as digital droplet PCR, which recently allowed to demonstrate the expression also of the angiotensin converting enzyme (ACE)-1 and the ACE-2 in the human adrenal cortex [11].
ACE-2, a carboxypeptidase which differs from ACE-1, cleaves one amino acid residue from Ang I or Ang II, to form Ang 1-9 and Ang 1-7, respectively. Ang 1-9 can be cleaved to Ang 1-7 by ACE-1 or via the action of alternative endopeptidase, such as neutral endopeptidase or prolyl endopeptidase [12].
The heptapeptide Ang 1-7 has well proven vasodilatory actions, which counteracts Ang II vasoconstriction [13], and exerts cardiovascular protective effects via the G protein-coupled receptor Mas receptor (MasR, reviewed in [14]). In rat cardiac fibroblasts, Ang 1-7 inhibited proliferation and collagen production by reducing extracellular signal-regulated kinase (ERK) 1 and ERK2 kinase activity induced by Ang II [15]. Moreover, in Ang II-infused mice, Ang 1-7 attenuated cardiac hypertrophy, decreased the production of cardiac reactive oxygen species, and increased NO production [16]. Along with results of other studies, these findings suggested that the balance between ACE-1, forming Ang II, and ACE-2, generating Ang 1-7 can be crucial for controlling not only the tissue levels of Ang II, but also the net cardiovascular and modulatory effects of the RAS [17].
Altogether they also suggested the potential for local production of angiotensin peptides, including Ang I, Ang II, and Ang 1-7, in the human adrenal cortex.
Measurements of angiotensin peptides with liquid chromatography tandem-mass spectrometry analysis (LC-MS/MS) in human adrenocortical tissues, comprising aldosterone-producing adenoma (APA), and APA-adjacent tissue, and in plasma collected during adrenal vein sampling from the adrenal veins and from the infrarenal inferior vena cava showed that both APA and APA-adjacent tissues have detectable levels of Ang II and Ang III. Furthermore, both Ang II and Ang I showed measurable levels in adrenal vein blood draining both from APA side and from the contralateral normal adrenal gland [11]. However, in spite of the gene expression of ACE-2 in adrenocortical tissues and in plasma draining from the adrenals, Ang 1-7 was by no means detectable. Considering that ACE-2 is the receptor of SARS-CoV2, these findings indicated that the adrenal cortex is a potential target for SARS-CoV2 infection, which can account for cases of adrenal insufficiency in infected patients [18]. Of note, Mao et al. [19] reported the colocalization of ACE2 and transmembrane serine protease 2 (TMPRSS2) in zona fasciculata and zona reticularis of adrenal cortex suggesting that SARS-CoV2 can directly interfere with cortisol secretion and increase the rate of critical illness-related corticosteroid insufficiency in COVID-19 patients.
These novel data, along with the detection of angiotensin receptors in the adrenal cortex, described in the next section, established the basis for a better understanding of the role of the RAS in the adrenal gland.
Soon after the purification and sequencing of Ang II, the peptide was demonstrated to potently stimulate the secretion of aldosterone [20]. It is now well established that Ang II, by acting through the angiotensin type 1 receptor (AT1R), a member of the seven-transmembrane, G protein-coupled receptor family, stimulates the expression of the aldosterone synthase coding gene (CYP11B2) in a concentration-dependent manner. These results confirmed that Ang II is a potent secretagogue of aldosterone as the increase of CYP11B2 mRNA induced by Ang II is consistently followed by the release of aldosterone in the cell supernatant. What perhaps is less known and investigated is the effect of Ang II on CYP11B1, the cortisol-forming enzyme, as discussed later.
In a systematic search for the different receptor mRNAs, we recently found that all the angiotensin receptors, including the angiotensin type 2 receptor (AT2R), the MasR, and the alamandine receptor (MrgD) are expressed, albeit at a much lower level than the AT1R in the human adrenal cortex (Figure 1). This rank of expression is in agreement with the compelling evidence that the secretagogue effect of Ang II on aldosterone involves activation of AT1R [21].
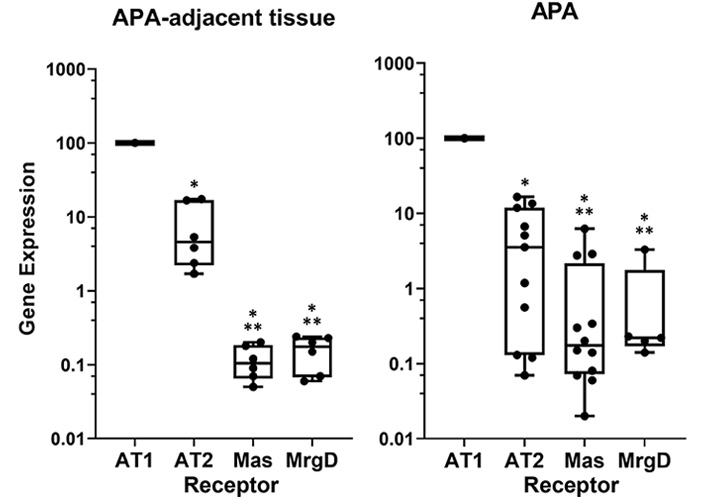
Relative expression of the different angiotensin receptors in human adrenal tissues. Data were obtained by the real-time reverse transcription-polymerase chain reaction (RT-PCR); the different gene expression is calculated with ∆∆Ct method using porphobilinogen deaminase as housekeeping gene and are presented by setting AT1R expression at 100% for comparison. * P < 0.0001 vs. AT1R; ** P < 0.01 vs. AT2R
Ang II stimulates aldosterone production by activating multiple intracellular signaling pathway, the best characterized of which involves an increase of intracellular Ca2+ via activation of the phospholipase C (PLC) and production of diacylglycerol and inositol triphosphate as second messengers (Figure 2). The AT1R-mediated Ang II-stimulated aldosterone production involves a depolarization of the zona glomerulosa cells through modulation of the opening status of two key K+ channels: the G protein-coupled inwardly rectifying potassium channel (GIRK)4, and the Twik-related acid-sensitive K+ (TASK) channels (Figure 2). This depolarization opens the voltage-dependent T-type Ca2+ channels.
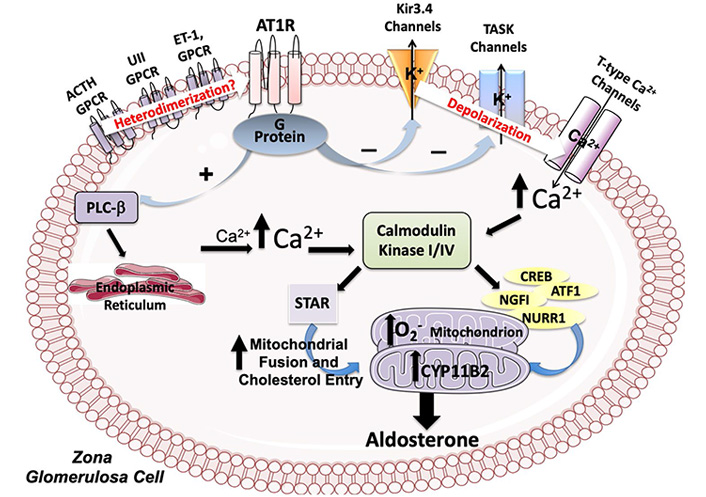
Molecular pathways modulated by Ang II in adrenal zona glomerulosa cells. Ang II, by acting through the AT1R stimulates the expression of the aldosterone synthase coding gene (CYP11B2) by increasing intracellular Ca2+ through the modulation of the activity of the PLC-β and of the GIRK4, and of the TASK channels. The increase of intracellular Ca2+ activates the calmodulin kinase (CaMK) cascade which acts on CYP11B2 expression by phosphorylating the transcription factors Activating Transcription Factor 1 (ATF-1), cyclic adenosine monophosphate response element (CRE)-binding protein (CREB) and nuclear receptor related 1 (NURR1). Ang II also increases the aldosterone synthase activity by activating NAPDH oxidase and, thereby, by elevating reactive oxygen species (O2–) in the mitochondria and by promoting fusion of mitochondria and translocation in these organelles of relevant steroidogenic acute regulatory. Finally, heterodimerization of AT1R with other G protein-coupled receptors (GPCRs) is supposed to be involved in aldosterone synthesis
While the GIRK4 channel functions by transferring K+ from inside to outside of zona glomerulosa cells, the TASK channels, a widely distributed family of channels encoded by KCNK genes, generates background, or leak, K+ currents. Both channels are essential for maintaining the resting membrane potential of adrenal zona glomerulosa cells at the negative value of –80 mV [22].
By interrupting these currents, Ang II depolarizes the cell membrane, thus causing opening of the voltage sensitive T-type Ca2+ channels, whose open probability state is proportional to the degree of cell membrane depolarization, and, thereby, inducing activation of aldosterone synthesis (Figure 2) [23]. Mutations in the sequence of the coding gene (KCNJ5) altering GIRK4 channel activity, which lead to excess aldosterone production in both familial and sporadic hyperaldosteronism, results in membrane depolarization and, therefore, in opening of these channels [24].
The increase of intracellular Ca2+ is key for aldosterone synthesis because it activates the CaMK cascade which, through phosphorylation of the STAR protein, increases cholesterol transport in the mitochondria [25]. CaMKI and CaMKIV also act on CYP11B2 expression by phosphorylating the transcription factors ATF-1 and CREB, which bind to CREs and Nerve growth factor-induced clone B (NGFIB), and NURR1 that bind to the Ad-5 and NBRE-1 cis elements [26, 27].
Considering that the AT2R is expressed in the adrenal gland, we explored its role in aldosterone secretion with a pharmacological approach using the AT2R agonist C21 [28] to determine if activation of the AT2R could counter-regulate the AT1R-mediated secretagogue effect of Ang II. These experiments showed that AT2R activation with C21 had no effect on aldosterone production in human adrenocortical cells under unstimulated conditions and also when aldosterone secretion was up-regulated by Ang II [29]. Of note, at high concentration (10–5 M) C21 markedly increased CYP11B2 gene expression and enhanced the effect of Ang II on expression of this gene. This effect was abolished by pre-treatment with the AT1R antagonist irbesartan, indicating that at high concentration (10–5 M) C21 acts as an AT1R agonist [28].
In steroid-producing cells, cholesterol transport from the outer to the inner mitochondrial membrane is the first and rate-limiting step for the synthesis of all steroid hormones. In fact, several of the enzymes necessary for the biosynthetic pathways are located in mitochondria. Thus, these organelles have been recently pointed out as key in the regulation of aldosterone synthesis mediated by Ang II.
Ang II, by elevating reactive oxygen species in the mitochondria of human and rat adrenocortical cells, provides the oxidative environment that is needed to convert deoxycorticosterone into aldosterone and increased the aldosterone synthase activity by activating nicotinamide adenine dinucleotide phosphate (NAPDH) oxidase [30]. This effect was abolished by either cell treatment with an AT1R antagonist (losartan) and also with an antioxidant (polyethylene glycol-catalase).
Moreover, it has been showed that Ang II is able to stimulate aldosterone production in adrenal cells also by modulating mitochondria fusion/fission events. Mitochondrial dynamics are implicated not only in maintaining the equilibrium of organelles by repairing or eliminating defective mitochondria [31], but also in regulating steroidogenesis [32].
Accordingly, Ang II was demonstrated to promote aldosterone synthesis in H295 cells by increasing fusion of mitochondria and translocation in these organelles of relevant steroidogenic proteins, such as STAR, that is responsible for cholesterol transport from the outer to the inner mitochondrial membrane, and protein kinase C (PKC) ε, mitogen-activated protein kinase (MEK) and ERK, that drive the activation signaling in mitochondria [33]. Whether and how this occurs in APA, where, as mentioned above, Ang II is low, remains to be investigated [11].
Adrenal zona fasciculata secretes glucocorticoids mainly in response to adrenocorticotropic hormone (ACTH), but not only. It also responds to Ang II [34–36] in line with the notion that the AT1R is expressed in this zona in different species [6–8, 37].
As proof-of-concept that Ang II can regulate cortisol production, we provided in vivo evidences for a stimulatory effect of Ang II by treating rats with Ang II (700 μg/kg·day) for 1-week and demonstrating an increase of immunodetectable 11-β hydroxylase in the adrenal cortex (Figure 3) [38]. Furthermore, by acting on AT1R, Ang II, significantly increased CYP11B1 gene expression in adrenocortical cell line H295R and its sub-clone HAC15, and also in strips obtained from APA-adjacent tissue and APA [11, 28].
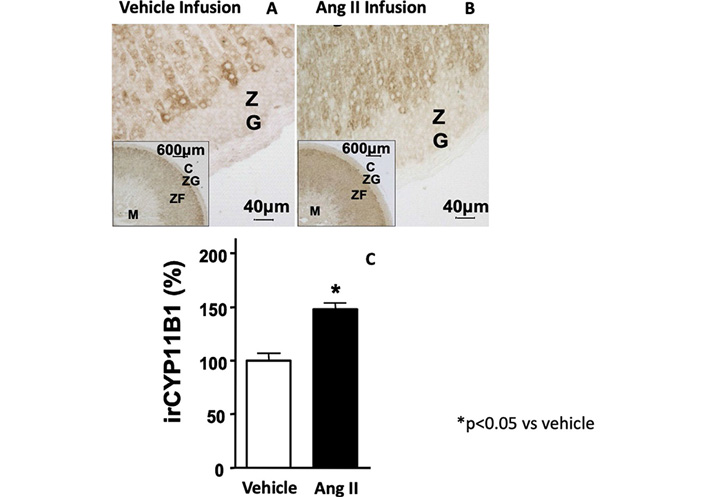
11-β hydroxylase immunostaining of rat adrenal cortex after the treatment with vehicle (Panel A) and Ang II (700 μg/kg·day) for 1-week (Panel B). Quantitative analysis of immunostaining showed a significant increase of the 11-β hydroxylase protein expression after Ang II treatment as compared to vehicle (Panel C)
Note. Adapted with permission from “Expression and functional role of urotensin-II and its receptor in the adrenal cortex and medulla: novel insights for the pathophysiology of primary aldosteronism.” Giuliani L, Lenzini L, Antonello M, Aldighieri E, Belloni AS, Fassina A, et al. J Clin Endocrinol Metab. 2009;94:684–90. (https://doi.org/10.1210/jc.2008-1131). © Oxford University Press
Like in zona glomerulosa cells, a pivotal role for cortisol synthesis is played by depolarization-dependent Ca2+ entry; moreover, both T-type and L-type Ca2+ channels were identified as mediators of Ang II-stimulated cortisol secretion in bovine and human zona fasciculata cells [39, 40]. Noteworthy, Ang II receptor activation is coupled to membrane depolarization through inhibition of the potassium two pore domain channel subfamily K member TWIK-related potassium channel (TREK)-1 [41].
To date, notwithstanding the AT1R expression in the androgen-producing adrenocortical zona reticularis [42], information on whether Ang II affects the biosynthesis and secretion of androgen is lacking.
Ang III has been found to increase aldosterone secretion from rabbit and rat adrenal gland [43, 44]. This effect was partially blunted by the AT2R antagonist PD123319 and was affected by the AT1R antagonist candesartan[45]. A secretagogue effect of Ang III on aldosterone has been also reported in human HAC15 cells, but, at variance with what found in rat adrenocortical zona glomerulosa, in humans Ang III-stimulated aldosterone production was abolished by losartan, suggesting an AT1R-mediated regulation [46]. Of note, Ang II was far more potent than Ang III in stimulating aldosterone secretion in all models investigated thus far.
As human adrenocortical tissues express not only the AT1R but also, albeit at much lower levels, the AT2R and the MasR, we recently investigated the effects of Ang 1-7 on aldosterone and cortisol production in H295R cells. We found that at concentrations ranging from 10–8 to 10–7 M, Ang 1-7 elicited no effects on CYP11B1 or CYP11B2 gene expression [11]. We observed a significant increase in CYP11B1 and CYP11B2 mRNA at concentrations ranging from 10–6 to 10–4 M. This effect was blunted by the MasR antagonist A779, and abolished by the AT1R antagonist irbesartan, suggesting that at high concentration Ang 1-7 stimulates CYP11B2 and CYP11B1 via activation of the AT1R.
Of note, when added on top of Ang II, Ang 1-7 did not blunt Ang II induced expression of CYP11B2 and CYP11B1 mRNA [11]. Opposite to our results, Itcho et al. [47] reported a weak blunting effect of 10–8 mol/L Ang 1-7 on aldosterone secretion and CYP11B2 gene expression induced by 10–8 mol/L Ang II in HAC15 cells. However, those authors did not use purified or and/or synthetic Ang 1-7 but conditioned medium obtain after 6 hours incubation of HAC15 with Ang II. Moreover, they could only presume that their cells would have generated Ang 1-7 as neither they measured Ang 1-7 concentration in the medium nor did they rule the presence of other aldosterone release modulators [47]. However, it was also reported that at 10–6 mol/L Ang 1-7 blunted Ang II- and ACTH-induced aldosterone production by acting through MasR in rat zona glomerulosa cells [48]. As this is a high concentration of Ang 1-7 and the relative quantity of the AT1R, AT2R, and MasR was not determined in rat zona glomerulosa [48], a possible off target effect of Ang 1-7 cannot be ruled out. Moreover, these divergent results could be explained by species differences, given that for example Ang II receptors do not play the same role in rodents and humans.
Even though on the whole, the available evidences indicate that Ang II is the main secretagogue of aldosterone, research in the last decades showed that at least a dozen of other factors regulates the biosynthesis and secretion of aldosterone in the adrenal zona glomerulosa and interact in a complex way. For example, endothelin (ET)-1 was in vitro shown to be equipotent to Ang II in stimulating aldosterone secretion in human adrenocortical cells and in strip preparations acting via both ETA and ETB receptors [49–51]. Moreover, the incubation of zona glomerulosa cells with Ang II on top of ET-1 resulted in a significant potentiation of Ang II effect on aldosterone secretion suggesting a cross-talk between ET-1 and Ang II [52]. Of note, Ang II by acting via AT1R stimulates ET-1 release and expression [53, 54] and in turn, ET-1 increases Ang II formation [55].
Besides their secretagogue action on the secretory activity of the adrenal gland, Ang II and ET-1 promote hypertrophy and hyperplasia of the zona glomerulosa [56, 57] via AT1R [56]- and ETA- [51, 58] dependent mechanisms, respectively. Both peptides exert their mitogenic effects through PKC dependent activation of the p42/p44 mitogen-activated protein (MAP)-kinase cascade [58, 59]. The expression of a chymase-mediated biosynthetic pathway alternative to the endothelin converting enzyme-1 in the human adrenal cortex capable of generating ET-1-31 also suggested that conversion of pro-endothelin 1 to ET-1-31, a predominant endothelin A receptor agonist, or to ET-1, a mixed endothelin A and B agonist, could direct adrenocortical stem cells in the cambium layer toward differentiation into a proliferogenic/hypertrophic phenotype or a secretory phenotype [51]. Hence, the cross-talk between Ang II stimulating formation of ET-1 and chymase generating ET-1-31 could be relevant for controlling the differentiation of zona glomerulosa cells according to the physiological needs.
Information on the interactions between Ang II and other factors are much more scant, although studies have raised the possibility that association as heteromeric complexes of AT1R with other receptors may be one of the mechanisms by which AT1R can diversify and modulate its signaling [60]. A heteromerization between the AT1R and other GPCRs had been demonstrated in several tissues and cell types, but not in the adrenal cortex, until we could show this phenomenon to occur in vitro in HAC15 cells [61]. In these human adrenocortical carcinoma cells, heterodimerization of AT1R with the G protein-coupled estrogen receptor (GPER) potentiated the GPER-mediated effect on CYP11B2 expression. Thus, it is conceivable that heterodimerization of Ang II receptors, particularly of AT1R, with other GPCRs known to stimulate aldosterone, such as the endothelin receptors [50], adrenocorticotropic hormone receptor [21] and urotensin II receptor [38], can represent a putative additional mechanism capable of finely tuning aldosterone synthesis in the adrenal cortex both in physiological and disease conditions.
In summary, compelling evidences have confirmed the role of Ang II as an important secretagogue for aldosterone and also for cortisol. Moreover, accumulating data have shown that the regulation of adrenocortical hormone biosynthesis is a far more complex process involving a number of additional factors, among which ET-1 and other peptides may act through different receptors and complex interactions of their receptors. Improved technology will undoubtedly provide a better understanding of these complex processes and, therefore, should be a goal of future research resources.
ACE: angiotensin converting enzyme
Ang: angiotensin
APA: aldosterone-producing adenoma
AT1R: angiotensin type 1 receptor
AT2R: angiotensin type 2 receptor
CaMK: calmodulin kinase
ERK: extracellular signal-regulated kinase
ET: endothelin
GIRK: G protein-coupled inwardly rectifying potassium channel
GPCRs: G protein-coupled receptors
MasR: Mas receptor
RAS: renin angiotensin system
STAR: steroidogenic acute regulatory
TASK: Twik-related acid-sensitive K+
GPR conceived and revised the manuscript; LL and BC wrote the manuscript; GR and TMS revised the manuscript.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2021.
Copyright: © The Author(s) 2021. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Rafael Luzes ... Adalberto Vieyra
Natalia L. Rukavina Mikusic ... Mariela M. Gironacci
Sukhwinder K. Bhullar ... Naranjan S. Dhalla
Kristin E. Reeve ... Babbette LaMarca
Eran S. Zacks ... Richard B. Devereux
Casper N. Bang ... Peter M. Okin
Casper N. Bang ... Peter M. Okin
Marcello Chinali ... Richard B. Devereux
