Affiliation:
1General Medicine and Metabolic Diseases, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 20122 Milan, Italy
2Department of Pathophysiology and Transplantation, University of the Study of Milan, 20122 Milan, Italy
Email: rosa.lombardi@unimi.it
ORCID: https://orcid.org/0000-0001-6958-927X
Affiliation:
1General Medicine and Metabolic Diseases, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 20122 Milan, Italy
ORCID: https://orcid.org/0000-0002-7723-3801
Affiliation:
2Department of Pathophysiology and Transplantation, University of the Study of Milan, 20122 Milan, Italy
ORCID: https://orcid.org/0000-0003-0631-6666
Affiliation:
1General Medicine and Metabolic Diseases, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 20122 Milan, Italy
2Department of Pathophysiology and Transplantation, University of the Study of Milan, 20122 Milan, Italy
ORCID: https://orcid.org/0000-0001-5918-0171
Explor Med 2021;2:20–38 DOI: https://doi.org/10.37349/emed.2021.00030
Received: October 20, 2020 Accepted: November 21, 2020 Published: February 28, 2021
Academic Editor: Lindsay A. Farrer, Boston University School of Medicine, USA
The article belongs to the special issue Exploring NAFLD/NASH
Patients submitted to liver transplantation (LT) are exposed to high risk of cardiovascular (CV) complications which are the main determinants of both short-term and long-term morbidity and mortality in LT. Non-alcoholic fatty liver disease (NAFLD) is a very frequent condition in general population and is associated with a high risk of cardiovascular disease (CVD) which represents the first cause of death of these patients. NAFLD is predicted to become the first indication to LT and nowadays is also frequently detected in patients submitted to LT for other indications. Thus, the risk of CVD in patients submitted to LT is forecasted to increase in the next years. In this review the extent of CV involvement in patients submitted to LT and the role of NAFLD, either recurring after transplantation or as de novo presentation, in increasing CV risk is analysed. The risk of developing metabolic alterations, including diabetes, hypertension, dyslipidemia and weight gain, all manifestations of metabolic syndrome, occurring in the first months after LT, is depicted. The different presentations of cardiac involvement, represented by early atherosclerosis, coronary artery disease, heart failure and arrhythmias in patients with NAFLD submitted to LT is described. In addition, the tools to detect cardiac alterations either before or after LT is reported providing the possibility for an early diagnosis of CVD and an early therapy able to reduce morbidity and mortality for these diseases. The need for long-term concerted multidisciplinary activity with dietary counseling and exercise combined with drug treatment of all manifestations of metabolic syndrome is emphasized.
Liver transplantation (LT), the only effective treatment for end stage liver disease, has spread in the past 50 years in Europe, plateauing in recent years, with about 7,300 LTs performed in Europe and 8,000 in the United States annually [1, 2]. Non-alcoholic fatty liver disease (NAFLD) [3] is becoming the leading cause of LT in the USA, and rate of LT caused by NAFLD is likely to further increase in next years as a consequence of metabolic syndrome (MS) diffusion and the absence of established therapies [2]. Survival rates from United Network for Organ Sharing registry at 1, 5 and 10 years are approximately 85%, 70% and 50% [1, 4], with the critical period for post LT outcome represented by the first year during which 46% of deaths occur, nearly 60% of which within 6 months [1]. More than 10% of LT recipients have cardiovascular disease (CVD) which together with hepatic and cancer, are the most common causes of death after LT [5]. Nowadays, despite the marked improvement in immunosuppressive therapies and organ preservation techniques [6] post-transplant death rate remains elevated because of CVD.
The aim of this review is to clarify the extent of cardiovascular (CV) involvement in post LT patients, defining the role of NAFLD in increasing CV risk. Indeed, as depicted in Figure 1, beyond being a cause of LT, NAFLD can reappear after LT (recurrent NAFLD) and even arise after LT in patients without steatosis before transplantation (NAFLD). Reported data in this review were identified by search and selection database of MEDLINE, Google Scholar, PubMed, Elsevier, by using the search term “LT” combined with “CV risk” and “NAFLD”. Relevant articles were selected. Review articles are cited to provide more details and references.
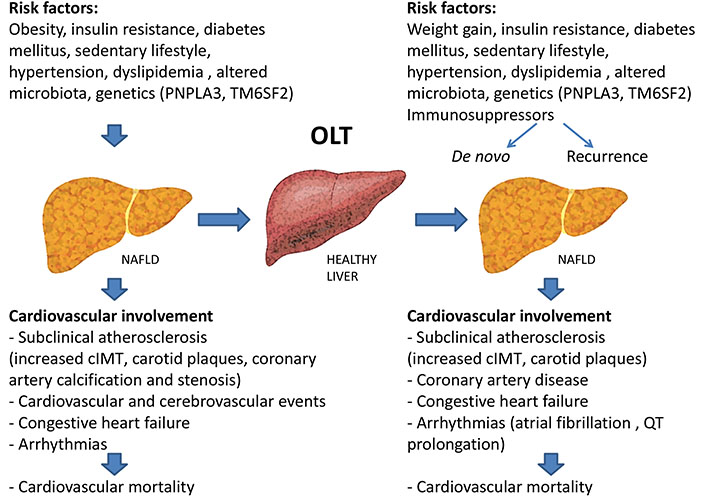
Risk factors for the development of NAFLD and cardiovascular damage before and after liver transplantation. Obesity, insulin resistance, diabetes mellitus, hypertension, dyslipidemia, sedentary lifestyle, altered microbiota and genetics [patatin-like phospholipase domain containing 3 (PNPLA3) and transmembrane 6 superfamily member 2 (TM6SF2)] favor the onset of NAFLD. NAFLD could progress into advanced liver disease requiring liver transplantation (LT). However, NAFLD can develop even after liver transplantation, sustained by the same pathogenetic factors of the pre-LT. Either in the pre-LT or post-LT period NAFLD exposes patients to high cardiovascular morbidity and mortality
The link between NAFLD and CV disease is well established since both diseases share many metabolic risk factors such as obesity, insulin resistance, type 2 diabetes (T2DM), hypertension, dyslipidemia, as well as a sedentary lifestyle, genetic predisposition (PNPLA3 and TM6SF2 gene) [7–9] and gut microbiota impairment which favours either hepatic steatosis or inflammation and atherosclerosis [10, 11].
NAFLD is a risk factor for either subclinical or established CVD and mortality. In fact, a higher prevalence of subclinical atherosclerosis [12] represented by increased carotid artery intima-media thickness (cIMT) and presence of carotid plaques [13], and coronary artery calcification and significant coronary stenosis at coronarography [14] have been demonstrated. Interestingly, there is a relation between severity of liver and CV damage, being a more advanced liver disease associated with a more serious vascular damage [11, 15]. In addition, NAFLD patients experience more CV events than the overall population. In 2016, Fracanzani et al. [16] evaluated the incidence of cardiac and cerebrovascular events in patients with NAFLD and in a control cohort, followed-up over a 10-year period, showing a higher prevalence of CV events in the NAFLD group [15]. In addition, NAFLD subjects are more likely to develop congestive heart failure and cardiac arrhythmias [mainly atrial fibrillation and corrected QT interval (QTc) interval prolongation] compared to the general population [17].
As a consequence, CV-related death appears to be the leading cause of mortality in patients with NAFLD, as demonstrated by Ekstedt et al. [18], who evaluated mortality from all causes in 229 patients over a period of 30 years.
Additionally, T2DM and morbid obesity, which are very prevalent in NAFLD, have been reported to impact on death/removal from the LT waiting list of patients with liver disease of different etiology [19]. On the basis of this evidence, screening for CV disease all patients with NAFLD, irrespectively of the presence of other traditional risk factors [20] is highly recommended by the European Association for the Study of the Liver [21].
Recent literature data indicate a different gender-related presentation of NAFLD. The prevalence of NAFLD is higher in males compared to premenopausal women becoming comparable after menopause, when women tend to gain weight, to have a different distribution of fat, mainly visceral, with an increased risk to develop NAFLD and CVD [22, 23].
Data on the prevalence of CV complications are controversial and not conclusive. An independent male association with coronary artery calcifications has been described in general population [13]. A large Korean cross-sectional study reported that men had a higher prevalence of NAFLD, carotid plaque and cIMT values [24]. On the contrary data obtained from German and Austrian populations indicate a close association between NAFLD and CV events (myocardial infarction and coronary heart disease) regardless of gender [25, 26].
In postmenopausal women a correlation between NAFLD (evaluated by computer tomography) and prevalence of coronary artery calcifications has been described, however the association was lost after correction for the known CVD risk factors [27]. Finally, although not conclusive, literature data suggest that while in the general population female sex appears to be protective for ischemic CV events, in women with NAFLD is not [28]. Indeed, in a recent meta-analysis considering 108,711 patients with NAFLD (44% females) all-cause mortality was about 1.5 times higher in women than in men and CV events 2 times higher [29].
Future studies on the different ways of evaluating metabolic alterations in women compared to men are needed in consideration of the increased number of transplants performed in women. In fact, in recent years, non-alcoholic steatohepatitis (NASH) represents the leading cause of transplantation in the female population [30, 31].
Despite it is clear that CV complications determine either short-term or long-term morbidity and mortality in LT [9, 32, 33], studies exploring prevalence and risk factors for specific CV events after LT are lacking and often CV assessment is evaluated as composite including coronary artery disease (CAD), heart failure and arrhythmias without considering cirrhosis associated cardiomyopathy. Also in a recent systematic review of 29 studies including 57,493 patients, definitions of CV outcomes were highly inconsistent [34] and only 3 studies evaluated CV-related mortality [5, 9, 35].
We reported the most consistent data on the onset of CVD post LT and the role of NAFLD in this setting as depicted in Table 1.
Evaluation of cardiovascular outcomes in transplanted patients with or without assessment of pre-LT steatosis, either receiving a graft with steatosis and/or with de novo/recurrent NAFLD post LT
| Authors | Year | Type of study | Nation | Population | F-up after LT | CV endpoint | NAFLD | Results |
|---|---|---|---|---|---|---|---|---|
| Evaluation of CV outcomes in transplanted patients without data on pre-existing NAFLD | ||||||||
| Alves et al. [35] | 2019 | Cross sectional | Brasil | 79 | 1.4–6.3 years | cIMT | NA |
|
| Bargehr et al. [53] | 2018 | Retrospective Case control | USA | 717-32 cases (AF)-63 controls | 5–8 years | Intraoperative and postoperative cardiac complications (ventricular tachycardia, hemodynamic instability, cardiac arrest, death) | NA |
|
| Josefsson et al. [44] | 2014 | Retrospective | UK | 234 | 2–20 years | -CV events (arrhythmias, CAD) -Mortality from CV events | NA |
|
| Dowsley et al. [43] | 2012 | Retrospective | USA | 107 | 2.6 ± 1.4 months | HF | NA |
|
| Eimer er al. [42] | 2008 | Prospective | USA | 86 | 2 weeks-2 years | Systolic HF | NA |
|
| Evaluation of CV outcomes in transplanted patients with data on pre-existing NAFLD but without analysis on the impact of NAFLD on CV assessment | ||||||||
| Memaran et al. [71] | 2019 | Cross sectional | Germany | 104 (children) | 6.9 years | -Carotid-femoral pulse PWV-cIMT-LVMI | Pre-LT NASH 6% |
|
| Sonny et al. [47] | 2018 | Case control | USA | 1,284-45 cases (LVEF < 45% within 6 months from LT) -180 controls | 6 months | Systolic HF (LVEF < 45%) | -Pre-LT NASH case: 22%-Controls: 23% |
|
| Roccaro et al. [62] | 2018 | Retrospective | USA | 994 | 2-12 years | Major CV events (cardiac arrest, MI, stroke, PAD) | Pre-LT NASH 10% |
|
| Perito et al. [37] | 2018 | Cross sectional | USA | 88 (children) | 11.2 ± 5.6 years | cIMT | Pre-LT NASH 17% |
|
| VanWagner et al. [38] | 2017 | Retrospective | USA | 1,024 | 10 years | Hospitalization or mortality from major CV events (MI, AF, HF, cardiac arrest, PE, TIA, stroke) | Pre-LT NASH 16% |
|
| VanWagner et al. [32] | 2016 | Retrospective | USA | 32,810 | 90 days | Major CV events (MI, AF, PE, HF, cardiac arrest, stroke) | Pre-LT NASH 9.7% |
|
| Fussner et al. [39] | 2015 | Retrospective | USA | 455 | 8–12 years | CVD (CAD, arrythmias, congestive HF, symptomatic PAD) | Pre-LT NASH 10% |
|
| VanWagner et al. [9] | 2014 | Retrospective | USA | 54,697 | 30 days | Mortality from CV events | Pre-LT NASH 5% |
|
| Qureshi et al. [45] | 2013 | Prospective | USA | 970 | 5.3 ± 3.4 years | HF | Pre-LT NASH 4.5% |
|
| Watt et al. [5] | 2010 | Retrospective | USA | 798 | 12.5 (9–13) years | Mortality from all causes and CV causes | Pre-LT NASH 29% |
|
| Evaluation of CV outcomes in transplanted patients with pre-existing NAFLD and with analysis on the impact of NAFLD on CV assessment | ||||||||
| Kwong et al. [29] | 2020 | Retrospective | USA | 1,023 | 1–3 years | -Survival-CV events (AF, MI, HF, stroke) | Pre-LT NASH in 21% |
|
| Nagai et al. [95] | 2019 | Retrospective | USA | 32,660 (6,344 NASH) (17,037 HCV) (9,279 ALD) | 1–2 years | All cause and CV mortality | Pre-LT NASH 19% |
|
| D’Avola et al. [98] | 2017 | Prospective | Spain | 1,819 | 5 years | All cause and CV mortality | -Cryptogenetic cirrhosis 2.9%.-Data on NASH NA |
|
| Piazza et al. [104] | 2016 | Retrospective | Italy | 143 (65 ALD) (78 NASH) | 3 years | -All-cause mortality-CV events (sudden cardiac death, CAD, congestive HF, AF or arrhythmia, valvular heart disease, PAD, or stroke) | Pre-LT NASH 54% |
|
| VanWagner et al. [8] | 2012 | Retrospective | USA | 242 (115 NASH) (127 ALD) | 5 years | -Survival-CV events (death from any cardiac cause, MI, acute HF, arrhythmia, stroke) | Pre-LT NASH in 47% |
|
| Contos et al. [83] | 2001 | Prospective | USA | 58 (30 NASH) (16 ALD) (12 PBC) | 30 days | Survival | Pre-LT NASH 51% |
|
| Evaluation of CV outcomes in transplanted patients receiving liver graft with NAFLD | ||||||||
| Kulik et al. [79] | 2017 | Retrospective | Germany | 1,205 [77 requiring re-LT, 39 due to primary non function (PNF) and 38 to vascular and biliary disease] | 3 months-11 years | -In-hospital mortality in patients with re-LT-Survival in patients with re-LT | NAFLD in 69% of graft of with PNF |
|
| Andert et al. [80] | 2017 | Retrospective | Germany | 94 | 30 days-1 years | All cause mortality | Donor graft hepatic steatosis: < 30% (n = 27), 30%–60%(n = 41) > 60% (n = 26) |
|
| de Graaf et al. [76] | 2012 | Retrospective | Australia | 291 | 3 months | Mortality | -NAFLD in 72% of graft-Data on pre-LT NASH NA |
|
| Evaluation of CV outcomes in transplanted patients with de novo/recurrent NAFLD | ||||||||
| Pisano et al. [36] | 2020 | Prospective | Italy | 54 | 2 years | -Carotid IMT, plaques and PWV-Diastolic dysfunction (E/A) -EAT | -New onset steatosis 26%-Pre-LT NAFLD 19%-Graft with steatosis 20% |
|
| Bhati et al. [91] | 2017 | Retrospective | USA | 103 | 5–15 years | -All cause and CV mortality-Survival | -Recurrent NAFLD 87–88%-Pre-LT NASH 47%, criptogenetic cirrhosis 53% |
|
| Hejlova et al. [85] | 2016 | Retrospective | Czech republic | 548 | 15 years | Survival (comparison between grade 0–1 steatosis vs. 2–3 grade steatosis) | De novo NAFLD in 56% (17% grade 3) |
|
| Yalamanchili et al. [90] | 2010 | Retrospective | USA | 2,052 | 1–10 years | Survival | -De novo NAFLD in 31%-Pre-LT NASH/criptogenetic cirrhosis in 12% |
|
| Dureja et al. [94] | 2011 | Cohort-study | USA | 88 | 1–7 years | -All causes mortality and CV mortality-Survival | -Recurrent NAFLD 39%-Pre-LT NAFLD/criptogenetic cirrhosis 100% |
|
PAD: peripheral artery disease; BMP: brain natriuretic peptide; MI: myocardial infarction; AF: atrial fibrillation, PE: pulmonary emobolism; PWV: pulse wave velocity; LVMI: left ventricular mass index; HF: heart failure; LVEF: left ventricular ejection fraction; TIA: transient ischemic attack; NAFL: non-alcoholic fatty liver; BP: blood pressure; GFR: glomerular filtrate rate; NS: not statistically significant; NA: not available; LDL: low density lipoproteins; BNP:brain natriuretic peptide; ALD: alcoholic liver disease; HCV: hepatitis C virus; HCC: hepatocellular carcinoma; PBC: primary biliary cholangitis; PNF: primary non function; E/A: E wave A wave ratio; EAT:epicardial adipose tissue
The presence of early atherosclerosis assessed by either cIMT or by the presence of carotid plaques in patients undergoing LT has been recently documented not only in adults but also in pediatric patients. Indeed, endothelial damage has been demonstrated to onset very early after LT, with increase in carotid IMT and stiffness after 6 months from transplant [36, 37], both in children and adolescents [38]. In adults, presence of subclinical atherosclerosis was associated with an increased prevalence of features of MS, namely diabetes, hypertension and dyslipidemia [37].
CAD is the most studied CVD in patients post LT because of its highly negative prognostic impact on patient’s survival. In fact, a study following up patients post LT for 10 years showed an incidence of CAD, either with or without myocardial infarction, of approximately 40%, with increasing incidence over time (i.e. 15% at 3 years and 30% at 8 years post LT). In particular, among all patients who experienced CAD, 12% underwent a revascularization procedure in the first year after LT [39]. Interestingly, in subjects without pre-existing CVD, pre-transplant troponin I elevation (> 0.07 ng/mL) before LT was predictive of occurrence of CVD after LT [40], as well as of higher mortality in the first month post-transplant, possibly indicating that even subtle undiagnosed CAD (i.e. subclinical or microvascular), could predispose to future CV events [41].
Heart failure after LT is often reported, with transient cardiac decompensation occurring in 7–43% of patients during postoperative period [42–49]. Cirrhotic cardiomyopathy (CCM), described as a cardiac dysfunction (systolic or diastolic) in patients with end-stage liver disease without prior heart disease, includes a hyperdynamic circulation, a blend of systolic and diastolic dysfunction, along with prolonged ventricular repolarization, and blunted inotropic and chronotropic response to stress [32]. CCM is possibly due to fibrosis and hypertrophy of the myocardium and to subendocardial oedema [50, 51].
Pre-transplant diastolic dysfunction seems to be linked with graft rejection and failure [47], post-transplant mortality [44, 47] and post-transplant systolic heart failure [44, 48]. Indeed, in the absence of an overt clinical manifestation it is often challenging to establish whether subclinical CV damage was already present before the transplant or whether it is a new onset. In addition, some cardiac alterations of patients with cirrhosis are due to coexisting obesity or diabetes, thus making the diagnosis of CCM even more confusing. The recent availability of new methods for the assessment of CCM in patients with end-stage liver disease modified the criteria for the diagnosis and follow-up of the patients before and after LT [52].
A prolonged QT interval is very frequently reported in the ECG of patients listed for LT [50], and it is associated with a high risk of sudden cardiac death (SCD), especially when the interval is more than 0.5 s. On the other hand, the prognostic role of prolonged QTc in cirrhotic patients not requiring LT is not defined [50]. However, QTc often normalizes after LT [50], whereas its persistent prolongation is associated with an increased rate of post LT fatal and non-fatal CV events [45, 53].
Among all tachyarrhythmias, atrial fibrillation, either before or after LT, is the most widely observed. Its prevalence in LT candidates ranges from 1.4% to 6% [33, 54] and it is associated with post LT increased CV complications, graft failure and mortality [33, 54, 55]. Interestingly increased long-term risk of atrial fibrillation has recently been described in NAFLD patients [56] and more severe the liver disease (i.e. NASH or cirrhosis) higher its prevalence. Few data are available on the development of atrial fibrillation after LT in patients with cirrhosis of which the etiology is not metabolic.
In order to define the prognostic role of CV complications, CV risk assessment is essential in LT recipients, so that scores predictive of both early and late CV atherosclerotic complications are accumulating.
Among predictors of short term occurrence (i.e. within 1 year after LT) of CV events, the most widely used is the CV risk in orthotopic liver transplantation (OLT), which is based on pre-LT demographic, social, and clinical variables [57].
Conversely, scores for the assessment of the risk of late atherosclerotic complications tailored for LT recipients are missing, so that currently those applied in the general population are used, including the Framingham general CVD score (FRS) [58], the pooled cohort equations (PCEs) [59], the Reynolds Risk Score [60], the Prospective Cardiovascular Münster Study (PROCAM) [61] and the Systematic Coronary Risk Evaluation Project (SCORE) [62]. On the contrary, no validated scores for the prediction of heart failure after LT are available.
Along with risk scores, also the presence of metabolic comorbidities may help clinicians in stratifying CV risk in LT recipients. In fact, T2DM, especially if persistent after LT, has been demonstrated a key prognostic factor for CV morbidity, with an incidence of major CV events of 13% and 27% at 5 and 10 years [63].
Unfortunately, clear guidelines about CV follow-up after LT are missing, as well as about evaluation of subclinical CV changes. Usually, the follow-up consists of a clinical and biochemical control performed semesterly or annually and referral to a specialist only in the presence of hypertension or diabetes. If on one hand the onset CV events after LT has been widely studied, on the other hand only few studies and a meta-analysis [37, 64–72] demonstrated an increase in subclinical atherosclerosis after solid organ transplantation.
Patients who undergo LT can receive a liver graft with steatosis, can develop steatosis which was absent before LT (steatosis) and can have recurrence of steatosis in the new liver (patients with NAFLD pre-LT).
Given the increased prevalence of NAFLD worldwide, along with a shortened organ pool donation in many countries, utilization of donor grafts with hepatic steatosis is now more common [73]. Hepatic steatosis is seen in the biopsies of a consistent percentage of potential liver donors, reaching up to 75% if overweight is present [74].
As a consequence of reperfusion, alterations in microcirculation and hepatocytes are induced by steatosis in the graft, with consequent necrosis and impaired regenerative processes [75, 76]. As a result, hepatic steatosis in donor livers exposes recipients to increased morbidity and mortality. Necessity of intensive care unit, longer hospitalization, as well as increased risk of graft failure [77–79], especially for steatosis in more than 60% of the graft [79–82], is usually observed. Viceversa, presence of moderate steatosis seems to affect significantly neither the long-term liver-related outcome [83] nor the CV outcome [37].
The term de novo NAFLD indicates the occurrence of steatosis in the transplanted livers of patients who did not have steatosis before LT, its prevalence ranging from 25% to 60% [37, 84–86] depending on follow-up duration and populations studied. Interestingly, prevalence of de novo steatosis increases over time (30% at 1 year up to nearly 50% after 10 years) with 5–10% progressing towards NASH and 2.5% to cirrhosis [85–89].
Risk factors for de novo steatosis include presence in LT recipients of sarcopenia and features of MS (especially insulin resistance, hypertension and obesity), tacrolimus based immunosuppressive therapy, hepatitis C virus and genetic predisposition as the genotype [83–85], as well as hypoadiponectinemia and high levels of free fatty acids [90]. Indeed, in transplanted patients who develop de novo steatosis, CV events are common with nearly 40% of transplant recipients experiencing an event within 10 years, one-third occurring within the first year.
Recurrent NAFLD is the onset of steatosis in the graft of a patient needing LT for the liver complications of hepatic steatosis in a dysmetabolic setting, with a recurrence rate of 30–60% within 1–5 years after LT, and with progression towards NASH of 10–33% and advanced fibrosis of 5–10% [91]. Other data report a higher prevalence, with a recurrence rate as high as 90–100% [84, 92]. Differences in the prevalence of steatosis recurrence are likely related to the diagnostic methodology to assess steatosis, the time from transplant, and presence of pre and post-transplant risk factors.
In addition, patients who need a liver transplant because of metabolic cirrhosis are likely to have recurrence of NAFLD, and classically they present features of MS and pre-existing CV disease [91, 93], thus being exposed to higher CV risk by default [94, 95].
De novo and recurrent NAFLD are indeed two distinct entities. In particular, patients with recurrent NAFLD present higher prevalence of obesity and diabetes compared with patient with de novo NAFLD, are more likely to progress to advanced forms of NAFLD, suggesting a more aggressive course of the disease [87], likely because of a longer exposure to metabolic alterations. In addition, it has been reported that steatosis resolves in one-fifth of patients with de novo and only rarely in those with recurrent NAFLD [87]. However, data on the impact of recurrent NAFLD on long-term outcomes are conflicting, some showing a similar overall survival in patients with and without recurrent NAFLD [84, 91, 95], even in the presence of NASH [92], others an increase in mortality, mainly if patients had developed NASH [83, 92, 96].
Although there are no concordant data on the increase in overall mortality in NAFLD transplant patients compared to those of other etiologies, CV complications after LT are higher in NAFLD patients. In fact, a higher incidence of major cardiac and cerebrovascular events was reported in NAFLD subjects related to age, pre-transplant T2DM and other features of MS and a history of post-transplant CAD [10, 97].
Furthermore, de novo and recurrent steatosis are related to weight gain post LT. Weight gain is observed in almost all patients after 3 months from LT, with patients with pre-transplant NAFLD gaining more weight than non-NAFLD patients [98]. Moreover, new onset obesity was found related with a higher incidence of CV disease [99].
The interplay between metabolic and genetic factors in the CVD of patients with NAFLD is known [100] conversely the relevance of genetic factors in CV complications post OLT is still not defined. A dated paper which analyzed the role of the C677T-methylenetetrahydrofolate reductase (MTHFR)-polymorphism on vascular complications in 47 liver transplant recipients reported that this polymorphism was significantly associated with an increased incidence of vascular complications [101]. However, the sample size was small and no other study confirmed these results. In addition, recently variants in the MTHFR gene have been recently demonstrated as not associated with fatty liver disease making unlikely the role of this variant in post OLT CVD [102]. As previously mentioned, genetic factors, including the major genetic determinant of NAFLD, and the TM6SF2 E167K polymorphisms, as well as the membrane-bound O-acyltransferase domain-containing 7 (MBOAT7) genetic variant facilitate NAFLD occurrence before transplant [103].
It is very likely that the same polymorphisms will increase the risk of CVD after OLT. In a small study performed in China it was reported that the coexistence of obesity and positivity for I148M GG was strongly associated with de novo NAFLD occurrence post OLT [104]. Thus, even if longer follow up was not available to assess the risk of CVD in positive patients it can be expected that similarly to patients with NAFLD, transplanted subjects are at higher risk for CVD. It is possible that genetic polymorphisms may even play a major role given the presence of multiple environmental factors, after OLT, increasing CV risk. It will be interesting to define whether carriers of polymorphisms known to facilitate NAFLD occurrence but protect from CVD, such as the TM6SF2 E167K, will reduce the risk of CVD post OLT [100].
In summary, given the epidemic of NAFLD and consequently the fastest growing indication to LT, some authors have evaluated whether NAFLD and NASH per se constitute an increased risk of CVD but results are contrasting. Piazza et al. [105] found that NASH is not an independent risk factor for CVD in transplanted patients and a recent meta-analysis including 4,237 transplanted patients, 717 with NASH, from 9 studies [106] confirmed these data. In contrast, another meta-analysis pooling data from 16 observational studies, demonstrated that NAFLD was a risk factor for fatal and nonfatal CV events, and the more advanced the liver disease the higher the risk [107].
Thus, findings are far from being conclusive. While there is a general agreement that the metabolic alterations prevalent in NAFLD patients have an impact on death/removal during the LT waiting list, survival, CV events and renal failure rates were similar in NASH and non-NASH patients undergoing LT [30].
In conclusion, NAFLD represents one of the main indications for LT, it is often present also in patients in whom the indication for LT recognizes other etiologies and can develop after transplantation. Therefore, NAFLD seems to confer an increased risk of CV morbidity and mortality, mainly when associated with T2DM and MS.
Patients referred to LT for NAFLD-related complications need aggressive management of risk factors before LT to reduce waiting list morbidity/mortality and to reduce post LT CV damage related to de novo development or recurrent NAFLD, weight gain and MS.
Prevention of CVD morbidity and mortality requires long-term concerted multidisciplinary activity with dietary counseling and exercise associated with therapy for hypertension, T2DM and dyslipidemia.
CAD: coronary artery disease
CCM: cirrhotic cardiomyopathy
cIMT: carotid artery intima-media thickness
CV: cardiovascular
CVD: cardiovascular disease
LT: liver transplantation
MS: metabolic syndrome
NAFLD: non-alcoholic fatty liver disease
NASH: non-alcoholic steatohepatitis
OLT: orthotopic liver transplantation
PNPLA3: patatin-like phospholipase domain containing 3
T2DM: type 2 diabetes
TM6SF2: transmembrane 6 superfamily member 2
RL and GP revised the literature, focusing on full text paper regarding CV involvement in post LT patients and role of NAFLD in increasing the CV risk and wrote the draft of the manuscript. SF and ALF were involved in the critical revision of the manuscript to its final form and contributed to the review for important intellectual content.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2021.
Copyright: © The Author(s) 2021. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 5124
Download: 45
Times Cited: 0
Noel C. Salvoza ... Natalia Rosso
Amedeo Lonardo, Stefano Ballestri
Marvin Ryou ... Gyorgy Baffy
Michael Doulberis ... Stergios A. Polyzos
Marica Meroni ... Paola Dongiovanni
Ivana Mikolasevic ... Sandra Milic
Valerio Rosato ... Marcello Persico
Carlo Acierno ... Ferdinando Carlo Sasso
Angelo Di Vincenzo ... Marco Rossato
Angelo Colucci ... Claudia Mandato
