Affiliation:
1School of Cellular and Molecular Medicine, University of Bristol, BS8 1TD Bristol, UK
2Sitryx Therapeutics Ltd, OX4 4GA Oxford, UK
Email: laura.mcmillan@sitryx.com
ORCID: https://orcid.org/0009-0004-0295-9298
Affiliation:
1School of Cellular and Molecular Medicine, University of Bristol, BS8 1TD Bristol, UK
Email: Christoph.Wuelfing@bristol.ac.uk
ORCID: https://orcid.org/0000-0002-6156-9861
Explor Immunol. 2023;3:148–157 DOI: https://doi.org/10.37349/ei.2023.00094
Received: December 09, 2022 Accepted: January 29, 2023 Published: April 27, 2023
Academic Editor: Noah Isakov, Ben Gurion University of the Negev, Israel
The article belongs to the special issue The Role of Adaptor Proteins in Lymphoid Cell Signaling
Linker for activation of T cells (LAT) is a central adaptor protein in proximal T cell activation. A key element of its adaptor function is the efficiency with which LAT interacts with its binding partners. Such efficiency is controlled by the local concentration of LAT as well as the vicinity to up- and downstream interaction partners, i.e. LAT localization. Several factors control LAT localization. LAT is a palmitoylated transmembrane protein and traffics between vesicular compartments and the plasma membrane. Membrane heterogeneity and protein-protein interactions can drive LAT clustering, at scales from a few to hundreds if not more molecules. LAT vesicular trafficking through the small, crowded cytoplasm of a T cell and the commonly nm scale clusters are difficult to access experimentally, in particular in the physiological interaction of T cells binding to antigen presenting cells (APCs) with a highly undulating interface. Only in recent years have technological advances begun to provide better access. Based on such advances, three elements of LAT localization are discussed in conjunction: vesicular trafficking as it regulates LAT transport towards, insertion into, and removal from the plasma membrane; LAT clustering as it increases local LAT concentrations; LAT-anchored supramolecular signaling complexes as they embed LAT in a dense network of interaction partners. Consistent with the important role of LAT localization for its function, each of these processes regulates LAT activity and the efficiency of T cell activation.
Linker for activation of T cells (LAT) is required for T cell activation from thymocyte development through T cell effector function. LAT mutations can lead to substantial immune disease [1]. LAT is a transmembrane protein with two palmitoylation sites. It links the activation of lymphocyte-specific protein tyrosine kinase (Lck) and zeta-chain-associated protein kinase-70 (ZAP-70) upon ligand engagement of the T cell receptor (TCR) to a host of downstream signaling processes [2, 3]. The key signaling motifs of LAT are four tyrosine residues that can be phosphorylated by ZAP-70. A LAT proline-rich motif allows Lck to recruit LAT to the TCR and ZAP-70 through its Src homology 3 (SH3) domain [4]. The phosphorylated LAT tyrosine residues then serve as docking sites for various SH2 domain-containing signaling intermediates. LAT, thus, is the central signal distribution point in proximal TCR signaling.
In addition to the biochemical generation of binding motifs for SH2-containing signaling intermediates, the phosphorylation of the four key tyrosine residues, LAT function is regulated by its localization. Local concentrations of signaling intermediates, as governed by localization, control proximity to up- and downstream interaction partners and the efficiency of interaction with them. Three mechanisms that control LAT localization. As a transmembrane protein, LAT traffics through vesicles. Its amount and localization are discussed at the plasma membrane depend on the extent and localization of plasma membrane insertion of vesicles carrying LAT and on its removal from the plasma membrane by endocytosis. In addition, the localization of LAT as a transmembrane protein is also regulated by plasma membrane heterogeneity in conjunction with protein-protein interactions which promote clustering and spatial segregation. Finally, as a tetravalent signaling intermediate LAT nucleates highly crosslinked networks of multiple signaling intermediates which have the potential to grow to supramolecular dimensions. The investigation of LAT localization is technically challenging as it requires the resolution of nm scale structures in the crowded small cytoplasm and on the undulating plasma membrane of T cells interacting with antigen presenting cells (APCs), i.e. at the immunological synapse. Despite substantial recent progress, the investigation of LAT localization remains limited by experimental accessibility. Therefore, approaches used including potential limitations and key questions left unanswered are highlighted.
Using imaging approaches, it is estimated that 30–75% of the cellular pool of LAT resides in vesicles that are clearly separate from the plasma membrane [5–7]. Additional vesicles close to or docked at the plasma membrane are unlikely to be included in this quantification as they are within the diffraction limit of detection in light microscopy. Vesicular trafficking of LAT thus has to be a key regulator of LAT localization. In T cells vesicular trafficking is exceedingly difficult to study as individual vesicles and their movement cannot be resolved as separate objects by fluorescence microscopy in the small and densely packed cytoplasm of the T cell. As discussed in molecular detail below, most insight has been generated by colocalizing clusters of LAT-containing vesicles with key vesicular markers and regulators of trafficking at single time points. Further data were generated by biochemical purification of LAT-containing vesicles and dynamic imaging of the plasma membrane and vesicles directly associated with it by total internal reflection fluorescence (TIRF) microscopy in the activation of T cells by flat APC substitutes. The complementary strengths and limitations of these studies generate an initial picture of LAT trafficking (Figure 1).
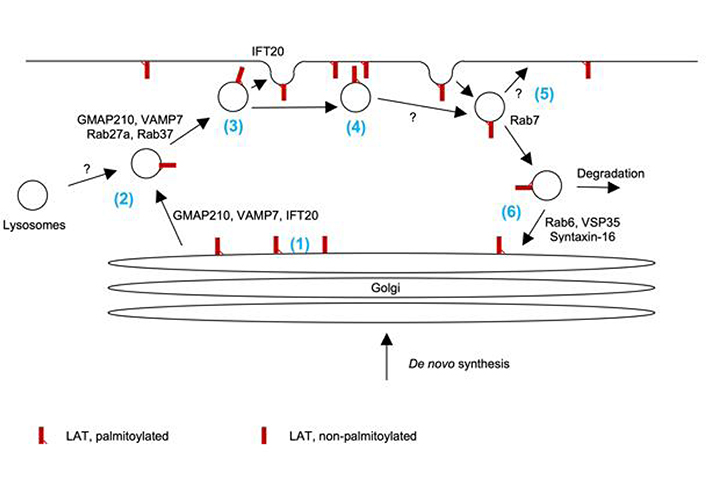
Vesicular trafficking of LAT. (1) Palmitoylation of LAT and the ability of its transmembrane domain to mediate association with lipid-ordered domains are required for Golgi exit. (2) LAT trafficking to the vicinity of the plasma membrane is regulated by Rab27a, Rab37, Golgi-microtubule-associated protein of 210 kDa (GMAP210), and vesicle-associated membrane protein 7 (VAMP7). Given the role of Rab27a, VAMP7, and the ciliary transport machinery in the trafficking of lysosome-derived secretory vesicles, LAT may traffic with such vesicles. (3) IFT20 mediates the fusion of docked LAT vesicles at the plasma membrane. It is uncertain which fraction of docked vesicles fuse. (4) It is likely that both LAT embedded in the plasma membrane and localized on docked vesicles can contribute to proximal signal transduction. (5) LAT is endocytosed in vesicles associated with Rab7. It is uncertain which fraction of these vesicles uses the recycling endosome pathway to reinsert into the plasma membrane is uncertain. Similarly, the long-term fate of docked but not fused vesicles is unresolved. (6) Part of endocytosed LAT uses retrograde traffic to the Golgi as regulated by Rab6, vacuolar protein sorting-associated protein 35 (VSP35), and syntaxin-16. Part of endocytosed LAT is degraded in a ubiquitin-dependent fashion
Specifically, the palmitoylation of LAT and the ability of its transmembrane domain to mediate association with lipid-ordered domains are required for exit from the Golgi apparatus to the plasma membrane [8, 9]. Exocytic LAT vesicles are distinct from vesicles carrying TCRζ and Lck. They are characterized by association with Rab27a, Rab37, and the vesicle soluble N-ethylmaleimide sensitive factor attachment complex receptor (SNARE) vesicle-associated membrane protein 7 (VAMP7) [5]. The association of LAT-containing vesicles with Rab27a is intriguing, as Rab27a plays a key role in the docking of lysosome-derived lytic granules with the plasma membrane in cytotoxic T cells [10]. LAT may thus travel with such vesicles. VAMP7 is required for the docking of LAT-containing vesicles at the plasma membrane [11]. VAMP7 is associated with lysosome-derived secretory vesicles in B cells and NK cells [12, 13], again suggesting that LAT could travel with such vesicles in T cells. It is still unclear how many of the LAT-containing vesicles docked at the plasma membrane subsequently fuse with the plasma membrane. Biochemical studies suggest that such fusion is slow and not substantial within 30 min of T cell activation but detectable after hours [11]. Imaging studies as detailed below though provide direct evidence for some vesicle fusion within minutes. Nevertheless, even without fusion docked LAT-containing vesicles can associate with clusters of the TCR and the downstream adaptor SH2 domain-containing leukocyte protein of 76 kDa (SLP-76) at the cytoplasmic side of the plasma membrane [6] and, thus, could contribute to signal transduction. Combined TIRF and confocal imaging studies show that intraflagellar transport 20 (IFT20), a key component of the ciliary transport machinery, regulates the fusion of LAT-containing vesicles with the plasma membrane but not their transport to the vicinity of the plasma membrane [14]. Further supporting the role of the ciliary transport machinery in LAT trafficking, the expression of LAT, but not TCRζ in a ciliated inner medullary collecting duct cell line results in its transport to the cilium [15]. As the ciliary transport machinery plays a key role in T cell synapse organization including that of cytotoxic T cells [16], these data are again consistent with the role of lysosome-derived granules in the delivery of LAT to the plasma membrane. Linking various stages in LAT transport, Golgi exit, ciliary transport, and secretory vesicles, Golgi-microtubule-associated protein of 210 kDa (GMAP210) marks vesicles that are positive for LAT, VAMP7, and IFT20 and is required for LAT transport to the plasma membrane [15]. LAT is removed from the plasma membrane by endocytosis. A fraction of LAT vesicles contain the endosomal markers transferrin [17] and, up to 70%, Rab7 [6]. At least some of these vesicles engage in retrograde transport to the Golgi. Biochemical purification of LAT-containing vesicles demonstrates enrichment of the retrograde transport markers Rab6, vacuolar protein sorting-associated protein 35 (VSP35), and the target SNARE syntaxin-16 [18]. Such transport is dependent on TCR engagement. Some of the internalized LAT is degraded in a ubiquitin-dependent fashion [19].
Together these studies paint a picture of LAT trafficking in three key steps (Figure 1). (i) Newly synthesized LAT and LAT retrieved through retrograde transport are moved from the Golgi to the immunological synapse, possibly in lysosome-derived secretory vesicles. (ii) LAT-containing vesicles docked at the immunological synapse can fuse to insert LAT into the plasma membrane or function as non-fused distinct vesicular signaling sites. (iii) LAT is removed from the immunological synapse by endocytosis for degradation or retrieval through retrograde transport with an uncertain role of recycling endosomes. Turnover through all these steps is enhanced by TCR signaling.
To determine the importance of vesicular transport for LAT and T cell function, regulators of vesicular transport associated with LAT have been deleted. While such deletion likely affects the transport of large numbers of proteins in addition to LAT, a consistent picture is emerging across these studies. Deletion of various components of the LAT vesicle transport machinery, e.g., VAMP7, Rab6, and ITF20, consistently leads to diminished LAT amounts at the immunological synapse and less efficient T cell activation [11, 14, 18]. LAT vesicular transport thus is critical for LAT function.
Important questions remain. Which fraction of docked LAT-containing vesicles fuse with the plasma membrane? Is there a preferred localization of LAT vesicle docking and fusion? Can LAT in docked vesicles participate in T cell signal transduction? Which fraction of plasma membrane-localized LAT is endocytosed during T cell activation? Does LAT continue to signal after endocytosis? Which fraction of endocytosed LAT engages with retrograde transport to the Golgi as opposed to direct reinsertion into the plasma membrane through recycling endosomes or lysosomal degradation?
The local concentrations of signaling intermediates govern their interaction probabilities and thus the efficiency of signaling processes. Clustering at the nm scale enhances local concentrations and thus is an important determinant of signaling function. Historically, such clustering could only be resolved by electron microscopy (EM), requiring extensive sample processing. However, more recent single molecule localization microcopy approaches, as discussed in detail below, have made molecular clustering accessible in gently fixed or even live cells. Nevertheless, these highly data-rich imaging approaches are more readily executed in a single imaging plane rather than in three dimensions. Therefore, a substantial part of the single molecule imaging data is generated in T cells activated on flat surfaces, cover slips coated with antibodies, or supported lipid bilayers. Based on such studies, LAT is not evenly distributed in the plasma membrane but undergoes T cell activation-regulated clustering. However, it remains uncertain how such data apply to T cell APC couples with their highly undulating, deformable interface [20].
Specifically, pioneering EM work on plasma membrane sheets of a mast cell line before and after activation through the Fcϵ receptor has demonstrated LAT clusters smaller than 20 molecules before activation that grow to 50 to 150 molecules upon mast cell activation. LAT clusters are adjacent to but not mixed with Fcϵ receptor clusters, thus forming separate signaling islands in the plasma membrane [21]. Initial spinning disk confocal microscopy of T cells activated on flat surfaces established dynamic LAT clustering at the scale of 0.5–1 μm [22]. Subsequent single molecule localization microscopy showed that only a fraction of LAT was in such clusters, 8% before and 24% after T cell activation [23]. Such clusters increase in size and number upon T cell activation as at least in part driven by lateral LAT recruitment within the plasma membrane, as opposed to vesicular insertion [24]. LAT clusters only partially overlap with upstream TCR clusters and downstream SLP-76 clusters [23, 25], extending the concept of LAT signaling islands to T cells. Single molecule tracking showed that individual LAT molecules can integrate into clusters of the costimulatory receptor cluster designation 2 (CD2) or bounce off the edges of such clusters [26]. The lack of cluster entry is consistent with the existence of discreet signaling islands; integration of some LAT molecules into the CD2 clusters illustrates a potential means of communication between clusters. A detailed analysis of fluorescence intensity as a function of the distance from the plasma membrane allows a distinction between LAT in the plasma membrane versus vesicles close to it. Such work showed a 2.7-fold increase in LAT amounts in the plasma membrane upon T cell activation with enhanced LAT clustering to a cluster size of 30 to 50 molecules as largely driven by the insertion of vesicular LAT [27]. The dynamics and relative contributions to LAT cluster formation of lateral diffusion versus insertion from VAMP7+ vesicles were further investigated by a combination of advanced imaging approaches, lattice light sheet microscopy, structured illumination TIRF microscopy, and correlative light EM with focused ion beam scanning EM as the three-dimensional EM component [28]. Within 2 min of T cell activation LAT clusters formed in the plasma membrane with very few vesicles in their vicinity. At 5 min of T cell activation, VAMP7+ vesicles are abundant in the LAT cluster vicinity with evidence of LAT plasma membrane insertion from the vesicles [28]. It remains uncertain what drives LAT clustering. One suggestion is plasma membrane heterogeneity, where proteins are driven into membrane islands of 30–300 nm that are cholesterol-enriched as characterized by EM of T cell plasma membrane sheets [29]. Again, LAT was found to be pre-clustered, clusters grow in size upon T cell activation and are adjacent but distinct from TCR clusters [25]. Extending a potential role of membrane heterogeneity to vesicles, LAT-containing vesicles show a weak but significant correlation with higher lipid order [30].
Concepts developed in separate work on TCR clustering may be applicable to LAT. TCR clustering was verified to occur on T cells in vivo [31]. Signaling-active TCRs were shown to be enriched in TCR clusters [32], consistent with the idea that molecular clustering enhances signaling efficiency. Finally, a careful reanalysis of single molecule localization microscopy data suggests that technical limitations can lead to overcounting of TCR clusters [33]. Contrary to previous work, TCRs were found not to be clustered prior to T cell activation but only thereafter. Together the studies on LAT and the TCR paint a picture where LAT may or may not be clustered in resting T cells. Upon T cell activation LAT clusters are formed and/or grow into distinct LAT signaling islands with distinct contributions from LAT lateral diffusion and vesicular insertion. LAT phosphorylation is enhanced in such islands. LAT islands are adjacent to clusters of the TCR and other signaling intermediate, prominently SLP-76, such that individual molecules at the edges of the clusters can move between them to connect the various signaling processes (Figure 2).
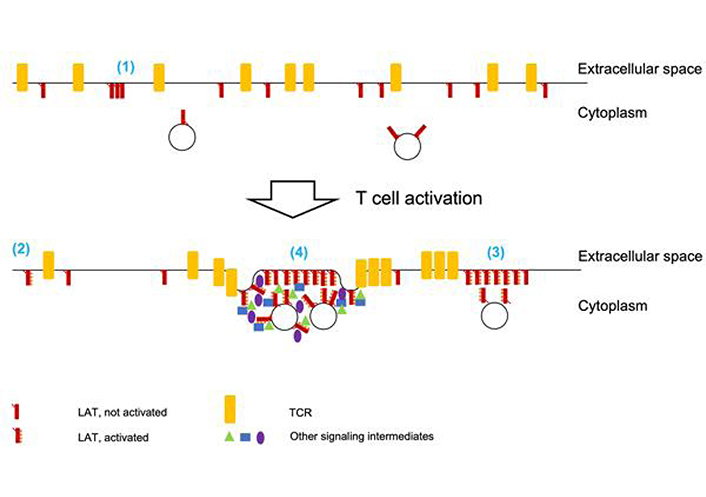
LAT clustering upon T cell activation. (1) Prior to T cell activation, some LAT clustering has been described. Parallel work on TCR clustering raises doubts that technical challenges in single molecule localization microscopy may overestimate such clustering. (2) T cell activation, as dominated by TCR engagement, leads to LAT phosphorylation. For clarity TCR engagement is not depicted. It is unclear to which extent non-clustered LAT molecules are phosphorylated. (3) Upon T cell activation, LAT forms signaling islands where LAT activation is likely enhanced. It is unclear whether all LAT molecules in such clusters are equally phosphorylated. The extent to which docked LAT-containing vesicles are part of such islands is unresolved. LAT islands are adjacent to but rarely mix with TCR clusters. (4) Upon strong T cell activation including costimulation through CD28 LAT clusters into supramolecular signaling complexes that are enriched in a substantial number of signaling intermediates and associated with increased membrane undulations. The extent to which LAT-containing vesicles are part of such complexes is unresolved
Important questions remain. Is LAT signaling restricted to LAT islands or only more efficient in them? How do LAT clusters look in the undulating plasma membrane at the interface between T cells and APCs? Can LAT clusters form on vesicles adjacent to the plasma membrane? Are LAT clusters retained upon endocytosis? How is the association of downstream signaling intermediates with LAT changing when LAT clusters?
Complexes of mutually interacting signaling intermediates can reach ‘supramolecular’ dimensions of hundreds of molecules or more [34–37]. The key driving forces of supramolecular protein complex assembly are high local concentrations and high valence of the interacting signaling intermediates. In supramolecular assemblies, interactions can become more efficient through the formation of reaction crucibles, be limited through phase separation-driven sequestration, and the nature of protein interactions can qualitatively change [34, 37]. While supramolecular complexes formed by a small number of components are often characterized by defined structures such as lipid droplets or protein fibers [37], structural properties of supramolecular assemblies built from a large number of components remain largely undefined. In such supramolecular assemblies, the same set of components likely assembles into a spectrum of complexes with somewhat varying stoichiometry and size. Nevertheless, because of their size supramolecular complexes are a comparatively accessible element of LAT localization, as they can commonly be detected as discreet objects in light microscopy.
LAT valence depends on the extent of tyrosine phosphorylation as determined by the strength of a T cell stimulus. Fully phosphorylated LAT is tetravalent. Even delayed phosphorylation of a single of these tyrosine residues has already functional consequences in the response to attenuated T cell stimulation [38]. Through the association of LAT with the adaptor proteins growth factor receptor-bound protein 2 (Grb2)-related adaptor protein 2 (Gads) and SLP-76 the number of binding sites for downstream signaling intermediates and thus the valence of a LAT-based signaling complex is further increased [39]. In addition, Gads can dimerize [40]. Interaction partners of a LAT-based signaling complex themselves can be multivalent. For example, Grb2 together with Son of sevenless 1 (Sos1) has been shown to oligomerize LAT [41, 42]. In such oligomerization, Grb2 can be replaced or complex formation can be further enhanced by the addition of phospholipase Cγ1 or its SH2-SH2-SH3 domains [43]. As such highly interconnected and multivalent protein interaction network LAT-based signaling complexes have the properties required for supramolecular complex assembly. Using in vitro reconstitution of signaling complexes with purified proteins as initiated by membrane-associated LAT, supramolecular complexes can be formed with different compositions, LAT, Grb2, Sos1 or LAT, Gads, SLP-76, non-catalytic region of tyrosine kinase (Nck) [44]. Such complex formation is dependent on the number of phosphorylated tyrosine residues in LAT and the concentration of the interaction partners. Reconstitution of a LAT Grb2 Sos1 complex in giant unilamellar vesicles triggers lipid segregation into more and less ordered phases [45]. Such segregation can further stabilize the supramolecular complexes and allows for the more effective recruitment of lipid phase-segregated signaling intermediates, prominently K-Ras [45]. In such reconstitution experiments, LAT Grb2 Sos1 aggregates form in two steps, a very rapid linear association of cross-linked LAT molecules followed by slower aggregation into two-dimensional structures [46].
In T cells activated on supported lipid bilayers presenting major histocompatibility complex (MHC) peptide and the costimulatory ligand intercellular cell adhesion molecule-1 (ICAM-1) a single MHC peptide complex could trigger a LAT condensate of 258 ± 65 [standard deviation (SD)] molecules [47]. In T cell APC couples stimulated emission depletion microscopy has detected LAT-associated signaling complexes up to a volume of 1 μm3 associated with plasma membrane undulations [48]. LAT mobility in these complexes is reduced, their formation is diminished upon the attenuation of T cell simulation and complex formation can be enhanced through the synthetic increase of LAT valence, all key properties of supramolecular complexes. The composition of these LAT-based complexes changes dynamically during T cell activation [48]. Many signaling intermediates, prominently SLP-76, only associate with the LAT complexes during 2 min of T cell activation. Complex formation during that time was associated with efficient interleukin-2 (IL-2) generation. Together these data suggest that LAT-based signaling complexes can reach supramolecular dimensions in live T cells that mediate efficient signal transduction in response to strong stimuli.
Important questions remain. Is all LAT in supramolecular complexes embedded in an undulating plasma membrane or are LAT-containing vesicles part of these complexes? Is LAT complex formation associated with the formation of a lipid-ordered phase in cells? What drives the dissociation of key signaling intermediates, prominently SLP-76, from these complexes after only 2 min of T cell activation? Do these complexes simply make LAT signaling more efficient or do they convey new signaling properties?
LAT function is regulated at two interlinked levels: biochemically through the extent of phosphorylation of its key four cytoplasmic tyrosine residues and through the control of its subcellular localization in vesicular trafficking, clustering, and supramolecular complex formation. Moving forward, three questions seem pertinent. How are the biochemical and localization-based mechanisms of the regulation of LAT function integrated? How do these mechanisms play out in the physiological interaction of primary T cells with APCs? How are these mechanisms employed when LAT function adapts to the multitude of functional T cell states in health and disease? Despite an extensive existing literature, there is plenty left to discover about LAT.
APCs: antigen presenting cells
CD: cluster designation
EM: electron microscopy
Gads: growth factor receptor-bound protein 2-related adaptor protein 2
GMAP210: Golgi-microtubule-associated protein of 210 kDa
Grb2: growth factor receptor-bound protein 2
IFT20: intraflagellar transport 20
LAT: linker for activation of T cells
Lck: lymphocyte-specific protein tyrosine kinase
SH3: Src homology 3
SLP-76: Src homology 2 domain-containing leukocyte protein of 76 kDa
Sos1: Son of sevenless 1
TCR: T cell receptor
TIRF: total internal reflection fluorescence
VAMP7: vesicle-associated membrane protein 7
ZAP-70: zeta-chain-associated protein kinase-70
LEM: Writing—review & editing. CW: Writing—original draft, Writing—review & editing.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This work was supported by BBSRC grant [BB/P011578/1]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2883
Download: 28
Times Cited: 0
Tadashi Matsuda ... Kenji Oritani
Mari Hikosaka Kuniishi ... Takanori So
Shulamit Katzav
Xiaoyu Jiang, Izidore S. Lossos
