Affiliation:
1Department of Medical Biology and Central Electron Microscope Laboratory, Medical School, Pecs University, 7624 Pecs, Hungary
2János Szentágothai Research Centre, Pecs University, 7624 Pecs, Hungary
3MTA-PTE Human Reproduction Research Group, 7624 Pecs, Hungary
4National Laboratory for Human Reproduction, 7624 Pecs, Hungary
Email: Szekeres.julia@pte.hu
ORCID: https://orcid.org/0000-0001-7395-9667
Explor Immunol. 2021;1:406–417 DOI: https://doi.org/10.37349/ei.2021.00027
Received: September 14, 2021 Accepted: November 15, 2021 Published: December 31, 2021
Academic Editor: Satish Kumar Gupta, Indian Council of Medical Research, India
The article belongs to the special issue Human Reproduction: Involvement of the Immune System
The foetus expressing paternal antigens ought to be “rejected” by the maternal immune system. However, the immunological relationship of the mother and the foetus does not follow the rules of transplantation immunology. Maternal immune functions are re-adjusted during pregnancy, to create a tolerant environment for the developing foetus. Progesterone and its downstream mediator; the progesterone induced blocking factor (PIBF) are important in this process. The mRNA transcribed from the PIBF1 gene contains 18 exons, and codes for a 90 kDa protein. The 90 kDa form is associated with the centrosome and plays a role in cell cycle regulation, while smaller isoforms produced by alternative spicing are secreted, and bind to the glycosylphosphatidylinositol (GPI) anchored PIBF receptor. Upon ligation, the former forms a heterodimer with the alpha chain of the interleukin-4 (IL-4) receptor and activates the Janus kinase/signal transducers and activators of transcription (Jak/STAT) pathway, via which, PIBF induces increased production of T helper2 (Th2) cytokines. PIBF regulates natural killer (NK) cytotoxicity, by inhibiting perforin release from the cytoplasmic granules of NK cells. During normal human pregnancy, the serum concentrations of PIBF increase with gestational age, and lower than normal serum levels predict spontaneous pregnancy termination. Depletion of PIBF during the peri-implantation period in mice, results in lower implantation and increased resorption rates, together with increased decidual and peripheral NK activity, downregulation of the genes implicated in T cell activation in CD4+ cells, and Th1 differentiation of the T cells. PIBF is expressed in rapidly proliferating immature cells as well as several tumours, and regulates invasion. The PIBF gene has been identified in the chromosomal region 13q21-q22—which is a common site for somatic deletions in a variety of malignant tumours. These data suggest that PIBF might be involved in tumorigenesis.
Progesterone is critical for pregnancy in most mammalian species. In humans, progesterone production gradually rises during gestation to reach a level of 100–500 nm in the sera of pregnant women and 1–10 μm in placental tissue. Decades ago, it became evident, that in addition to its endocrine effects, progesterone mediates interactions between the endocrine and immune systems. It has been shown that high concentrations of progesterone inhibit the activation and killing of T cells [1–3], and extend the survival of allogeneic grafts implanted in hamster uteri [4, 5]. In 1997, Siiteri et al. [6] reported that progesterone was able to influence the function of peripheral lymphocytes. The lowest effective concentration was 10 μg/mL, which is approximately 100-fold higher than the concentrations found in maternal blood in the 3rd trimester of pregnancy, but relevant to those, found at the foeto-maternal interface [7]. They assumed that the immune-modulating effects of progesterone might account for the acceptance of the semi-allogeneic foetus by the maternal immune system, and they called progesterone “nature’s immunosuppressant”.
By comparing the effect of progesterone on pregnancy- and non-pregnancy lymphocytes, it became obvious that the former responded to 100-fold lower concentrations than non-pregnancy lymphocytes [8]. The remarkable difference in progesterone sensitivity suggested the presence of specific binding sites in lymphocytes of pregnant, but not in those of non-pregnant women.
The existence of progesterone receptors (PRs) in pregnancy lymphocytes was only confirmed later, when progesterone receptor-specific antibodies became available. In 1989, together with Gerard Chaouat we demonstrated PRs in peripheral pregnancy-, but not, in non-pregnancy lymphocytes, and showed that the percentage of PR+ peripheral lymphocytes increase throughout normal gestation [9, 10]. The percentage of PR-expressing lymphocytes from women with recurrent miscarriages, pre-term delivery was significantly lower than in those from healthy pregnant women of corresponding gestational ages [9], suggesting a relationship between lymphocyte PR expression and pregnancy outcome.
PR induction in the lymphocytes is an activation-related phenomenon. Exposure of non-pregnancy lymphocytes to mitogenic or alloantigenic stimuli increases PR expression [11]. In circulating lymphocytes of liver transplanted patients of both sexes, the expression of PRs is comparable to that of pregnant women [12], suggesting that allogeneic stimulation by itself is sufficient for inducing PRs. Lymphocyte PR expression is thus, not a consequence of the altered hormonal environment during pregnancy, rather that of the alloantigenic stimulation by the fetal antigens. This concept gained further support from the finding, that paternal leukocyte immunization of women with recurrent miscarriages increased PR expression in lymphocytes [13]. Taken together, these data indicate that lymphocyte activation is responsible for the induction of PRs in immune cells.
The effect of progesterone on the immune system of pregnant women is at least in part receptor-mediated [9, 10, 13–16], however not all immune cells have been confirmed to express PRs. Purified decidual NK cells do not express PRs [17], while both classical PR-A and B have been demonstrated in killer cell immunoglobulin-like receptors (KIR+), but not in KIR− peripheral blood NK cells [18]. Almost 70% of decidual T cells express the γ/δ T cell receptor [19, 20], γ/δ T cells are able to recognize unprocessed foreign antigens in a major histocompatibility complex (MHC) non-restricted manner, and most decidual γ/δ T cell are activated [19–22], therefore, they are likely candidates for recognizing trophoblast presented fetal antigens. The majority of PR+ circulating pregnancy lymphocytes express the γ/δ T cell receptor [23, 24]. These cells might be of decidual origin, where they became activated by trophoblast-presented foetal antigens.
Hormone binding induces structural alterations in the steroid receptor, which enables DNA binding [25] and the induction of genes, resulting in protein synthesis. The progesterone-induced blocking factor (PIBF) is one of the progesterone-regulated genes and the resulting protein is responsible for the immunomodulatory effects of progesterone [26, 27].
The mRNA transcribed from the PIBF1 gene contains 18 exons, and codes for a 90 kDa protein [28] which is a source of several smaller isoforms. The full length PIBF and the small isoforms exert different functions. The 90 kDa form is associated with the nucleus and has been identified as a component of the peri-centriolar satellite [29, 30]. The smaller forms are localized in the cytoplasm [29]. The former has a role in cell cycle regulation, while the small isoforms are secreted and bind to the glycosylphosphatidylinositol (GPI) anchored PIBF receptor [31]. Digesting the anchoring region of the receptor results in a loss of the immunological effects of PIBF [31], furthermore, GPI deficiency is associated with infertility in female mice [32]. The receptor does not have a trans-membrane domain, therefore, following ligand binding forms a temporary association with the alpha chain of the interleukin-4 (IL-4) receptor and signals via the Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway [31].
The two major subsets of T helper (Th) cells include Th1 and Th2 cells with different cytokine production profiles. The Th1 subset secretes the Th1 cytokines, e.g., interferon-γ (IFN-γ), tumor necrosis factor-β (TNF-β), TNF-α, IL-2, which induce strong cell-mediated and inflammatory reactions. Th2 cells, on the other hand, secrete the Th2 cytokines IL-4, IL-5, IL-6, IL-10, and IL-13, which stimulate antibody production. During the major part of normal human pregnancy, the balance of Th1 and Th2 cytokines is shifted towards Th2. The absence of the Th2 dominant cytokine pattern might result in reproductive failure [33, 34], as shown by the fact, that peripheral blood mononuclear cells from women with recurrent spontaneous pregnancy loss secrete Th1 type cytokines upon activation [35, 36].
Upon engagement the PIBF receptor forms a heterocomplex with the alpha chain of the IL-4 receptor, and this explains the effect of PIBF on cytokine synthesis. Signalling via the IL-4 receptor results in increased production of Th2 type cytokines, by which PIBF contributes to the Th2 dominant cytokine pattern during normal pregnancy (Figure 1).
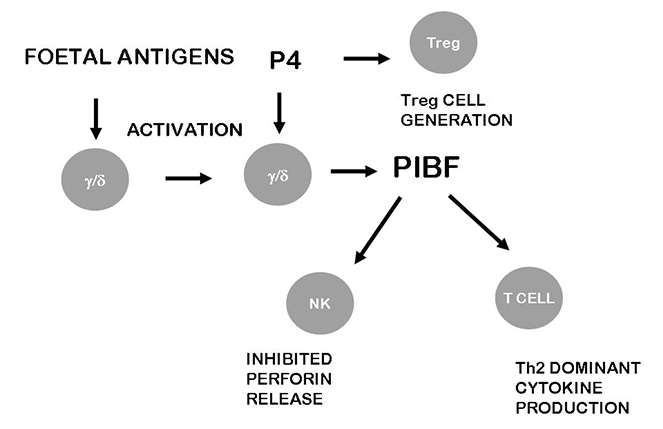
The effects of PIBF on the immune system. Following activation by foetal antigens, maternal lymphocytes express PRs, and in the presence of progesterone, produce PIBF. The small PIBF isoforms bind to the PIBF receptor and activate signaling pathways that lead to increased production of Th2 cytokines. By inhibiting degranulation of NK cells, PIBF contributes to the low NK activity. These together with the positive effect of progesterone on regular T (Treg) cell generation are crucial for moderating anti foetal immune reaction
PIBF treatment results in elevated IL-4 and IL-10 production by peripheral lymphocytes of non-pregnant mice [37]. A prospective clinical trial [38] on women with recurrent miscarriage showed that dydrogesterone treatment induced PIBF production, which down-regulated peripheral Th1 and stimulated Th2 type cytokine production.
PIBF treatment of lymphocytes from women with pre-term delivery inhibited the originally high production of Th1 type cytokines and stimulated the production of Th2 type cytokines [38, 39]. A recent study revealed that the T cells of PIBF-deficient pregnant mice differentiate towards Th1 [40].
Th1/Th2/Th17 immunity [41–43] and Treg cell generation [44] are also affected by progesterone. Tregs isolated from pregnancy blood express membrane PRs. Furthermore, the number of membrane progesterone receptor positive Tregs increases throughout gestation and drops before delivery, suggesting that the pregnancy protective effect of progesterone is partly mediated via Treg cell action [45].
PIBF also affects NK activity in several ways. Several studies reported increased peripheral NK activity in women suffering from miscarriages of unknown aetiology [46–50].
In mice high peripheral NK activity results in fetal loss [51]. Adoptive transfer of high NK activity spleen cells induces resorptions in pregnant Balb/c mice [52], and the high resorption rates can be corrected by PIBF supplementation [53]. Conversely, the development of high resorption rates is counteracted by anti-NK antibody treatment [54], suggesting that PIBF protects pregnancy by keeping NK activity under control.
Granulated decidual NK cells, constitute a dominant lymphocyte population in the early decidua both in human and mice. Though these cells are fully equipped with the cytotoxic enzymes, their killing activity is low [55–57], but secrete angiogenic factors and cytokines instead [58]. PIBF is present in the cytoplasmic granules of mouse decidual NK cells [59] and by inhibiting perforin release [60, 61] contributes to the low cytotoxic activity of these lymphocytes (Figure 1).
Progesterone maintains uterine quiescence until term, when the loss of progesterone results in increased prostaglandin production and the latter initiates parturition by stimulating myometrial contractility [62]. PIBF inhibits phosholipase A2, the enzyme required for the liberation of arachidonic acid [63], thus reduces the availability of the precursor for prostaglandin synthesis.
Insufficient PIBF production increases the risk of preterm labour [64], however, intervening at a different point of the mechanism might allow pregnancy to go to term.
Treatment of women at risk for preterm delivery with low dose aspirin, inhibiting the cyclooxygenase enzyme, resulted in a significantly lower rate of preterm delivery [65]. In line with this, a recent randomized controlled trial showed that low dose aspirin treatment of almost 600 patients significantly prevented preterm birth, and improved perinatal mortality rates [66].
PIBF is present in the urine and the sera of pregnant women. In serum samples of in vitro fertilization (IVF) patients PIBF was detected 14 days after embryo transfer [67], and later the serum concentrations increased by gestational age in uneventful pregnancies [68]. Lower than normal concentrations predict spontaneous pregnancy termination [69, 70] as shown by the finding that not only decidual PR and PIBF expression, but also the serum concentrations of PIBF are characteristically low in women with unexplained miscarriages in [71]. The predictive value of low PIBF levels was suggested in an earlier study showing that in the absence of detectable PIBF at three to five weeks, even seemingly normal pregnancies are at risk of miscarriage [72]. The same group demonstrated a higher percentage of PIBF+ lymphocytes in pregnant than in non-pregnant women [73], as well as a positive effect of lymphocyte immunotherapy on the percentage of PIBF+ cells [74]. Elective pregnancy termination with progesterone receptor antagonists resulted in a decreased percentage of PIBF positive peripheral lymphocytes [75], suggesting a relationship between the availability of progesterone-induced PIBF and the outcome of pregnancy.
In early pregnancy, PIBF induces decidual transformation of mouse endometrial stromal cells, and the endometrial expression of this protein is markedly increased during the implantation window [76], suggesting that PIBF might play a role in implantation.
Depletion of PIBF during the peri-implantation period results in impaired implantation and increased resorption rates in mice, together with high decidual and peripheral NK activity. PIBF deficiency also causes a significant downregulation of the genes implicated in T cell activation and a Th1 type differentiation of the T cells [40].
PIBF treatment normalized the Th1/Th2 ratio, reduced the inflammation, corrected the blood pressure and prevented foetal growth restriction in a rat preeclampsia model [77].
Taken together, these data show that the immunomodulatory effects of progesterone play a role in the maintenance of normal gestation.
RNA expression analyses of several human cell lines revealed an overexpression of PIBF mRNA in highly proliferating, immature cells. Check et al. [78] and Srivastava et al. [79], reported that several human leukemia cell lines expressed PIBF mRNA and some of them the PIBF protein. The addition of progesterone to the media increased the expression of PIBF while blocking the progesterone receptor downregulated its expression.
In tumour cells, the full-length PIBF shows a co-localization with the centrosome [29], and has been identified as a component of the pericentriolar satellite [30]. These data, together with the identification of the PIBF gene on chromosome 13, close to the site for somatic deletions in several malignant tumours [80]—suggest that PIBF might be involved in tumour development (Figure 2).
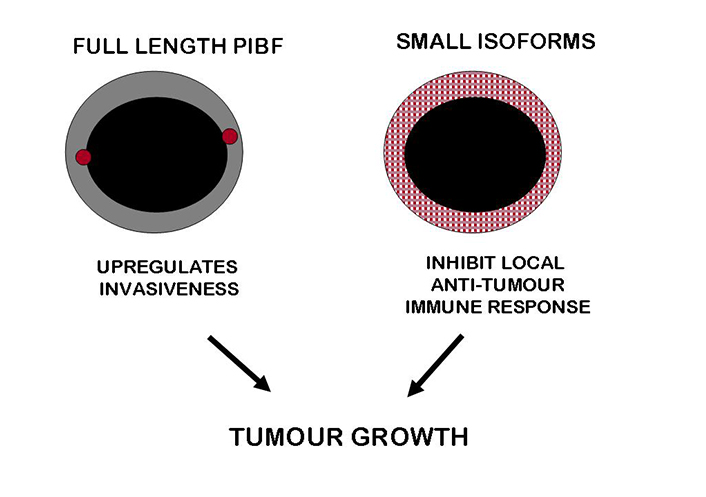
PIBF acts in favor of tumour progression. The full length PIBF is associated with the pericentriolar satellite and upregulates the invasiveness of tumour cells. The smaller isoforms present in the cytoplasm are secreted, and by inducing a Th2 dominant cytokine production as well as reduced NK activity, inhibit local anti-tumour immunity. These together, result in enhanced tumour growth
Controlled trophoblast invasion is a key process during human placentation and a prerequisite of successful pregnancy [81]. PIBF is expressed by the trophoblast, and also by tumours and regulates invasiveness of both tissues. The distribution of PIBF within the first trimester decidua coincides with trophoblast invasion [82]. PIBF is expressed in both normal 1st trimester villous trophoblast and in partial mole, but PIBF expression markedly decreased in complete mole and absent in choriocarcinoma [83]. These data suggest that PIBF expression is inversely related to trophoblast invasiveness.
Invasiveness is a common feature of trophoblast and malignant tumours; however, while tumour invasion is unlimited, trophoblast invasion is strictly controlled. Silencing of PIBF increased invasiveness as well as matrix metalloproteinase-2 (MMP-2) and MMP-9 secretion of trophoblast, and decreased those of tumour cells. Invasion-related signaling by PIBF was in both cell types initiated through the IL-4Rα/PIBF receptor complex [84].
In vivo experiments suggest that PIBF might indeed contribute to tumour development [85]. Treatment with progesterone receptor blockers increased the length and quality of life of mice with spontaneous leukemia. Within 2 weeks of therapy only 11.4% of the mifepristone treated mice died vs. 50% of controls [86].
Taken together, these data show that PIBF promotes tumour growth.
Starting from the peri-implantation period, PIBF plays a key role during pregnancy.
PIBF induces decidual transformation of endometrial stromal cells, and its expression in the mouse endometrium peaks during the implantation window, suggesting that this molecule is required for successful implantation. PIBF deficient mice are characterized by low implantation, and later on, by high resorption rates. The latter is due to the loss of PIBF-associated immunological effects, e.g., the Th2 dominant cytokine balance and down-regulated NK activity, both of them crucial for creating a friendly immunological environment for the developing foetus. Furthermore, the lack of PIBF results in dysregulated trophoblast invasion, which might result in trophoblastic diseases or preeclampsia.
PIBF is highly expressed by malignant tumours and tumour cell lines, suggesting its role in tumour development. This molecule might promote tumour growth via different pathways. The immunological effects of the short isoforms could dampen local anti-tumour responses; the full-length molecule associated with the centrosome might interfere with the normal cell cycle, and upregulate the invasiveness of tumour cells.
Taken together, by using the same mechanisms, PIBF which promotes normal gestation, acts as a foe in malignant tumours.
GPI: glycosylphosphatidylinositol
IL-4: interleukin-4
NK: natural killer
PIBF: progesterone induced blocking factor
PRs: progesterone receptors
Th2: T helper2
Treg: regular T
The author contributed solely to the work.
The author declare that she has no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This work was supported by European Social Fund (EFOP-3.6.3.-VEKOP-16-2017-00009) and the Research Grant of the University of Pecs (PTE Á OK-KÁ 2017–22 EFOP-3.6.1.-16-2016-00004). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2021.
Copyright: © The Author(s) 2021. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Gustaaf Albert Dekker, Pierre Yves Robillard
Kushaan Khambata ... Satish K. Gupta
Gursaran P. Talwar ... Krishna M. Ella
Shigeru Saito ... Sayaka Tsuda
Noémie Abisror ... Arsene Mekinian
Ruchi Sachdeva, Rahul Pal
Shibin Cheng ... Surendra Sharma
Betcy Susan Johnson, Malini Laloraya
Alison McCallion ... Chandrakant Tayade
Pier Luigi Meroni ... Francesco Tedesco
Marijke M. Faas
Alaa Kazhalawi ... Nathalie Lédée
Chiara Agostinis ... Roberta Bulla
Mickey V. Patel ... Charles R. Wira
Thanh Luu ... Joanne Kwak-Kim
