Affiliation:
Immunoendocrinology group, Division of Medical Biology, Department of Pathology and Medical Biology and Department of Obstetrics and Gynecology, University of Groningen and University Medical Center Groningen, 9713 GZ Groningen, the Netherlands
Email: m.m.faas@umcg.nl
ORCID: https://orcid.org/0000-0002-5860-3993
Explor Immunol. 2022;2:518–539 DOI: https://doi.org/10.37349/ei.2022.00065
Received: February 02, 2022 Accepted: April 27, 2022 Published: August 25, 2022
Academic Editor: Satish Kumar Gupta, Indian Council of Medical Research, India
The article belongs to the special issue Human Reproduction: Involvement of the Immune System
Uterine natural killer (uNK) cells, a specific type of natural killer (NK) cells, are important cells at the foeto-maternal interface in humans as well as in mice. uNK cells are part of the innate lymphoid cells group 1. Especially in the mouse, but also in the rat, many in vivo studies have been performed to evaluate the role of uNK cells in placental development. These studies have shown that uNK cells are not indispensable to pregnancy, but that they play an important role in optimal decidual angiogenesis in early pregnancy, trophoblast invasion and spiral artery remodelling in the mouse placenta. Based on the mouse studies, various in vitro studies, as well as immunohistological studies of the human placenta from elective abortions, have shown that uNK cells have similar functions in the human placenta. In the present narrative review, the role of the uNK cells in the development of the mouse and rat placenta will be discussed first. Thereafter, studies on the role of human uNK cells in the human placenta will be reviewed and these studies will be discussed in the light of the knowledge on mouse uNK cells.
Natural killer (NK) cells are innate immune cells with cytolytic functions [1]. They belong to the group of innate lymphoid cells (ILCs). ILCs are a diverse group of cells, which are important for immunity and tissue development and remodelling [2]. They are subdivided into 3 groups based on the similarity with cytokine production and transcription factors with the various T cell subsets [2]. NK cells belong to the group 1 ILCs [2, 3]. Next to their cytolytic function, NK cells also produce proinflammatory and regulatory cytokines. Human NK cells are characterized by the expression of CD56 and lack of expression of CD3 [1]. In the peripheral circulation, two major types of NK cells can be distinguished: CD56bright and CD56dim NK cells [1]. The CD56dim NK cells are mature cells, co-express CD16, have a cytolytic function and make up 90% of the NK cells in the peripheral circulation [1]. CD56bright NK cells are more immature NK cells, do not co-express CD16, comprise about 10% of all circulating NK cells and are considered poorly cytotoxic, but cytokine producers [1].
In the uterus, group 1 ILCs are important for placental and foetal development. The main cells of this group 1 ILCs are uterine NK (uNK) cells [4]. The first detection of uNK cells in the placenta was in mice in the 1980s [5, 6]. Soon thereafter, large granular lymphocytes (LGL), were detected in the human decidua [7]. These cells were uNK cells. Since then a lot of research has been done on uNK cells and their function at the foeto-maternal interface. uNK cells have important functions in for instance decidual angiogenesis and in spiral artery remodelling [8]. Although they are CD56bright and produce cytokines and other factors, they have a different phenotype and function as compared with circulating CD56bright NK cells—they are granulated and produce various molecules, such as angiogenic molecules [9]. Similar to uNK cells in the human placenta, uNK cells in the mouse placenta are granulated and produce various cytokines, angiogenic factors and growth factors [10, 11].
One of the first studies showing the role of uNK cells in placental development and therefore foetal survival was a study by the group of B. Anne Croy, showing that foetal loss occurred in mice lacking uNK cells [12]. Since then many studies have been performed on mouse and human uNK cells and their role in healthy placental and foetal development and growth as well as in pregnancy complications. In various pregnancy complications, different numbers and functions of uNK cells have been observed. For instance women with preeclampsia, it has been suggested that excessive inhibition of uNK cell function may play a role in incomplete trophoblast invasion and spiral artery remodelling in these patients [13]. Also, the numbers of uNK cells may be decreased in preeclampsia as well as in foetal growth restriction [14]. Other complications of pregnancy have been associated with increased numbers of uNK cells, such as in women with recurrent miscarriage or unexplained fertility [15–17]. The studies on uNK in pregnancy complications suggest the importance of uNK cells for normal placental and foetal growth and development.
This narrative review aims to give an overview of the role of uNK cells in normal placental development and function. We will first focus on the role of uNK cells in the mouse placenta since the role of the uNK cells in placenta development has mostly been studied in this species. We will also discuss some studies in rat placentas, which are additive to the mouse studies. This will be followed by reviewing studies on the role of uNK cells in the human placenta and indicating the role of mouse studies in defining the role of uNK cells in human placental development.
After fertilization and various cell divisions, the fertilized oocyte develops into the blastocyst, which implants into the endometrium [18]. The embryo develops from the inner mass of the blastocyst, the embryoblast, while the placenta develops from the outer layer of the blastocyst, i.e. the trophoblast [18]. The placenta supports the foetus during pregnancy by exchange of gasses and nutrients and waste products. This takes place in the chorionic villi, of which the syncytiotrophoblasts, i.e. the outer lining, are in direct contact with maternal blood. This part of the placenta is also called the foetal part of the placenta. The foetal part of the rodent placenta has a labyrinthine structure, rather than a villous structure. Also in the labyrinth, foetal syncytiotrophoblast is in direct contact with maternal blood [19].
Rodents, such as mice and rats, like humans, have a haemochorial placenta, indicating intimate contact between foetal and maternal tissue [19]. This intimate contact is observed in the foetal part of the placenta but also at the foeto-maternal interface. This interface is not identical between human and rodent pregnancy (see also Figure 1). The uterine response to pregnancy, i.e. to the implanting blastocyst and the invading foetal trophoblast cells is, however, similar between human and rodent pregnancy [20]. This response includes implantation of the blastocyst, which induces endometrial decidualisation, an increase in the numbers and composition of maternal immune cells at the site of implantation as well as placental vascular remodelling [20]. Thus, the foeto-maternal interface consists of decidualised maternal endometrium, with many maternal immune cells, such as uNK cells, as well as invaded foetal trophoblast cells. This part is sometimes also called the maternal part of the placenta.
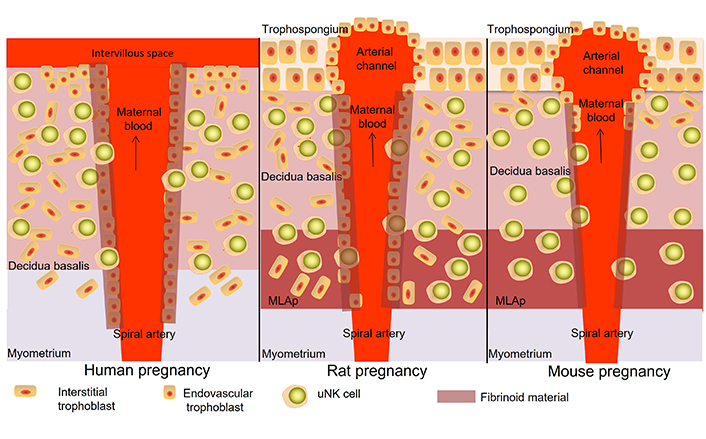
Location of uNK cells in the placenta of early to mid-pregnancy of humans (left), rats (middle) and mice (right). In mice and rats, uNK cells can be found in the decidua basalis and mesometrial lymphoid aggregate of pregnancy (MLAp). They can be found close to trophoblast cells, around the spiral arteries and in the arterial wall as well as scattered throughout the decidua and MLAp. In mice, trophoblast invasion is only seen in the first part of the decidua, close to the trophospongium, while in rats, trophoblast invades throughout the whole decidua and into the MLAp. In the human placenta, uNK cells can be found in the decidua basalis, closely related to trophoblast cells, around and in the wall of the spiral arteries as well as scattered throughout the decidua. In human, rat and mouse placentas also other immune cells can be found, such as macrophages, dendritic cells and T cells (not shown in this figure)
Note. Adapted from “Uterine NK cells and macrophages in pregnancy,” by Faas MM, de Vos P. Placenta. 2017;56:44–52 (https://www.sciencedirect.com/science/article/pii/S0143400417301790?via%3Dihub). CC BY.
Foetal trophoblast invasion at the foeto-maternal interphase is different between human and rodent placentas. Foetal trophoblast invasion is very invasive in humans, even into the myometrium, while in mice trophoblast invasion is very restricted with only limited invasion into the decidua [21]. In the rat, trophoblast invasion is more similar to humans and is observed throughout the decidua and into the MLAp, also called the mesometrial triangle [22, 23]. The localisation of uNK cells in the placenta is different between humans and rats/mice since in human pregnancy, uNK cells are mainly found in the decidua [21], while in mouse and rat pregnancy uNK cells are found in the decidua as well as in the MLAp [21]. The MLAp develops in the myometrium in early to midgestation in mice and rats from decidualised endometrial cells and maternal immune cells and can be compared with the deeper part of the maternal placental bed in humans (see also Figure 1) [24].
Despite the differences in early placental development and uNK cell location between rodents and human placentas, much of the knowledge on uNK in placental development and function is derived from rodent studies, especially from mouse studies. Many of the mouse studies on uNK cells have been performed by the group of B. Anne Croy from Kingston, Canada and therefore much of our knowledge on uNK cells is derived from this group, supplemented and confirmed by others. This will be described below. Studies on rat uNK cells if they are supplemental to the knowledge on uNK cells from the mouse studies will also be described.
After implantation of the blastocyst, decidualisation is initiated and as a result of the decidualisation, immune cells are attracted to the decidua and angiogenesis is initiated and the decidual vessels undergo structural and molecular changes [25]. This is followed by invasion of foetal trophoblasts and further increased numbers of leukocytes into the decidua basalis and further development of the placenta [25]. The main populations of immune cells found in the decidua basalis in early pregnancy in the mouse are uNK cells, macrophages and dendritic cells [10]. Unique to the mouse placenta is the presence of B-cells in the early placenta [10]. Helper T cells and cytotoxic T cells are hardly found [10].
The dominant immune cell in the decidua in early pregnancy is the uNK cell [10]. Although these cells are rare immediately after implantation in mice (day 4.5 of gestation), there are many uNK cells in the decidua basalis on the mesometrial side as of day 5.5 of gestation, with the number of uNK cells peaking between day 10.5 and day 14 of gestation in the mouse [10]. At this peak, uNK cells can be found in the decidua basalis and the MLAp [26] and are localised around and in the arterial wall of spiral arteries and scattered throughout the tissue (see Figure 1) [26]. After day 14 of gestation, the numbers of uNK in the placenta decline towards the end of pregnancy [26].
Although not so well studied as in the mouse placenta, in the rat placenta, uNK cells also increase in number in early pregnancy in the decidua [27]. The peak of the uNK cells in the decidua of and the MLAp of rats is around day 13–14 after which there is a demise of the uNK cells in the direction of the MLAp and at the end of pregnancy, there are only a few uNK cells in the MLAp, only at the outer border, close to the myometrium [21]. uNK cells are found throughout the decidua and MLAp but are mainly concentrated around the spiral arteries [21].
uNK cells in the mouse placenta were classically defined by the presence of periodic acid shift (PAS) positive granules [28]. Later it was shown that uNK cells in the mouse, but not other NK cells, were positively stained with Dolichos biflorus agglutinin (DBA) lectin [29]. DBA lectin stained both the uNK cell granules and the cell membrane [29]. Further studies have shown that mouse uNK cells are either PAS+DBA+ (and also NK1.1–DX5–) [30] or PAS+DBA– (and NK1.1+DX5+) [26, 30]. The latter subset resembles peripheral NK cells [30] and is smaller than the DBA+ subset [30]. The PAS+DBA+ subset is unique to the decidua basalis and MLAp of pregnancy [26]. These two subsets are present in equal numbers in the decidua basalis in early pregnancy (gestational day 6), but at later pregnancy days, i.e. by midgestation, much more PAS+DBA+ uNK cells can be found in the decidua as compared with PAS+DBA– uNK cells [26]. In the MLAp, also more DBA+ as compared to DBA– uNK were found at midgestation [26]. Especially the DBA+ uNK cells were associated with the vascular wall [26]. The DBA+ and DBA– subsets do not only differ in their presence and localisation, but they also differ in gene expression. The DBA+ subset expresses more angiogenic factors, such as vascular endothelial growth factor (VEGF)A and placental growth factor (PLGF) and perforin and granzyme [11, 31], while the DBA– subset is the main producer of interferon (IFN)γ [11].
In more recent papers, uNK cells are subdivided into uterine tissue-resident NK (trNK) cells and uterine conventional NK (cNK) cells. The uterine trNK cells are mainly PAS+DBA+ uNK cells and produce angiogenic factors, while the uterine cNK cells are mainly PAS+DBA– NK cells, producing more IFNγ [32]. In the remainder of this review, the terms trNK and cNK cells will be used rather than DBA+ and DBA– uNK cells (see also Figures 2 and 3).
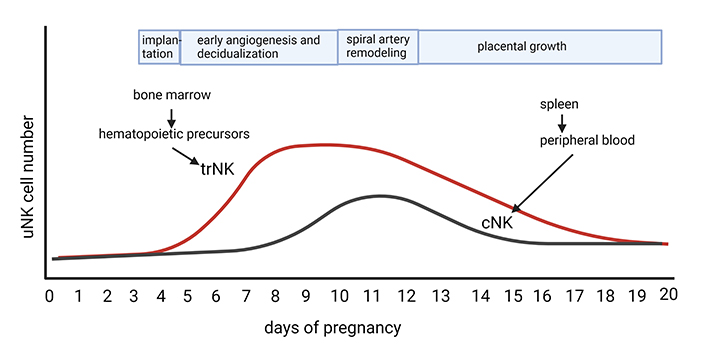
Number of uNK cells at different time points of pregnancy in the mouse. The mouse placenta has two uNK cells subsets, trNK and cNK cells. trNK cells are derived from hematopoietic precursors from the bone marrow and proliferate in early pregnancy and peak in the placenta from day 8–12 and thereafter decrease towards the end of pregnancy. These cells are important for decidual angiogenesis and spiral artery remodelling. cNK cells are recruited from the peripheral blood to the placenta around day 9 and peak on days 11–12. They produce IFNγ, which is important for spiral artery remodelling (Created with BioRender.com)
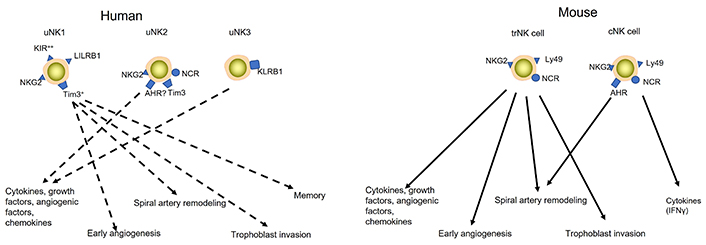
uNK cells subsets in humans (left) and mice (right). Humans have three uNK cell subsets: uNK1, uNK2, and uNK3. Although current knowledge indicates that human uNK cells are involved in decidual angiogenesis, trophoblast invasion and spiral artery remodelling, which subsets execute which function is not completely known. Therefore, dashed lines indicate which uNK cell subset may be involved in which function. Mouse uNK cells consist of two subsets, trNK and cNK cells. Various studies have shown that trNK cells are involved in decidual angiogenesis, trophoblast invasion and spiral artery remodelling, while cNK cells produce IFNγ, which is involved in spiral artery remodelling (indicated by uninterrupted lines). At this time uNK subsets have not been studied in the rat. KIR: killer-cell immunoglobulin-like receptor; NKG2: NK group 2; AHR: aryl hydrocarbon receptor; Tim3: T cell immunoglobulin and mucin domain-containing protein 3; NCR: natural cytotoxicity receptor; LILRB1: leukocyte immunoglobulin-like receptor B1; KLRB1: killer cell lectin-like receptor B1; ?: it is not sure that this receptor is expressed on these cells
The source of uNK cells has been of interest for a long time. The question of whether uNK cells are derived from an in situ uterine precursor or home from the periphery to the uterus during pregnancy is still not completely solved, since this was and remains a difficult subject to study. The use of alymphoid mice and NK cell-deficient mice have given insights into the source of uNK cells in mice. Using alymphoid mice, the group of B. Anne Croy has shown that no uNK (no trNK nor cNK) cells were found in the implantation sites of day 12 alymphoid pregnant mice [26]. Following bone marrow transplantation of normal lymphoid mice, uNK cells were relatively normal in the decidua basalis and the MLAp at day 12.5 of gestation and almost similar in numbers and location to control immune-competent pregnant mice at this day [26]. These uNK cells were exclusively PAS+DBA+ (trNK cells), no PAS+DBA– uNK cells (cNK cells) were found in the decidua basalis and MLAp of these mice [26]. This suggests that trNK cells are derived from homed progenitor/precursor cells from the bone marrow and that most uNK cells at this stage of pregnancy, i.e. trNK, are of non-uterine origin. They may ultimately originate from a hematopoietic precursor from the bone marrow [33]. It may also suggest that the cNK cells do not arise from the bone marrow. Since earlier studies have shown that uNK cells may be derived from the spleen, it may be suggested that the cNK cells are peripheral NK cells derived from the spleen and home to the decidua basalis and MLAp [34].
Using another elegant model, i.e. Ncr1iCreXRosaMt/MG reporter mice, mice were studied from day 5.5 until day 11.5 of pregnancy and it was shown that, in line with previous experiments, the pregnant uterus contained cNK cells and trNK cells during this pregnancy period [32]. The trNK cells were the only NK cells able to proliferate and they were PAS+DBA+ uNK cells. Although the cNK cells in the uterus increased in number, this was not due to proliferation [32], suggesting that they home to the uterus from other sources. As indicated above, this other source may be the spleen [34].
Further studies into the cNK cells and trNK cells in the decidua and MLAp used knockout (KO) mice for nuclear factor interleukin 3-regulated (Nfil3), which lack peripheral NK cells, but still have trNK cells [35]. In these mice, although both cNK and trNK cells differentiate and uNK cells localise and mature at implantation sites similar to control mice, the abundance of uNK cells is much lower compared to control mice, especially for cNK cells (decreased by about 75–80%) [35]. The authors suggested that less uterine trNK were present in Nfil3 KO mice. This has indeed been shown by Boulenouar et al. [36]. Since the uNK cells show relatively normal, terminal maturation, it is suggested that the uNK cells do not require (Nfil3) for their maturation [35]. Despite this, it was suggested that the uNK cells in Nfil3 KO mice were functionally disturbed since spiral artery remodelling was diminished [35]. An alternative explanation may be that diminished spiral artery remodelling was not due to diminished function of the uNK cells present in these mice, but was due to a lack of cNK cells in these KO mice; these cNK cells are the cells mainly involved in IFNγ production [11], which is necessary for spiral artery remodelling [37]. Since these cNK cells are derived from peripheral NK cells, which infiltrate into the decidua basalis and MLAp during spiral artery remodelling, these uNK cells are not present in Nfil3 KO mice [35].
Together these data may suggest that there may be different sources for different subtypes of uNK in the mouse and that the different subtypes may be present at different times of pregnancy. The trNK cells mainly accumulate in early pregnancy by the proliferation of already present trNK cells. This subtype may be important for decidualisation and decidual angiogenesis. The cNK cells accumulate later, are recruited from the periphery, and are important for spiral artery remodelling (see also Figure 2).
In the virgin uterus, NK cells are small, agranular lymphocytes and mature uNK cells are only found in the uterus after blastocyst implantation and decidualisation [38, 39]. Maturation involves uNK cell proliferation and acquiring cytoplasmic granules. For this maturation process, interleukin (IL)-15 is indispensable, since in IL-15 KO mice, uNK cells do not mature [40]. IL-15 is produced by stromal cells in the decidualising uterus [41].
While IL-15 is important for uNK cell maturation and maintenance, other stimuli are important for the activation of uNK cells and there their functional activities. uNK cells can become activated in various ways. uNK cells can become activated by integrating positive and negative signals from inhibitory and activating Ly49 receptors that bind to major histocompatibility complex (MHC) molecules [42]. Ly49 receptors in mice have similar functions to KIRs in humans. Both trNK cells and cNK ells express Ly49 [42]. In broad-spectrum Ly49 knockdown mice, the numbers of uNK cells were not affected, although these mice were usually infertile [42]. When pregnancy developed, decidual angiogenesis was lagging, with decreased VEGFA production [42]. However, the production of IFNγ or perforin was not reduced [42], suggesting that the production of IFNγ or perforin is not regulated by the binding of Ly49 receptors to its ligands.
The MHC dependent receptors on the uNK cells can bind to classical and non-classical MHC-I. Most classical MHC-I is expressed on maternal cells in the placenta, while non-classical MHC-I is expressed on the trophoblast. Education of NK cells is the priming and calibrating of NK cell function, which is regulated via inhibitory receptors on NK cells [43]. Studies have indicated that in the mouse uNK cells education mainly takes place via CD94/NKG2, a natural-killer group 2 receptor, which has an important role in NK cell cytotoxicity [44]. NKG2 member A (NKG2A) is an inhibitory receptor and is involved in uNK cells education at the foeto-maternal interface [44]. NKG2A expression was observed in both uNK cells subsets in the mouse [44]. uNK cells positive for NKG2A are involved in spiral artery remodelling, probably related to IFNγ production, foetal growth and brain development [44]. Education of uNK cells via NKG2A is induced by binding to receptors on maternal cells and not to receptors on foetal cells, since the ligand for NKG2A in the mouse (Qa-1) is not expressed on mouse trophoblast [44, 45]. Also, NKG2D is expressed by mouse uNK cells. NKG2D is an activating receptor and is more strongly expressed in cNK cells as compared to trNK cells [30].
In addition to MHC-dependent receptors, other NK cells receptors, i.e. MHC-independent receptors, have been shown to play a role in uNK cells activation in the mouse, such as NCR1, the homologue of NKp46 in humans. The NCR receptor was most expressed by trNK cells in the decidua as compared to cNK cells [46]. KO mice lacking this receptor showed normal numbers of NK cells in the uterus [46]. However, uNK cells (both trNK and cNK) remained smaller, indicating impaired maturation and activation [46, 47]. The immaturity of uNK cells in NCR KO mice is also reinforced by the finding that uNK cells in these mice were lytically inert [47]. The impaired maturation is also in line with the finding that early implantation sites in NCR1 KO mice showed limited angiogenesis [46], and trNK cells showed increased VEGF production with decreased PLGF production [47]. As the impairments in early decidual angiogenesis in the NCR1 KO mice are comparable to PLGF deficient mice [48], PLGF may be the most important angiogenic factor for early decidual angiogenesis, which cannot be substituted by VEGF.
The AHR is also expressed on uNK in the mouse and rat [46, 49]. AHR is a ligand-dependent transcription factor responding to environmental pollutants, such as aromatic hydrocarbons, and endogenous metabolites, for instance, products produced by the gut microbiota (f.i. indole derivatives) [50]. In mice, AHR expression is mainly seen on cNK cells [46]. Lack of this receptor in pregnancy does not result in decreased amounts of uNK cells, but the proportion of cNK cells is decreased [46], suggesting that AHR is important for recruiting cNK cells. In mice lacking the AHR, uNK were smaller and spiral artery remodelling was deficient [46]. This suggests a role for AHR in uNK cells maturation; the lack of cNK cells seems to be in line with deficient spiral artery remodelling. Recent studies in pregnant rats have shown that dioxins, a well-known ligand of AHR, resulted in placental adaptions and changes in uNK cells. This study, however, showed that endothelial cells were the predominant direct cellular site of dioxins and effects on uNK cells may be indirect [49].
Thus, both Ly49 receptors and NKG2D receptors as well as MHC independent receptors are important for the maturation and activation of uNK cells (see also Figure 3). AHR may be especially interesting since it links uNK with deficient spiral artery remodelling, which is a hallmark of the important pregnancy complication preeclampsia.
Blastocyst implantation into the mouse uterus is at gestation day 4.5 and initiates massive structural changes in the uterus. The first changes include decidualisation. The decidua is visible from gestational day 5.5 [10]. At that time, also decidual angiogenesis starts with the development of decidual capillaries [10]. There is an increase in the number of vessels and CD31+ endothelial cells showing blebbing [10], which is a sign of endothelial tip cell differentiation and sprouting. Initially, the role of uNK cells in early decidual angiogenesis was shown by histological studies indicating that at the time of decidual angiogenesis uNK cells can be found in the neighbourhood of the developing vasculature in the decidua basalis [51, 52]. These uNK cells are mainly trNK cells [11]. To aid in the angiogenic process in early placentation, these trNK cells in the decidua produce various angiogenic factors, such as VEGFA, VEGFC, PLGF, transforming growth factor (TGF)β1, metallomatrix proteinases (MMPs), tumour necrosis factor (TNF)α and inducible nitric oxide synthase (iNOS) [11, 51, 53], but also the delta-like ligand (DLL)1 [52]. The role of VEGF in decidual angiogenesis is most studied: it is important for sprouting [54] and VEGF expression is widespread in uNK cells as well as in epithelial and stromal cells immediately surrounding the blastocyst on day 5, and in the decidual cells as of day 6 [55]. The definite role of VEGF in implantation, angiogenesis and placentation in the mouse is seen from the fact that embryos in VEGF KO mice are lethal [56–58].
The role of uNK cells in decidual angiogenesis has been shown by in vivo studies in which alymphoid mice were used (Rag2–/–/Il2rg–/– mice lacking NK cells and T and B cells) [26]. Although decidual angiogenesis occurs in these mice, indicating that uNK are not an absolute requirement for decidual angiogenesis, the onset of angiogenesis was delayed, while also a delay in vessel maturation was observed [59]. This suggests that uNK cells not only produce VEGF or other angiogenic factors but also factors that induce vessel maturation [59]. The definite role for uNK cells in decidual angiogenesis was shown by subsequent transplantation studies, in which the Rag2–/–/Il2rg–/– mice were reconstituted preconceptionally with Rag2–/– bone marrow (containing NK cells but not T and B cells) and which showed complete normalisation of the decidua basalis angiogenesis [12, 59].
During pregnancy, spiral arterioles in the decidua and MLAp are remodelled into high capacity, low resistance vessels with thin walls and large lumens [60]. In the mouse, this takes mainly place between day 9 and day 14 of pregnancy [24]. This remodelling is important since it is thought to be necessary for the increased nutritional demands of the foetus during pregnancy. In mice, 5–10 spiral arteries in the MLAp and decidua converge in the layer of trophoblast giant cells into only a few trophoblast-lined canals. These canals bring the maternal blood to the base of the placenta from where the blood flows into the intervillous spaces of the labyrinth [24]. Although foetal trophoblasts are important for spiral artery remodelling in both humans and mice, in mice the role of the trophoblast is less well established but may be less important since in the mouse trophoblast invasion is less deep [24]. Trophoblast cells only invade spiral artery segments in the decidua close to the main layer of trophoblast giant cells [24]. Since spiral artery remodelling is also found in the MLAp, in which uNK are found but no trophoblasts, uNK cells are thought to be the most important cell aiding in mouse spiral artery remodelling. This is in line with the fact that various NK-cell deficient mice models, also lacking uNK cells, have defective spiral artery remodelling [12, 31, 61]. Later it was established that IFNγ, produced by the uNK cells, but independent of the uNK cells, is important for this spiral artery remodelling [37]. The IFNγ is derived from cNK cells that infiltrate into the decidua during the time of spiral artery remodelling [11]. The mechanisms by which IFNγ induces spiral artery remodelling are not completely understood yet. However, IFNγ likely regulates gene expression by cells in and around the arteries, since IFNγ is known to regulate the expression of many genes [62]. Such genes could be VGEF, iNOS and alpha 2-macroglobulins which are known to destabilize vessels [62–64]. Alternatively, IFNγ can also activate macrophages [65], which can also be found close to spiral arteries [21]. Macrophages are known producers of MMPs, which may be involved in spiral artery remodelling [66].
In the rat, uNK cells are also involved in spiral artery remodelling, although in this species, trophoblasts seem to be more important in spiral artery remodelling [21, 67, 68]. UNK cells in the rat induce (partial) disruption of the spiral artery tunica media [68]. Our group found uNK cells in the presence of partially remodelled spiral arteries, in the absence of trophoblast [21]. This initial remodelling by uNK cells of the spiral arteries is followed by further remodelling of these arteries by invasive trophoblasts [27, 68].
In rodents, both mice and rats, uNK cells have been shown to suppress trophoblast invasion. Various studies have shown that the trafficking of trophoblast into the decidua and MLAp was inversely related to the presence of uNK cells [21, 67, 69, 70]. Further studies have shown that in mice lacking NK cells (such as the Tge26 transgenic mice), trophoblast invasion was accelerated [69]. Similar results were found in the rat by using anti-asialo GM antibodies, which deplete NK cells [68]. IFNγ null mutant and IFNγ receptor null mutant mice show early-onset trophoblast invasion, suggesting a role for IFNγ produced by uNK in the suppression of trophoblast invasion in the mouse [69]. The role of IFNγ on trophoblast was corroborated by in vitro studies [69].
Recent research may suggest that uNK cells possess a memory [71]. It has been suggested that in the mouse decidua memory cells exist, but these are not uNK cells but other cells of the ILC-1 groups of cells. These specific ILC-1 cells are 4–5 fold increased in midgestation in the second compared with the first pregnancy [4]. These cells express C-X-C motif receptor 6 (CXCR6), a marker associated with memory NK and NKT cells [71]. These data suggested that CXCR6+ ILC-1 are potential memory cells of pregnancy.
uNK cells in the human endometrium and placental bed do express high levels of CD56 and lack CD16 expression, akin to the CD56bright/CD16– peripheral NK cell population [9]. However, in contrast to the peripheral NK cells, uNK cells are granulated and express different markers [9, 72], such as higher levels of KIRs and NKG receptors as compared with blood NK cells [9, 72]. KIRs and NKG receptors are important for uNK function. The binding of these receptors to human leukocyte antigen (HLA) molecules on trophoblast affects uNK cell function, for instance for the production of certain growth factors [73] or their role in spiral artery remodelling [73].
CD56bright uNK cells are present in the endometrium before pregnancy and expand and become more granulated in the luteal phase of the menstrual cycle [74, 75]. In the early pregnancy decidua, their numbers increase even further [74, 75]. In early pregnancy, more than 70 % of the decidual leukocytes are uNK cells and therefore these cells are the dominant population in the decidua at that time [72]. uNK cells decrease after midgestation but remain present during all stages of pregnancy [76, 77]. In healthy pregnancy, uNK cells are usually found close to invading foetal trophoblast cells [78], as well as around spiral arteries that are being remodelled and scattered throughout the tissue (see Figure 1) [73, 74, 79].
Also in the human decidua, uNK cells are not a homogeneous population of cells, but heterogeneity exists in human uNK cells (see also Figure 3). These subsets, however, are less well characterized than in the mouse. Recent studies have shown that in the first-trimester decidua, three subsets of uNK cells can be found: uNK1, uNK2 and uNK3 [80, 81]. Each subset showed a unique transcriptome [80]. By looking at the expression profile of different markers, it was shown that uNK1 express higher levels of KIR as compared to the other subsets [80, 81]. uNK1 is also the only subset that expressed LILRB1, which can bind to HLA-G [80]. Both uNK1 and uNK2 express NKG2 receptors, which can bind to HLA-E molecules [80]. uNK3 expresses KLRB1, a member of the C-type lectin family (which is also expressed by uNK2, but at a much lower level), which may have inhibitory effects on trophoblasts [80]. Although at the moment it is difficult to relate the human uNK cells subsets to their mouse counterparts, the uNK1 cells may resemble the trNK cells in the mouse decidua [80, 81]. The peripheral blood NK (pbNK) clusters in the human decidua may resemble cNK cells in mice [81]. Based on the expression of IFNγ, also the uNK3 may resemble cNK cells in mice [81]. The exact function of the uNK subsets in the human decidua is unknown yet; however, it was shown that uNK1 cells have more cytoplasmic granules and express more granule proteins, such as perforin and granzyme [81]. They also have a more active glycolytic metabolism [81]. This together with their increased expression of KIR, the uNK1 subset is suggested to particularly interact with extravillous trophoblast [80]. This suggestion is in line with the suggestion that they resemble trNK cells in mice. The uNK2 and uNK3 subsets produced more cytokines/chemokines after stimulation [81]. In analogy with the mouse cNK cell subsets, it may be suggested that (one of) these subsets by producing cytokines [80] affect the function of other decidual cells such as macrophages, smooth muscle cells or uNK1 cells and in this way affect placental development. Unfortunately, it is difficult to test this hypothesis in vivo in the human decidua. Further studies are needed to identify the function of the different uNK cell subsets in humans. In view of the fact that at this time little is known about the role of the different uNK cell subtypes at the human maternal interface, in the remainder of this part of the review on human uNK cells, data will be discussed for the total population of uNK cells.
Like in the mouse decidua, for the human decidua two sources of uNK cells have been suggested: a local presence of precursor cells and trafficking of pbNK cells into the decidua. The presence of local hematopoietic precursor cells (HPCs; CD34+ positive cells) has been shown in the endometrium [82] as well as in the early decidua of pregnancy [83, 84]. They differed from peripheral blood or cord blood-derived CD34+ cells and expressed NK cell-specific transcription factors [84]. In the presence of IL-15 the main uNK cell activation/maturation factor, and other cytokines, or conditioned medium from decidual stromal cells, these HPCs isolated from decidua could be differentiated towards uNK like cells [83, 84]. Alternatively, another report suggests that uNK cell maturation does take place in the decidua, but not from HPCs, but from an immature uNK cells precursor present in the endometrium or decidua [85], which can locally differentiate into uNK cells.
In addition, the possibility of recruitment of pbNK cells into the decidua in pregnancy has also been suggested. Factors secreted from the decidua, such as C-X-C motif ligand 10 (CXCL10), CXCL12, and CX3C chemokine ligand 1 (CX3CL1) could attract pbNK cells to the decidua [86], where these NK cells could differentiate into uNK cells [87]. Evidence for this comes from various in vitro studies in which for instance pbNK cells could be converted into uNK like cells after culture with conditioned medium from decidual stromal cells [88–90].
In line with mouse uNK cells, IL-15 is important for the maturation and survival of uNK cells in humans [91]. Other triggers are necessary for the activation of uNK cells. It is well-known that uNK cells are activated by KIR receptors (both inhibitory and activating) [92]. KIR receptors bind to HLA-G and MHC-I molecules, which at the foeto-maternal interface is mainly HLA-C, which are expressed by the trophoblast [93]. KIR receptors are not the only receptors important for uNK cell activation. Also, other uNK cells receptors play a role in uNK cell activation. These are other for instance receptors that can bind to HLA-G, HLA-E and HLA-F on the trophoblast, i.e. LILRB [94], NKG2A and NKG2C [95]. Like in mice, NKG2A seems to be involved in uNK cell education and women that can better educate uNK cells via NKG2A have a lower risk of preeclampsia [44]. Also, non-MHC-dependent receptors are expressed on uNK in humans, such as the NCR [96]. Although it has been shown that the AHR, which plays a role in mouse uNK cell activation (see above), is expressed in human NK cells [97], only one report suggests that human uNK cells express the AHR [98]. Another molecule that may be important in uNK cells activity is Tim3 [99]. UNK cells expressed more Tim3 than pbNK cells and Tim 3 is important for cytokine production (f.i. IFNγ) and decreased cytotoxicity to trophoblast cells (see also Figure 3) [99].
One important function of uNK cells is the production of various factors, such as cytokines, growth factors and angiogenic factors. The diversity of factors produced by the uNK cells indicates the central role of uNK cells in placental development and maintenance. uNK cells produce various proinflammatory cytokines, such as TNFα, IFNγ, and IL-1β [100], but also anti-inflammatory cytokines, such as IL-10 [100]. uNK cells also produce chemotactic factors, such as IL-8, CXCL10 [101] and IL-6 [102]. Moreover, also various angiogenic factors are produced, amongst others VEGF-C, PLGF, angiopoietin-1 (Ang1) and Ang2 [103] and growth factors, such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) and TGFβ [101, 103]. uNK cells also have been shown to produce proteases, such as MMPs [104].
uNK cells are in close contact with foetal trophoblast cells but do not show cytotoxic activity to extravillous trophoblast cells [105]. They do show cytotoxic activity against an NK cell target K562 [106], which is lower than the cytotoxicity of peripheral NK cells against this target [106]. Therefore, the presence of uNK cells in the neighbourhood of invading trophoblast does not result in trophoblast killing or lysis, but, in accordance with the role of these cells in mouse and rat pregnancy, may suggest that they regulate trophoblast invasion. The presence of KIR receptors and the presence of the C-type lectin receptors (NKG receptors) on the uNK cells indicate that these cells can bind to trophoblasts, since trophoblasts express the ligands for these receptors, i.e. HLA-C, HLA-G and HLA-E [93]. The trophoblast does not express MHC class II molecules or the MHC class I molecules HLA-A or HLA-B [107].
In contrast to other functions of uNK cells, most of our knowledge on the interaction of uNK cells with trophoblast via KIRs and HLA molecules has been derived from human studies. HLA-C binds to KIR molecules on uNK cells; HLA-C1 binds to inhibitory KIR receptors, while HLA-C2 binds to KIR receptors that can be inhibitory or activating [13, 108, 109]. Thus, the result of the interaction between uNK cells and trophoblast does depend on the HLA-C expressed by the trophoblast and the KIR receptors expressed by the uNK cells. Correlation studies have shown that some combinations of trophoblast HLA-C and uNK cell KIR may not be optimal for uNK cell function and therefore trophoblast invasion and as a result may not be optimal for decidual angiogenesis and spiral artery remodelling [13, 108, 109]. Hiby et al. [13] found that women with uNK with mostly inhibitory KIRs (the KIR AA genotype), who carry a foetus with HLA-C2 are more likely to have aberrant placentation, with decreased trophoblast invasion and spiral artery remodelling and therefore more likely to develop preeclampsia.
HLA-G, HLA-E, and HLA-F are non-classical leukocyte antigens, mainly expressed by the trophoblast [110], indicating their role at the foeto-maternal interface. They can replace classical HLAs in providing a “self-signal” to uNK cells. This is important for the tolerance of invading trophoblasts that lack classical HLA molecules [107, 110]. HLA-G binds to KIR2DL4 [111] and immunoglobulin-like transcript 2 (ILT2) [112] on uNK cells. This binding of HLA-G on trophoblasts to its uNK cells receptor promotes cytokine production, such as IFNγ or VEGF, as well as uNK proliferation [113]. Trophoblast HLA-E binds to CD94/NKG2 receptors on uNK cells and may induce activation or inhibition of uNK cells, depending on the origin of the HLA-E linked peptide [114]. In addition, the expression of HLA-F on placental trophoblasts was shown. HLA-F was most highly expressed on the surface of extravillous trophoblast from the second trimester until term [115]. HLA-F has been shown to bind to KIR3DS1 [116], as well as to ILT2 [117]. The exact function of HLA-F in uNK cell activation is not known yet, but given the timing of expression of HLA-F, it may be suggested that HLA-F is involved in placental growth.
It is clear from the above that there is intimate contact between uNK cells and invading trophoblast and that these cells type reciprocally influence each other. Although difficult to study in vivo in humans, studies in rodents suggested that uNK cells regulated trophoblast invasion (see above). Various in vitro studies have demonstrated that uNK cells can induce trophoblast invasion [101, 118, 119], while also conditioned medium from uNK cells is a chemoattractant for trophoblasts [119, 120]. However, other studies have found that uNK cells limit trophoblast invasion [120] or did not affect trophoblast invasion [119]. Differences in outcome between the studies may be because uNK cells from different gestational ages may have been used since the study of Lash et al. [119] has shown that uNK isolated from week 8 to 10 decidua did not affect trophoblast invasion, while uNK cells isolated from the decidua in weeks 12–14 decreased trophoblast invasion. Moreover, different trophoblast sources have been used, such as isolated trophoblasts [101] or trophoblast explants [119, 120]. This may have affected the results. Finally, some studies stimulated the uNK cells with IL-15, while others did not. It thus seems clear that similar to mouse pregnancy, the uNK cells in humans regulated trophoblast invasion, although how they regulate this invasion remains under investigation.
Together uNK cells and trophoblast induce decidual angiogenesis and spiral artery remodelling. Unfortunately, understanding of early decidual angiogenesis is limited in humans. It seems likely, however, that uNK cells are involved, given the production of angiogenic factors by uNK cells [101, 103, 121]. uNK cells also have perforin present in the granules. Although perforin is mostly known for its lytic activity on virally infected cells [122], it may also be involved in angiogenesis [123]. As described in the section on mouse uNK cells, in the mouse, uNK cells are not essential for early decidual angiogenesis but do affect decidual angiogenesis [59]. Therefore, in view of the production of angiogenic factors by human uNK cells and data from mouse studies, it seems plausible that also human uNK cells play a role in early decidual angiogenesis.
Similar to the mouse, spiral artery remodelling takes place in humans in order to meet the increased nutritional demand of the foetus towards the end of pregnancy. In humans, spiral artery remodelling takes place between weeks 7 and 18 [124]. The first phase in human spiral artery remodelling is trophoblast independent remodelling. This phase is mainly mediated by immune cells, especially uNK cells [79, 125], although also other mechanisms are involved such as mechano-sensing by endothelial cells [126]. In this phase, a loss of the muscular-elastic structure and breaks in the endothelial cell layer are observed [79]. In the second phase, not only uNK cells play a role, but extravillous trophoblast cells are also important, as well as the collaboration between these 2 cell types [124]. The extravillous trophoblast is important in the loss of arterial smooth muscle and endothelial cells in this second phase [124].
In human pregnancy, first-time pregnancies are at a higher risk for complications like miscarriage and preeclampsia. Preeclampsia is a pregnancy-specific disease mainly characterized by hypertension and proteinuria or involvement of other organs, such as the liver, brain or kidney [127]. The most severe form of preeclampsia, early-onset preeclampsia, is associated with decreased trophoblast invasion and spiral artery remodelling [127]. Interestingly, not only is the percentage of preeclampsia higher in first-time pregnancies as compared with later pregnancies, but also the uterus and placenta differ between first time and later pregnancies [128]. In women who already had an uncomplicated pregnancy, early pregnancy trophoblast invasion into decidual vessels was more extensive [128]. These findings may suggest that there is a memory for pregnancy. Indeed, recent studies indicated the presence of pregnancy-trained decidual NK (PtdNK) cells [129]. These cells can only be found in the decidua of parous women [129]. They have a unique transcriptome and epigenetic signature and they uniquely express NKG2C and LILRB1 [129]. Interestingly, they produce more IFNγ and VEGF after stimulation [129]. Since IFNγ and VEGF are important in remodelling of the spiral arteries, it may be suggested that these PtdNK cells are better equipped for spiral artery remodelling than regular uNK cells. The finding of PtdNK cells was confirmed by a study by Vento-Tormo et al. [80], who showed the presence of three uNK cells subsets in the early human decidua, dNK1, dNK2 and dNK3 cells. The dNK1 cells are the only cells expressing both NKG2C and LILRB1 together with the expression of various KIR genes, indicating that these cells particularly interact with extravillous trophoblasts. It was suggested that these were the PtdNK cells [80].
As indicated at the start of this review, there are not only similarities between early placenta development in humans and mice/rats, but there are also differences in this early development between the species. Despite these differences, the studies on uNK cells in mice have often guided the studies on uNK cells in humans. For instance, the first detection of uNK cells in the placenta was in mice in the 1980s [5, 6], while only thereafter, LGL, which turned out to be uNK cells, were detected in the human decidua [7]. Also, uNK cell subsets were first described in mice and only much later in humans. Most of our evidence for the importance of uNK cells in placental and foetal development has come from mouse studies and mouse studies have been instrumental in our knowledge of the function of uNK cells in placental and foetal development. This is among others due to the fact that uNK cells can be studied at various time points of pregnancy in mice, especially in early pregnancy, at which uNK have their effects on placental development and growth. This is in contrast to human placental tissue, which is mainly available at the end of pregnancy, a stage in which little uNK cells are present.
The other advantage of mouse studies is the existence of KO mice, lacking NK cells or molecules, such as activation markers. Also, these studies have been indispensable in determining the role of uNK cells at the foeto-maternal interface. The use of KO mice lacking uNK cells, for instance, was the first study to show the definite role of uNK cells for placental and foetal development, since mice lacking uNK cells showed foetal loss [12]. Also, the use of various other KO mice showed the existence and function of uNK cell subsets in placental and foetal development, which was later confirmed in human studies.
Mostly mouse studies, rather than studies in the rat, have been used for studying uNK cell function. This is amongst others due to the fact that more KO mouse models were available than KO rat models. Moreover, in the mouse, trophoblast invasion is very shallow and only observed in the decidua closest to the embryo. Therefore, many of the changes in the decidua and MLAp, such as angiogenesis or spiral artery remodelling, are mainly induced by uNK cells, making it easier to study uNK cell function perse. Trophoblast invasion in the rat is much deeper, i.e. far into the MLAp and decidual angiogenesis and spiral artery remodelling is induced by a collaboration of uNK cells and trophoblast. This is more complicated to study. However, this advantage of mouse studies is at the same time a disadvantage, since the collaboration of uNK cells and trophoblast in the rat decidua and MLAp is much more similar to the human situation. Therefore, future studies should focus more on the rat to be able to study the role of the uNK cells in collaboration with trophoblasts.
Since the main role of uNK cells is in early placentation, to confirm the role of uNK cells in the human placenta, tissue from early pregnancy is needed. This confirmation has mostly been done using isolated uNK cells from placentas from elective abortion material or by immunohistochemical staining of these placentas. uNK cell function is mostly studied in isolated uNK cells, while the location of uNK in human decidua has been studied using immunohistochemistry. The disadvantage of early elective abortion material is that no information is available as to the outcome of the pregnancy. Some researchers have tried to overcome this problem by using uterine artery Doppler ultrasound screening to try to identify pregnancy outcomes [130]. This method is relatively good for identifying women at risk for preeclampsia or foetal growth restriction [130]; it does not identify some other pregnancy complications. In future studies, the use of biomarkers may help to discriminate between a healthy pregnancy and pregnancy complications to study uNK function from elective abortion material.
It seems very likely that in future studies, mouse or rat studies will remain necessary in further studies on the role of uNK cells in placental development and function, since it is and will remain difficult to study early placentation in humans. However, given the differences at the foeto-maternal interface between human and rodent pregnancy, it will always remain extremely important to confirm results from mouse or rat studies in human studies to be able to gain better insight into the role of uNK cells in human pregnancy. For now, not all functions of uNK cells have been confirmed in humans. For instance, although IFNγ is produced by both mouse, rat and human uNK cells, in mice this IFNγ is essential for spiral artery remodelling while in rats IFNγ plays a role in trophoblast invasion. It remains unclear what the role of IFNγ derived from uNK cells in humans is. Its role in human spiral artery remodelling is not clear, since in humans spiral artery remodelling is suggested to be mainly performed by extravillous trophoblasts in collaboration with uNK cells.
uNK cells are important cells at the foeto-maternal interface. Many mouse studies have shown that uNK cells are important for placental development; however, they are not indispensable for pregnancy. It is now known from these mouse studies, that uNK cells are important for early decidual angiogenesis, trophoblast invasion and spiral artery remodelling. To do so, they can produce various factors, such as cytokines, growth factors, angiogenic factors and chemokines. In the mouse, different uNK subsets exist, with different functions: a trNK subset, which is important for decidual angiogenesis, trophoblast invasion and spiral artery remodelling and a cNK cell subset, which produces IFNγ, which is necessary for spiral artery remodelling. Together with this knowledge from rodent studies, it is now well accepted that in human pregnancy, uNK cells have similar functions as uNK cells in the mouse—they are important for decidual angiogenesis, trophoblast invasion and spiral artery remodelling. The production of various cytokines, growth factors, angiogenic factors and chemokines is also important for uNK cell function in humans.
To be able to perform their function, uNK cells are activated via various mechanisms. uNK cells can be activated by MHC dependent and independent mechanisms. KIR (Ly49 in mice) and NKG receptors bind to classical and non-classical MHC-I molecules, and binding induces activation or inhibition of uNK cell function depending on the KIR (Ly49) or NKG receptor used. As an exception to the rule that most knowledge on uNK cells is derived from mouse studies, most of our knowledge on KIR and NKG receptors in uNK cell function is derived from studies into human uNK cells. These receptors are thought to be very important for the function of uNK cells. For instance, correlation studies have shown that specific combinations of KIR receptors and HLA-C molecules appear to be important for normal placentation, while other combinations are more present in women with preeclampsia. NKG receptors have shown to be involved in uNK cells education and these cells are also associated with complications like preeclampsia.
Less well studied are the MHC independent receptors on uNK cells, such as AHR receptors. Interestingly, these receptors bind to environmental pollutants, such as aromatic hydrocarbons, and endogenous metabolites, such as products produced by the gut microbiota. Therefore, through this receptor, uNK cells can also respond to environmental factors, indicating that environmental pollutants, but also bacterial products, may affect uNK cell function. This receptor may represent an option to therapeutically influence uNK cell function. Therefore, further study into the role of this receptor on uNK cells may lead to new therapeutical options for the treatment of pregnancy complications associated with aberrant uNK cells numbers or function. Aberrant numbers of uNK cells may result in decreased spiral artery remodelling, which is a hallmark of foetal growth retardation or preeclampsia. The presence of the AHR on uNK cells may also explain the recent findings on the role of the gut microbiome in pregnancy. We have recently shown that foetal and placental weight is decreased in germ-free mice as compared with conventional mice [131]. Pilot studies indicated decreased uNK cells in the decidua and MLAp of these mice (unpublished data). It may be suggested that the gut microbiome is important for the development of the placenta by affecting uNK cell function. Further studies are needed to substantiate this hypothesis.
Recent research has suggested that not only mouse uNK cells (trNK and cNK cells) but also human uNK cells consist of subsets. In humans, three subsets have been identified so far: uNK1, uNK2 and uNK3. Also, pbNK cells are found in the human placenta. Unfortunately, the function of these subsets has not been established yet. However, based on their receptor expression and similarity with mouse trNK cells, the uNK1 is thought to be similar to trNK cells, suggesting that they are involved in decidual angiogenesis, trophoblast invasion and spiral artery remodelling, while the pbNK cells, as well as the uNK3, may be similar to cNK cells, based on their production of IFNγ. Further studies are needed to evaluate the function of the different uNK cells subsets in the human placenta.
Interestingly, recently, a memory uNK cell has been discovered in the human placenta. This so-called PtdNK cell is mainly seen in second or later pregnancies and appears to be a uNK1 cell. These cells have a unique transcriptome and epigenetic signature and they uniquely express NKG2C and LILRB1. Further research is needed to evaluate how these PtdNk cells are generated, how they differ from other uNK cells and whether they are present in pregnancy complications, like preeclampsia. Also, mice seem to possess memory cells. They do not appear to be uNK cells but another cell type of the ILC-1 group.
In sum, although there is a huge literature on uNK cells in mice and humans and a lot of knowledge on the function of uNK cells, future studies should be focused on human uNK cells subsets and their respective functions, uNK cell memory cells, uNK cell education and the role of MHC-independent uNK cells receptors, such as AHR.
AHR: aryl hydrocarbon receptor
cNK: conventional natural killer
CXCL10: C-X-C motif ligand 10
DBA: Dolichos biflorus agglutinin
HLA: human leukocyte antigen
HPCs: hematopoietic precursor cells
IFN: interferon
IL: interleukin
ILCs: innate lymphoid cells
KIR: killer-cell immunoglobulin-like receptor
KO: knockout
LILRB1: leukocyte immunoglobulin-like receptor B1
MHC: major histocompatibility complex
MLAp: mesometrial lymphoid aggregate of pregnancy
MMPs: metallomatrix proteinases
NCR: natural cytotoxicity receptor
Nfil3: nuclear factor interleukin 3-regulated
NK: natural killer
NKG2: natural killer group 2
NKG2A: natural killer group 2 member A
PAS: periodic acid shift
pbNK: peripheral blood natural killer
PLGF: placental growth factor
PtdNK: pregnancy-trained decidual natural killer
Tim3: T cell immunoglobulin and mucin domain-containing protein 3
trNK: tissue-resident natural killer
uNK: uterine natural killer
VEGF: vascular endothelial growth factor
The author contributed solely to this paper.
The author declares that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2022.
Copyright: © The Author(s) 2022. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Gustaaf Albert Dekker, Pierre Yves Robillard
Kushaan Khambata ... Satish K. Gupta
Gursaran P. Talwar ... Krishna M. Ella
Julia Szekeres-Bartho
Shigeru Saito ... Sayaka Tsuda
Noémie Abisror ... Arsene Mekinian
Ruchi Sachdeva, Rahul Pal
Shibin Cheng ... Surendra Sharma
Betcy Susan Johnson, Malini Laloraya
Alison McCallion ... Chandrakant Tayade
Pier Luigi Meroni ... Francesco Tedesco
Alaa Kazhalawi ... Nathalie Lédée
Chiara Agostinis ... Roberta Bulla
Mickey V. Patel ... Charles R. Wira
Thanh Luu ... Joanne Kwak-Kim
