Affiliation:
1Department of Immunology, Innovita Research Company, 06116 Vilnius, Lithuania
2Department of Allergology and Immunology, Petrovsky National Research Centre of Surgery, 119991 Moscow, Russia
Email: seledtsov@rambler.ru
ORCID: https://orcid.org/0000-0002-4746-8853
Affiliation:
3Department of Immunology, Institute for Fundamental and Clinical Immunology, Novosibirsk 630099, Russia
ORCID: https://orcid.org/0000-0001-8072-6255
Affiliation:
1Department of Immunology, Innovita Research Company, 06116 Vilnius, Lithuania
ORCID: https://orcid.org/0000-0003-2978-4761
Explor Immunol. 2023;3:506–512 DOI: https://doi.org/10.37349/ei.2023.00117
Received: July 11, 2023 Accepted: August 18, 2023 Published: October 30, 2023
Academic Editor: Robert H. Miller, George Washington University School of Medicine & Health Sciences, USA
Immunotherapeutic treatment of autoimmune diseases should aim to inactivate autoaggressive memory T-cells and restore immune tolerance. It is envisaged that three approaches could be used to achieve this goal: stimulation of anti-idiotypic immune responses by vaccination with pathogenic T-cells; administration of suboptimal doses of antibodies (Abs) against two or more surface T-cell markers to provide selective Ab-mediated destruction of activated pathogenic memory T-cells; and induction of oral immune tolerance. The proposal entails the use of T-cell vaccination (TCV) or Ab-based therapy as an initial approach to reduce autoantigenic T-cell sensitization. Subsequently, the implementation of oral immunotherapy (OIT) is recommended to reinstate a consistent immune tolerance.
All naive T-cells go through thymic selection and gain measurable, albeit weak, reactivity to self-antigens (Ags). This weak self-reactivity is not strong enough to cause autoimmunity but suffices to maintain both T-cell survivability and reactivity to subsequent antigenic stimuli. With age, the proportion of naive T-cells decreases, while the proportion and number of memory T-cells increase. Compared to naive T-cells, memory T-cells are more sensitive to antigenic stimulation, less dependent on co-stimulation signals, and less sensitive to the action of immunosuppressive mechanisms. Therefore, the accumulation of memory T-cells inevitably results in an increase in T-cell reactivity to self-peptide/major histocompatibility complex (MHC) complexes, which increases the risk of autoimmune diseases [1].
Although nearly all available drug treatments are effective in alleviating autoimmune symptoms, conventional drug regimens used are characterized by: (i) non-specific immunosuppressive reactivity (i.e., they inhibit the functional activity of not only pathogenic autoaggressive immune cells but also non-pathogenic ones involved in protection against pathogens); (ii) metabolic activity that often injures internal organs; and (iii) the necessity for long-lasting drug intake. Obviously, other novel immunobiological approaches need to be developed to inactivate pathogenic lymphocytes specifically that would not negatively impact immune-mediated protection and function of internal organs and systems. It is argued that major efforts should be aimed at inactivating pathogenic T-cells, because pathological autoimmune processes are predominantly T-cell-dependent. In this respect, it is important to bear in mind that pathogenic T-cells differ from other “useful” T-cells in the variable regions (idiotypes) of their T-cell receptors (TCRs). Specifically, TCRs expressed by pathogenic T-cells are characterized by a higher avidity to self-peptide/MHC complexes and, consequently, higher autoaggressive reactivity [1, 2]. This implies that pathogenic T-cells can be specifically targeted by immunotherapeutics via their TCRs. Another approach to reducing the number of activated pathogenic T-cells in the body can be based on the use of suboptimal doses of cytotoxic antibodies (Abs) specific to different T-cell markers. The proposal includes the utilization of T-cell vaccination (TCV) or Ab-based therapy as an initial measure to reduce autoantigenic T-cell sensitization. Subsequently, it is recommended to implement oral immunotherapy (OIT) to reestablish a stable immune tolerance.
The unique determinants found in the variable regions of T-cell and B-cell receptors are known as idiotypes. These variable regions are formed during the postnatal period, allowing them to act as Ags that bypass innate immune tolerance and trigger idiotype-specific immune responses. This constantly active, adaptive idiotype-anti-idiotype mechanism plays a crucial role in controlling autoimmune reactivity, and its malfunction can lead to the development of autoimmune diseases [3, 4].
The TCV concept utilizes multiple immunizations with self-reactive T-cells to stimulate anti-idiotypic immune responses. The goal is to eliminate or deactivate these pathogenic immune cells within the body. Autoimmune memory T-cells, intended for TCV, can be isolated from a patient’s blood during a disease exacerbation. Clinical TCV studies have reported safety and immunological effectiveness in treating multiple sclerosis [3–5] and rheumatoid arthritis [6, 7], with the ability to induce anti-idiotypic CD4+ and CD8+ T-cells against hyper-variable TCR regions, including both the complementarity determining regions (CDRs) 2 and 3 [3]. In this scenario, CD8+ T-cells exhibited inhibitory and/or cytotoxic activity against CD4+ T-cells, with the latter inhibiting pathogenic immune cells by producing interleukin-4 (IL-4) and IL-10. TCV has also been shown to induce regulatory T-cells (Tregs) that control T-cell activation via cognate T-T interactions [4]. Additionally, inhibition of TCR-mediated immune reactivity can be achieved by surface TCR masking with specific Abs [8]. It is crucial to note that the TCV approach has no serious side effects and can induce anti-idiotypic immune reactivity in patients not only at the early stages but also at the advanced stages of autoimmune diseases when immunoregulatory mechanisms are significantly compromised due to long-standing illness and treatment-related drug burden [5, 7].
From an immunocytochemical standpoint, the cytotoxic effect mediated by Abs is characterized by a threshold phenomenon. This phenomenon suggests that immune complexes linked to cell membranes must achieve a specific density threshold to initiate the cytolytic process. This concept was validated through experimental evidence involving both polyclonal and monoclonal Abs that target a range of membrane-associated Ags [9, 10]. A novel immunotherapeutic concept of low Ab dose immunotherapy has been proposed, which makes use of more than one membrane Ag-specific Ab species administered in suboptimal dosages (that facilitate just partial Ab-mediated coverage of relevant antigenic determinants) for the selective destruction of pathogenic cells [9]. Theoretically, this technique facilitates the formation of Ag-Ab complexes on the cell surface that would reach a threshold cytolytic density only on pathogenic cells conditioned upon recognition of surface Ags by all individual Abs in the immunotherapeutic Ab formulation [9, 10]. In this scenario, non-pathogenic immune cells and surface markers expressed thereon would be recognized by some (but not all) Abs, thus preventing membrane-associated Ag-Ab complexes from reaching critical cytotoxic threshold levels with ensuing cytolysis. The therapeutic platform proposed could utilize two or more therapeutic Abs at suboptimal doses, with decreasing risks of serious complications resulting from any single Ab administered at the optimal therapeutic dose [9, 10].
The exacerbation of autoimmune diseases is caused by the activation of autoimmune memory T-cells. Generally, memory T-cells express a CD45RO+CD45RA− phenotype, naive T-cells are CD45RA+CD45RO−, and activated T-cells express the surface activation marker CD69. The hypothesis posits that administrating suboptimal doses of anti-CD45RO and anti-CD69 Abs during autoimmune disease exacerbations might prove effective in selectively eliminating activated, pathogenic memory T-cells (Figure 1). Therapeutic Ab effectiveness could be further enhanced by conjugation with artificial cytotoxic molecules. The main requirement for such molecules should be the existence of an achievable quantitative threshold for triggering their cytotoxic action. The Ab-based approach, in general, enables the reduction of activated pathogenic memory T-cells in the body while avoiding broad immunosuppression and its accompanying complications [9].
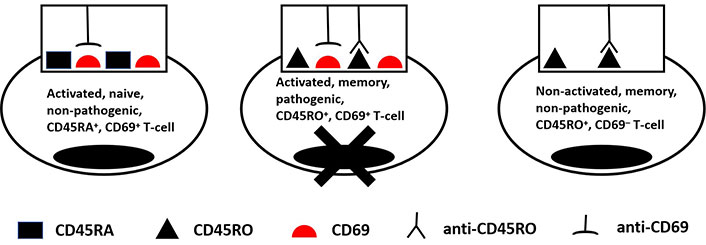
Selective destruction of an activated pathogenic memory T-cell due to the combined activity of anti-CD45RO and anti-CD69 therapeutic Ab-based drugs. The scenario shown here assumes that Abs used in suboptimal doses acquire the cytolytic threshold level of Ag-Ab immune complexes only on a pathogenic activated memory CD45R0+CD69+ T-cell, but not on non-pathogenic activated naive CD45RA+CD69+ and non-activated memory CD45R0+CD69+ T-cells
Clearly, the extremely powerful tolerogenic potential of the immune system should be fully mobilized for treating autoimmune diseases, whereby the oral auto-Ag administration route has turned out to be the most effective and convenient for stimulating Ag-dependent tolerogenic mechanisms. This allowed the formalization of OIT as a novel approach that uses the oral application of auto-Ags to stimulate tolerogenic immune mechanisms and gut immunoglobulin A (IgA) production. This immunotherapeutic concept is based on a physiological phenomenon that prevents pathological immune responses to food protein and commensal microbiota [11]. There have been reports of other forms of tolerogenic immunotherapy that utilize nasal, sublingual, and epidermic routes of Ag administration, with varying degrees of success in experimental and clinical settings [12]. However, when it comes to tolerance induction protocols, the oral route of auto-Ag administration holds a distinct position. This is mainly because it provides access to the largest immune system in the organism, known as the gut-associated lymphoid tissue (GALT), and allows for control over its predominantly tolerogenic immunoregulatory elements [12]. Indeed, the gut’s tolerogenic environment has been shown to be associated with various cell components, such as αβ or γδ CD8 intraepithelial lymphocytes, CD8+/CD4+ Tregs, tolerogenic CD103+ dendritic cells (DCs) [13], and novel population of retinoic acid receptor-related orphan receptor-γt (RORγt)+ Ag-presenting cells (APCs), called Thetis cells, residing within intestinal lymph nodes [14]. In contrast to immunostimulatory conventional APCs, tolerogenic APCs are immunosuppressive by virtue of low surface expression levels of MHC and co-stimulatory molecules, as well as the production of soluble immunosuppressive molecules. Hence, tolerogenic APCs have been shown to induce Ag-specific immune unresponsiveness and to maintain peripheral immune tolerance by inducing autoreactive T-cell anergy and deletion, as well as stimulating Treg differentiation [12, 15]. Tregs activated and propagated by tolerogenic APCs have been shown to suppress activation, expansion, and function of effector immune cells via cell contact-dependent mechanisms, as well as by producing immunosuppressive cytokines, such as IL-10, IL-35, and transforming growth factor-β (TGF-β) [12]. It is important to note that only a minor subpopulation of Tregs is actually Ag-induced, with Ag-specific Tregs being more potent in terms of immunosuppressive capacity than polyclonal Tregs. Furthermore, Ag-specific Tregs are responsible for bystander suppressor activity, characterized by the non-specific suppression of immune responses against Ags presented alongside the orally administered food Ags. Consistent with this notion, Ag-specific Tregs are more potent in preventing/treating autoimmune diseases in animal models, compared with polyclonal Tregs [12, 16]. The liver was described to play a pivotal role in oral tolerance, whereby administration of Ags in the portal vein was effective in preventing contact hypersensitivity response, delay-type hypersensitivity response, and in improving prognoses in surgical brain injury in mice [11]. The OIT paradigm also involves the application of various immunomodulatory agents, such as IL-4, vitamins A and D, and corticosteroids to improve effectively the functionality of tolerogenic APCs and regulatory lymphocytes [11].
Other factors are also critical in Ag-specific oral tolerance induction, such as Ag doses, frequency and regimens of feeding [17]. Thus, low-dose Ag administration was shown to induce T-helper 1 activation followed by tolerization, while high-dose Ags were capable of inducing tolerance without prior activation [18] thus being a more effective OIT protocol. Furthermore, high Ag doses were shown: (i) to direct responses towards deletion mediated by apoptosis or anergy of the specific T-cells; (ii) to be necessary to ensure sufficient Ag entry into the liver and more efficient recruitment of immunoregulatory cells in tolerance induction; (iii) to recruit and increase the frequency CD4+CD25+forkhead box protein 3 (Foxp3)+ Tregs, and (iv) to cause down-regulation of TCRs on the cell surface [19]. In experimental conditions, continuous supplementation of drinking water with Ag was more effective in tolerance induction with more long-lasting effects, compared to single/multiple Ag dose administration via the intragastric route by gavage [12, 20, 21]. Ag-specific OIT could be further enhanced by particular dietary components, such as phospholipids and polyunsaturated fatty acids (fish, nuts, seeds, soybeans, eggs, etc.), and vitamins D and A [11].
OIT effectiveness was substantiated by multiple published pieces of evidence that feeding auto-Ags, such as collagen and myelin basic protein, could prevent the development of arthritis [22] and multiple sclerosis [20, 23], respectively, in experimental animal models. There have been reports of clinical OIT usage for multiple sclerosis, rheumatoid arthritis, and type I diabetes with varying success rates [12, 24]. Hence, OIT remains a promising approach due to its lack of common side effects associated with traditional immunosuppression regimens and its potential for long-term clinical use. However, the clinical application of OIT on a wide scale is hindered by the fact that feeding Ags is more effective in naive, but not in primed individuals. Studies have shown that oral administration of Ags is only effective when given before disease induction [17, 25]. Therefore, a novel two-phase immunotherapy strategy for autoimmune disease (Figure 2) is proposed. During the initial phase, it is suggested to mitigate self-sensitization of the immune system by using TCV or Ab-based destruction of autoaggressive T-cells. The second phase focuses on restoring disturbed immune tolerance by feeding auto-Ags involved in the immunopathological process.
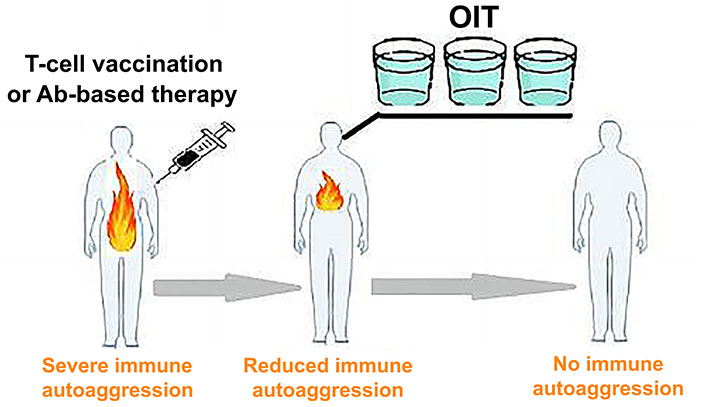
Two-phase immunotherapy for autoimmune disease. TCV (or Ab-based therapy) reduces immune self-sensitization, while subsequent long-term OIT restores immune tolerance to self-Ags
Restoration of impaired immune tolerance to auto-Ags remains the only feasible research and development (R&D) therapeutic strategy with the potential to cure the disease [2]. In spite of the fact that TCV and Ab-based therapy can down-regulate pathological autoimmune sensitization, these approaches are unable to fully restore defective immune tolerance to auto-Ags. It is suggested in this paper that OIT can be specifically employed to reinstate immune tolerance. However, as discussed in this paper, elevated autoimmune sensitization in patients poses a significant obstacle to achieving this goal. Consequently, long-term OIT may be adequately conducted in later treatment stages after TCV or Ab-based therapy. The hypothesis suggests that patients might derive benefits from consuming Ag-fortified drinkable concoctions, which would provide sustained support for the effective activation of Ag-specific tolerogenic immune mechanisms (Figure 2). Potentially, this proposed therapeutic approach, involving immune-based, multifactorial inactivation of pathogenic T lymphocytes, could be applicable in the treatment of various autoimmune diseases. Patients with severe comorbidities, including the elderly, could receive this immunotherapy at home without significant risks of major side effects. Furthermore, this treatment is relatively cost-effective, easily personalized, and can be repeated as needed. Additionally, the aforementioned immunotherapy could be combined with other treatments to enhance the likelihood of success. It’s important to emphasize that the innovative immunotherapeutic concept presented here lacks a foundation in robust clinical data. However, this immunotherapeutic approach could be tested in various established experimental models for treating different autoimmune diseases with a view to quickly introducing it in clinical practice.
Abs: antibodies
Ags: antigens
APCs: antigen-presenting cells
IL-4: interleukin-4
MHC: major histocompatibility complex
OIT: oral immunotherapy
TCRs: T-cell receptors
TCV: T-cell vaccination
Tregs: regulatory T-cells
VIS: Conceptualization, Writing—original draft. GVS and AAvD: Writing—review & editing, Methodology, Software. All authors have read and agreed to the published version of the manuscript.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2427
Download: 31
Times Cited: 0
Michail Athanasopoulos ... Ioannis Athanasopoulos
