Affiliation:
Department of Pediatrics, Hospital Nacional Edgardo Rebagliati Martins, Jesús María 15072, Peru
Email: ana.munoz.u@upch.pe
ORCID: https://orcid.org/0000-0001-5114-4133
Explor Asthma Allergy. 2024;2:111–117 DOI: https://doi.org/10.37349/eaa.2024.00033
Received: July 29, 2023 Accepted: March 06, 2024 Published: April 15, 2024
Academic Editor: Désirée Larenas-Linnemann, Hospital Médica Sur, Mexico
The article belongs to the special issue The Different Faces of Food Allergy
Nutritional therapy through exclusive enteral nutrition (EEN) is successful with Crohn’s disease (CD), but most patients relapse when returning to a normal diet. Personalized and sustainable diets over time have not been tried. This pioneering case report shows the successful response to the use of a skin prick test (SPT) with a 0.5 mm cutoff and a combination of parameters to guide the diet of a child with CD, ensuring continued remission and a regular diet over a follow-up period of 3 years. The 5-year-old patient had a history of chronic diarrhea. Laboratory showed anemia, hypoalbuminemia, high erythrocyte sedimentation rate (ESR), and fecal calprotectin (FCP) > 2,100 µg/g. Endoscopies revealed duodenal ulcer scar and ulcerative pancolitis. Simple endoscopic score for CD score (SES-CD) = 16 (severe). Pathology showed CD. EEN started with a polymeric formula, later moving to an elemental formula due to a suboptimal response. Medication included prednisolone, mesalazine, azathioprine, and methotrexate. Foods were introduced guided by the SPT and included 54 protein extracts from food tested every 3–4 months. The patient has clinical and histological remission despite having lamb, turkey, eggs, cereals (including wheat), and fish in his diet. FCP has been measured with every change in diet and maintained at < 100 µg/g with the reintroduction of food, with the exception of fish and eggs which, despite a negative SPT, gave mild symptoms and raised FCP to 223 µg/g. Both eggs and fish were successfully reintroduced (FCP < 100 µg/g) after 7 and 11 months respectively from failed reintroduction. This innovative approach based on SPT and strict clinical and follow-up inflammatory markers can potentially ensure remission, reintroducing foods with objective parameters, and improving the patient’s quality of life.
Currently, the cause of Crohn’s disease (CD) is unknown and its morbidity worldwide remains significant despite attempts to treat it. There is no available cure. Traditional treatment plans use corticosteroids and biologicals, but side effects and dependence are drawbacks. A potentially simple but effective approach in children is to use dietary therapy. This has adherence challenges, which is especially true with exclusive enteral nutrition (EEN), but there are no adverse effects, and it is very likely to improve patients’ symptoms.
The possible benefits associated with EEN are many, but in CD, two possible factors are relevant: the avoidance of enteral antigens in the gut and changes in the gut microbiome [1, 2]. However, EEN is not sustainable over time. Due to this, the scientific community is currently researching how modifications in dietary habits can maintain disease remission, mainly through exclusion diets [2, 3] and/or through the inclusion of anti-inflammatory foods [4]. Most exclusion diets are based on removing or reducing foods that scientific studies associate with “flares” either due to unfavorable changes to the microbiome or direct damage to the intestinal mucosa. Another perspective is that one must consider the known association of CD with allergy to cow’s milk protein [5], the finding of greater sensitization to food in patients with CD compared to controls [6], and successful therapeutic trials of personalized hypoallergenic diets based on food antigen testing [7, 8]. Thus, multiple food allergies may not only be a comorbidity in patients with CD but also the guide for a safe return to a “normal” diet.
Although the oral challenge is the gold standard for the diagnosis and management of food allergies, it is not useful in CD since responses to food may be delayed or worse, asymptomatic enteritis may occur [8]. This is the main reason to rely on an objective auxiliary exam such as the skin prick test (SPT).
The SPT consists of applying drops of allergen extracts on the patient’s forearm, and then gently pressing with a metal lancet, without bleeding. The test is indicated for allergies with suspected immunoglobulin E (IgE) mediation and measures sensitization to the allergen.
Conventionally, a wheal ≥ 3 mm is assumed to be positive [9]. The usefulness of this test in CD has not been previously evaluated. However, it is the method of choice for food allergy detection. Its positive predictive value is high to confirm a food allergy without employing oral challenge [10]. Therefore, if the SPT is negative, the possibility of successful reintroduction of food will be very high, unlike IgG antibody tests, which can only guide the withdrawal of food but are not useful for scheduling safe reintroduction. This means their use is limited for follow-up leading to regular diet and reintegration to the dining table.
This article presents encouraging results of a personalized diet guided by an SPT of the induced and maintained remission of CD in a child.
A 5-year-old male patient was admitted to the Pediatric Emergency Service of the Hospital Nacional Edgardo Rebagliati Martins (HNERM for its Spanish acronym). Personal history: cesarean delivery, birth weight 2.8 kg, mixed breastfeeding, infant colic, and “functional” constipation three months prior to being hospitalized. Family history: diabetes mellitus II (father). He had a history of diarrhea with bloodless mucus, vomiting, and fever in the month prior to being admitted. He received multiple antibiotic therapies which were unsuccessful. Fecal tests were negative for infection. He was hospitalized (month 0) in the Pediatric Gastroenterology Unit of the HNERM. On physical examination, he was pale and edematous. Laboratory exams showed anemia (hemoglobin: 9.3 g/dL), hypoalbuminemia (albumin: 2 g/dL), erythrocyte sedimentation rate (ESR): 38 mm/h, C-reactive protein (CRP): 15.4 mg/dL, fecal calprotectin (FCP) > 2,100 µg/g (abnormal > 100 µg/g), and fecal alpha 1-antitrypsin > 112.5 mg/g (abnormal > 26.8 mg/g) (Table 1).
Patient’s clinical follow-up: treatment (diet and medications) and inflammatory markers (FCP)
| Month | Diet | Drugs | FCP (µg/g) | Notes |
|---|---|---|---|---|
| 0 | EEN: polymeric formula | Prednisone, mesalazine, azathioprine | Baseline > 2,100After EEN = 185 | Purpuric rash with azathioprine |
| 4 | Polymeric formula + food group 1* | Prednisolone, methotrexate | > 2,100 | Greenish and abundant stools |
| 5 | Elemental formula + food group 1 | Prednisolone, methotrexate | < 50 | Yellow, normal stools |
| 10 | Elemental formula + food group 1 + food group 2** | Prednisolone, methotrexate | 60 | Asymptomatic |
| 13 | Elemental formula + food group 1 + food group 2 + food group 3*** | Prednisolone, methotrexate | 38 | Asymptomatic |
| 15 | Elemental formula + food group 1 + food group 2 + food group 3 + fish + egg (egg-yolk and egg-white) | Methotrexate | 223 | Vomit, mild constipation |
| 16 | Elemental formula + food group 1 + food group 2 + food group 3 + turkey (fish and egg removed) | Methotrexate | 13 | Asymptomatic |
| 22 | Elemental formula + food group 1 + food group 2 + food group 3 + turkey + egg-white (fish and egg-yolk removed) | Methotrexate | 32 | Asymptomatic |
| 26 | Food group 1 + food group 2 + food group 3 + turkey + egg-white + fish + lamb (egg-yolk removed, elemental formula removed) | Methotrexate | 120 | Suspected infection |
| 30 | Food group 1 + food group 2 + food group 3 + turkey + egg-white + fish + lamb (egg-yolk removed, elemental formula removed) | Methotrexate | 33 | Asymptomatic |
*: food group 1/first SPT: oats, cassava, barley, avocado, banana, pear, apple, and papaya; **: food group 2/second SPT: mandarin, beetroot, melon, orange, strawberry, peach, potato, sweet potato, quinoa, and rice; ***: food group 3/third SPT: broccoli, pumpkin, carrot, cauliflower, onion, garlic, corn, potato, tomato, passion fruit, watermelon, lemon, mango, pineapple, olive (olive oil), wheat. All foods were introduced one at a time after a negative SPT. Eight SPTs were performed in the 30 months of follow-up
An endoscopy revealed duodenitis and duodenal ulcer scar, and a colonoscopy ulcerative pancolitis with several longitudinal ulcerated lesions (5 mm to 10 mm) in the cecum, in the ascending, transverse, and sigmoid colon. Simple endoscopic score for CD score (SES-CD) = 16; where a value > 15 is severe. Pathologists reported histopathological findings compatible with CD (Figure 1).
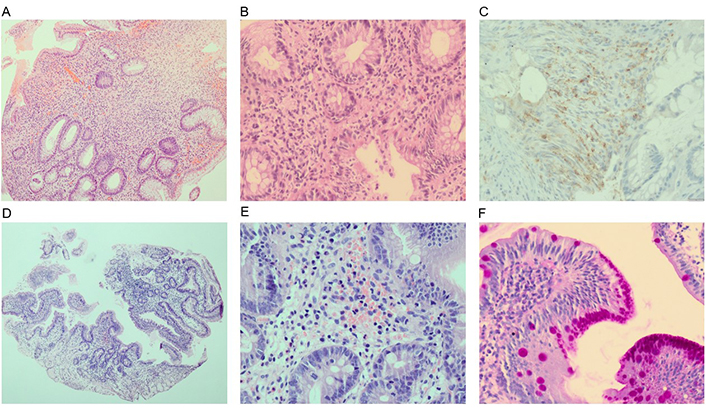
Patient’s baseline histopathology. (A) Colon: crypt architectural distortion and mucin depletion (hematoxylin eosin; HE, ×4); (B) colon: inflammatory expansion of the lamina propria with basal lymphoplasmacytosis and neutrophilic inflammation with cryptitis (HE, ×20); (C) colon: poorly-formed granulomas (CD68+; HE, ×40); (D) duodenum: decrease in villous height, alteration of normal crypt/villous ratio (2/1; HE, ×4); (E) duodenum: increased intraepithelial lymphocytes and eosinophilic inflammation in the lamina propria (HE, ×20); (F) duodenum: foveolar gastric metaplasia (peryodic acid of shiff+; HE, ×40)
Mycobacterium tuberculosis infection, toxoplasmosis, rubella, cytomegalovirus, herpes simplex, syphilis (TORCH), viral infections, and primary and secondary immunodeficiencies were ruled out. Treatment began that month with a pediatric CD activity index (PCDAI) score of 32.5. The standard approach of EEN was carried out with the patient receiving a polymeric formula until month 4, and subsequently, due to positive fecal inflammatory markers, moving to an elemental formula as the main source of protein until month 19.
Medication included oral prednisolone, with progressive weaning until withdrawal in month 14. Mesalazine was given for 14 days but was unsuccessful; azathioprine was tried but a purpuric rash appeared; as a result, methotrexate 15 mg/m2 was prescribed. After approximately 3 months of EEN (month 4), the patient was asymptomatic but always had greenish and abundant stools. However, he no longer had anemia (hemoglobin 12.9 g/dL) or hypoalbuminemia (albumin 5.03 mg/dL) and ESR decreased to 5 mm/h (PCDAI = 2.5). FCP was 185 µg/g (still high), but the fecal alpha 1-antitrypsin had normalized (7 mg/g). After month 3, several foods were introduced guided by an SPT. The SPT was scheduled every 3–4 months and included 54 protein extracts from foods including 9 types of meat, milk, egg (white and yolk), soy, 4 beans, 8 cereals, 3 tubers, 8 vegetables, 16 fruits, and peanuts (Alergofar®, Rio de Janeiro-Brasil). The foods were introduced with a cutoff of < 0.5 mm (this value was chosen as there is currently no method to guide diet for CD) and one at a time, every 3 days. All other foods that gave ≥ 0.5 mm or that were not tested were excluded. Protein intake through the elemental formula was maintained as long as animal proteins were not ingested. Aside from the aforementioned foods, only salt and sugar were permitted. The patient was monitored every 1–3 months, both to assess adherence to the diet and to measure growth, presence of symptoms, and monitoring of PCDAI and FCP.
With the introduction of foods, the FCP returned to a high level of > 2,100 µg/g. Consequently, at month 5, a decision was made to change to an elemental formula, which improved the consistency and color of the stools and lowered the FCP to < 50 µg/g, even with the same foods in the diet, which were oats, cassava, barley, avocado, banana, pear, apple, and papaya (food group 1/first SPT; Table 1). One of the key findings is that clinical remission and normal inflammatory markers have been maintained even with the introduction of additional foods, guided by an SPT.
There were no symptoms for most of the foods introduced based on the SPT results, but eggs and fish produced vomiting, mild constipation, and an increase in FCP (month 15), so they were removed; this resulted in a return to baseline values with clinical remission and normalization of the inflammatory markers. This reaction to egg and fish was due to the SPT not being 100% perfect in terms of specificity and sensitivity, just like any auxiliary test. Egg-white (month 22) and fish (month 26), with a negative SPT, were successfully reintroduced. This is possibly because the reactivity improved over time and the allergy to fish and egg disappeared. The FCP values have remained < 100 µg/g (remission) from month 16 to the present, except for a slight increase during month 26 (FCP = 120 µg/g) which can be attributed to an infectious intercurrence as several family members had colds at the time and because no changes in his diet had been made (month 30). However, no tests were done to diagnose or discard the cause of the infectious intercurrence.
The patient continues with a diet based on turkey, lamb, egg-white, fish, cereals (including wheat), tubers, fruits, and vegetables (Table 1), without elemental formula. At the time of writing (month 34), he has good weight and height gain and shows clinical and histological remission (PCDAI = 2.5) (Figure 2).
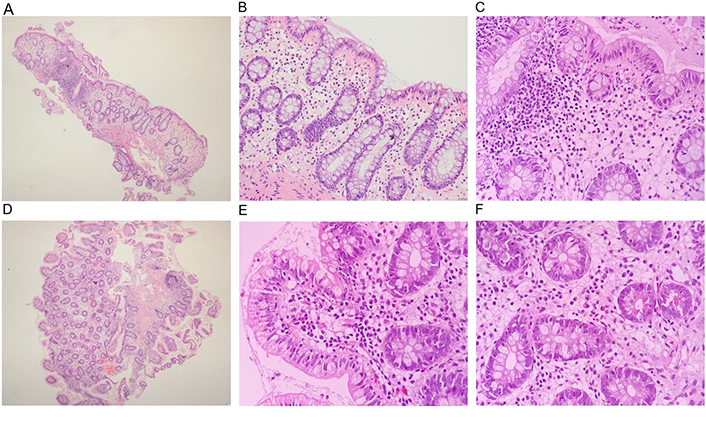
Patient’s follow-up histopathology. (A) Colon: mild crypt architectural distortion without mucin depletion (HE, ×4); (B) colon: mild lymphoplasmacytic inflammation in the lamina propria (HE, ×20); (C) colon: absence of neutrophils (HE, ×40); (D) duodenum: decrease in villous height, alteration of normal crypt/villous ratio (2/1; HE, ×4); (E) duodenum: mild lymphoplasmacytic inflammation in the lamina propria without increased intraepithelial lymphocytes (HE, ×20); (F) duodenum: absence of neutrophils (HE, ×40)
This case shows a consensus of sustained remission in clinical, laboratory, and histological parameters [11]. Having initially tried EEN with a polymeric formula, remission was later achieved with an elemental formula. The personalized diet based on an SPT allowed the reintroduction of wheat in the 13th month, a food prohibited in the CD exclusion diet (CDED) under the principle that causes inflammation [1]. This is due to the fact that it is a personalized and progressive diet. However, in the absence of an objective parameter, withdrawing or maintaining wheat is a matter of debate in the diets tested for CD [4].
The investigation demonstrates the usefulness of the SPT, under the perspective of sensitization to food in CD as the main cause of inflammation and SPTs, can be used to reintroduce food safely.
Intestinal inflammation per se implies increased intestinal permeability and the possibility of sensitization to dietary antigens. Antigens could even be microbial antigens (this is possible because there are allergens in all the five kingdoms of nature: animal, plant, fungi, protist, and monera) [12]. Diet may reduce antigen exposure, and, it could also quantitatively alter the gut microbiota. EEN has a mechanism which decreases T cell-mediated hypersensitivity and improves the regulatory T response [2].
These findings show that EEN with elemental formulas at the beginning of the nutritional management of CD could be a way for the digestive system to recover and reset itself in severe cases like the one seen in this article. This is, firstly, given that CD is a serious intestinal inflammation and that the ineffectiveness of partial enteral nutrition to achieve remission has been demonstrated and agreed upon [13]; and secondly, that nutritional management is needed to achieve a baseline of not only clinical remission but also of inflammatory markers which allow the re-introduction of food safely [1].
This case presents an alternative to modular diets such as the CDED, the carbohydrate-free diet [2], or the anti-inflammatory diet [4]. The CDED is a modular diet of wide acceptance worldwide, however, it requires the exclusion of foods such as wheat and its derivatives (a common food at the dining table and in daily social activity, with a great impact on the quality of life). On the other hand, the carbohydrate-free diet is very restrictive and carries a high risk of deficit, in addition to having adherence difficulties. Using SPT-based food reintroduction is a new and innovative personalized approach to the management of CD. Other personalized approaches were based on a serological test: Rajendran and Kumar [7] who published a successful experience using the IgG4 test for dietary guidance with symptomatology in patients with CD. Wang et al. [8] achieved a 3-month remission with a diet based on IgG antibodies to food. However, none of the studies has documented long-term improvement at both the clinical and histological levels, which this case has [2, 4, 7, 8].
Follow-up with the SPT is not easy, because, like any auxiliary test, SPT, despite the demanding cutoff, can have false positives and negatives, as evidenced by the frustrating introduction of eggs and fish (a rise in raised FCP despite maintaining the underlying treatment remaining unchanged); and the symptoms could be unseen or banal, with the patient having asymptomatic enteritis.
Furthermore, FCP is not 100% specific and sensitive, with elevated levels seen during benign intercurrences, which happened with the patient in this case. However, the SPT coupled with the follow-up by the FCP and the clinical picture enabled the required 100% certainty to be reached to maintain remission in cases of CD.
The follow-up limitation of SPTs and FCPs is the high cost related to personalized care. However, it is important to highlight that the management of CD is highly expensive if the use of biologics is included (which could be an alternative to the use of elemental formula in this patient). The strength of the approach in this patient lies in maintaining remission while liberalizing the diet, but also in making us foresee a future free of escalation therapy for him.
The design of a specific, successful, palatable, and long-term sustainable diet to induce and maintain remission of CD in children is an ongoing challenge. This is the first case of a successful response (clinical, endoscopic, and laboratory) to a personalized diet based on an SPT and strict clinical and inflammatory follow-up markers. The SPT enables new food to be introduced at regular intervals with few or no reactions which is not possible with serological tests. This approach has the potential to ensure that remission is maintained safely, scalation therapy is avoided, and the patient’s diet and quality of life are improved.
CD: Crohn’s disease
CDED: Crohn’s disease exclusion diet
EEN: exclusive enteral nutrition
FCP: fecal calprotectin
IgE: immunoglobulin E
PCDAI: pediatric Crohn’s disease activity index
SPT: skin prick test
AMU: Conceptualization, Investigation, Writing—original draft, Writing—review & editing.
The author declares that she has no conflicts of interest.
The research complies with the Declaration of Helsinki. The publication of the article was authorized by the Ethics and Research Committee of HNERM (authorization no. 86-CE-GHNERM-GRPR-ESSALUD-2023).
Informed consent to participate in the study was obtained from all participants.
Informed consent to publication was obtained from relevant participants.
Datasets generated by this study are available upon request from the author (ana.munoz.u@upch.pe).
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2464
Download: 24
Times Cited: 0
Angel Mazon ... Antonio Nieto
Valentina Faihs ... Knut Brockow
Polina Kostova ... Guergana Petrova
Leonel Pereira, Ana Valado
Purificación González-Delgado ... Anna Nowak-Wegrzyn
