Affiliation:
Department of Dermatology and Allergy Biederstein, School of Medicine, Technical University of Munich, 80802 Munich, Germany
ORCID: https://orcid.org/0000-0002-6019-2521
Affiliation:
Department of Dermatology and Allergy Biederstein, School of Medicine, Technical University of Munich, 80802 Munich, Germany
Affiliation:
Department of Dermatology and Allergy Biederstein, School of Medicine, Technical University of Munich, 80802 Munich, Germany
ORCID: https://orcid.org/0000-0002-1218-3399
Affiliation:
Department of Dermatology and Allergy Biederstein, School of Medicine, Technical University of Munich, 80802 Munich, Germany
ORCID: https://orcid.org/0000-0002-5352-5105
Affiliation:
Department of Dermatology and Allergy Biederstein, School of Medicine, Technical University of Munich, 80802 Munich, Germany
Email: knut.brockow@tum.de
ORCID: https://orcid.org/0000-0002-2775-3681
Explor Asthma Allergy. 2023;1:230–238 DOI: https://doi.org/10.37349/eaa.2023.00023
Received: July 11, 2023 Accepted: October 16, 2023 Published: December 28, 2023
Academic Editor: Ting-fan Leung, The Chinese University of Hong Kong, China
The article belongs to the special issue The Different Faces of Food Allergy
Aim: Most patients with wheat allergy dependent on augmentation factors (WALDA) show specific immunoglobulin E (sIgE) to ω5-gliadin. However, some WALDA patients may show negative results when testing for sIgE to total wheat extract. This is the first study to investigate potential clinical and serological differences in patients with ω5-gliadin-positive, challenge-confirmed WALDA dependent on their sensitization to total wheat extract.
Methods: Clinical and serological characteristics of patients with challenge-confirmed, ω5-gliadin-positive WALDA were analyzed based on the absence or presence of sIgE to wheat (cut-off 0.35 kUA/L).
Results: Thirty-six patients with challenge-confirmed WALDA were included (19 female; median age 50.5 years; median sIgE to ω5-gliadin 6.5 kUA/L). SIgE levels to grass pollen were related to the presence of any atopic comorbidity (P < 0.001) and showed a correlation with sIgE to wheat (P = 0.003), but not to the gluten-related allergens [all not significant (ns)]. Thirty-nine percent of patients (n = 14) showed sIgE levels to wheat lower than 0.35 kUA/L; in 19.4% (n = 7) levels were even below the detection limit of 0.01 kUA/L. WALDA patients without sIgE to wheat showed lower levels of total immunoglobulin E (IgE) and sIgE to wheat gluten, gliadins, and ω5-gliadin (all P < 0.001) as well as to grass pollen (P = 0.03). No significant differences in clinical characteristics like delay until diagnosis, the presence of an atopic condition, reaction severity, or threshold in the oral challenge test were observed.
Conclusions: SIgE to wheat extract was associated not only with sensitization against gluten allergens but also reflected total IgE production and concomitant grass pollen allergy, making it an insensitive and unspecific biomarker for WALDA. There were no clinical divergences between WALDA patients without or with sIgE to wheat. SIgE to total wheat extract does not appear to be clinically relevant and remains negative in a significant proportion of WALDA patients.
Wheat allergy dependent on augmentation factors (WALDA) is an immunoglobulin E (IgE)-mediated food allergy, with reactions being elicited when wheat consumption is combined with augmentation factors [1]. Symptoms may range from urticaria or angioedema alone to severe, life-threatening anaphylaxis [1–3]. Historically, in most cases, the disease has been described as wheat-dependent exercise-induced anaphylaxis (WDEIA). Exercise is the most frequently reported augmentation factor [4], however, numerous other augmentation factors exist, such as non-steroidal anti-inflammatory drugs, alcohol, stress, infections, or temperature extremes [5]. Additionally, some patients may present with skin-related symptoms only and do not fulfill anaphylaxis criteria. Thus, WALDA is the more accurate terminology when describing the whole patient population [6].
Unfortunately, in many cases, the patients still experience a delay of many years until they get the correct diagnosis [4, 5, 7–10]. However, the diagnosis and the patients’ education about the eliciting allergen and potential augmentation factors are the keys to preventing further potentially life-threatening reactions.
The diagnosis is based on a suitable clinical history, the sensitization profile, and an oral challenge test (OCT) using wheat gluten alone and with the addition of augmentation factors [1, 11, 12]. Potential causes for a delay in the correct diagnosis may include an inaccurate medical history taking (i.e., without the active question for augmentation factors or consumption of gluten-containing cereals) or an unspecific serological screening.
Component-resolved diagnosis has facilitated a detailed assessment of sensitization patterns over the last decades [13]. The detection of specific IgE (sIgE) to recombinant ω5-gliadin—one major allergen in the gluten fraction of wheat—has been shown to be a suitable screening parameter for WALDA with a reported sensitivity of 80% up to 98% [7, 8, 14, 15], so that the term ω5-gliadin-allergy is sometimes used as a synonym for WALDA [4]. SIgE to wheat, however, may remain negative in some patients with WALDA [16, 17]. On the other side, sIgE to grass pollen is a common cause for cross-reactivity to total wheat extract, leading to a clinically irrelevant co-sensitization against wheat in 60–65% of grass pollen-allergic patients [18, 19].
However, to date, nothing is known about whether the absence or presence of sIgE to wheat may be associated with differences in clinical phenotype or duration until diagnosis in patients with WALDA. Thus, this is the first study to investigate potential clinical and serological differences in patients with ω5-gliadin-positive, challenge-confirmed WALDA based on their sensitization to total wheat extract.
From January 2019 until May 2023, 36 patients with challenge-confirmed WALDA were included at the Department of Dermatology and Allergy Biederstein of the Technical University of Munich, Germany. Inclusion criteria were a history of allergic reactions after wheat consumption in combination with augmentation factors despite regular tolerance of wheat products alone, sIgE levels to ω5-gliadin ≥ 0.35 kUA/L (ImmunoCAP® assay, Phadia, Uppsala, Sweden), a positive OCT to wheat gluten ± augmentation factors and given informed consent to the study participation. The study received approval from the local medical ethics committee of the Technical University of Munich (approval number 477/21 S-NP).
Each patient underwent a comprehensive assessment of their medical history, including the severity of the most severe reaction [20], the timespan between the first allergic reaction until the final diagnosis, and any secondary diagnoses and atopic comorbidities. Blood samples were collected to determine total IgE and sIgE levels to grass pollen, wheat, gluten, gliadin, and recombinant ω5-gliadin as well as basal tryptase levels (ImmunoCAP® assay, Phadia, Uppsala, Sweden). A cut-off of 0.35 kUA/L was defined to compare WALDA patients with and without sIgE to total wheat extract according to the widely accepted clinical practice [11, 21] and corresponding to CAP class of 1 or above (ImmunoCAP®, Phadia, Uppsala, Sweden). Prick-to-prick skin tests (PPST) were conducted with native wheat flour (type 550) and wheat gluten. Histamine dihydrochloride (one drop; 10 mg/mL; ALK-Abello, Copenhagen, Denmark) and 0.9% saline were used as positive and negative controls, respectively. Results with a mean wheal diameter of 3 mm or greater compared to the negative control after 20 min were considered positive.
The OCTs were performed in an inpatient setting following a protocol previously published [12]. The reaction thresholds were assessed on an ordinal scale from 1 to 10. On the first day, the patients were challenged with wheat gluten alone, starting with 8 g, followed by 16 g and 32 g—corresponding to reaction thresholds 1, 2, and 3, respectively. On the second day, 1,000 mg of acetylsalicylic acid [ASA; two capsules with 500 mg acetylic salicylic with mannitol silicon oxide filler (99.5% mannitol and 0.5% aerosil) administered orally, produced by the Pharmacy of the Klinikum Rechts der Isar, Technical University of Munich, Germany] was added as an augmentation factor, and the patients were again challenged with 8 g, 16 g, and 32 g of wheat gluten (thresholds 4, 5, and 6). On the last day, 1,000 mg of ASA and 20 mL of 95% ethanol (Braun, Melsungen, Germany) in fruit or peppermint infusion (Marco Polo, Norden, Germany; ingredients fruit infusion: rosehip, hibiscus, apples, orange peel, elderberry, lemon peel; ingredients peppermint infusion: 100% peppermint) were administered, followed by 32 g of gluten (threshold 7) and 20 min of anaerobic exercise on a treadmill (threshold 8). In the last step, the challenge with ASA, alcohol, and exercise may be repeated with the ingestion of 64 g of wheat gluten (thresholds 9 and 10). In the event of an objective reaction, antiallergic treatment was performed. After confirmation of the diagnosis of WALDA, all patients were informed and educated about the disease, received dietary counseling, and were given an emergency set for self-treatment containing an adrenaline autoinjector (Fastjekt 300 μg, Viatris GmbH, Bad Homburg, Germany).
The Statistical Package for the Social Sciences (SPSS) Statistics 28.0 (SPSS Inc., Chicago, USA) and GraphPad PRISM version 9 (GraphPad Software Inc., La Jolla, CA, USA) were used for statistical analyses. Patient characteristics were described as median and range for continuous variables and as a number and/or percentage for dichotomous variables. After testing for normal distribution with the Shapiro-Wilk test, statistical analyses for non-normally distributed data were used. Differences between groups were calculated with Mann-Whitney U-tests. Correlations were assessed using Spearman’s rank correlation coefficients. A P-value of < 0.05 was considered statistically significant.
The study included 36 adult patients, comprising 19 females (53%) and 17 males (47%), with a median age of 50.5 years (range 23–81 years). Atopic comorbidities were reported by 36% of individuals (n = 13). As per inclusion criteria, all patients showed sIgE to ω5-gliadin (median 6.5 kUA/L; range 0.5–34.6 kUA/L) and objective reactions in the OCT. All patients showed basal serum tryptase levels below 11.4 μg/L (median 4.6 μg/L; range 2.3–10.7 μg/L). The median timespan between the first allergic reaction and the final diagnosis was 5 years (range 0.5–24 years), with a delay of 10 years or more observed in 10 patients (28%). Seventy-five percent of patients (n = 27) had experienced systemic anaphylaxis in their histories, while 9 patients (25%) reported only urticaria and/or angioedema after the consumption of wheat with augmentation factors.
In the OCT leading to the confirmation of the diagnosis, 33% (n = 12) showed objective reactions already after ingesting pure wheat gluten in supraphysiological doses with a higher ω5-gliadin content than normally consumed by an adult eating commercial, unconcentrated wheat products. Adding ASA as an augmentation factor could confirm the diagnosis in 50% of the patients (n = 18). One patient developed urticaria after ASA, alcohol, and wheat gluten, while additional exercise was needed in just four patients (11%) to elicit symptoms (data are missing from one patient previously diagnosed by OCT with a different protocol at an external institution).
The median sIgE to total wheat extract was 0.54 kUA/L (range < 0.1–4.65 kUA/L). Of the 36 included WALDA patients, 39% (n = 14) showed sIgE levels to total wheat extract lower than 0.35 kUA/L. In 19% (n = 7), wheat sIgE levels lay even below the detection limit of 0.01 kUA/L (Figure 1).
In the whole collective, sIgE levels to wheat showed a correlation with total IgE levels (P < 0.001), sIgE to grass pollen (P = 0.003), wheat gluten, gliadins, and ω5-gliadin (all P < 0.001) as well as with the wheat gluten PPST diameter (P = 0.018). No correlations with clinical characteristics were found.
No significant difference was found regarding sIgE to wheat when comparing patients with and without atopic comorbidities. However, patients with reported atopy showed a stronger sensitization against grass pollen (P < 0.001). Besides the presence of atopy, sIgE levels to grass pollen showed a correlation to total IgE and sIgE to wheat (P = 0.001 and P = 0.003, respectively) but not to the gluten-related allergens [sIgE to wheat gluten, gliadins, and ω5-gliadin, all not significant (ns)].
PPST with wheat flour was positive in 80% of patients (n = 29; mean wheal diameter 5.4 mm), compared to 86% (n = 31, mean wheal diameter 6.1 mm) of positive PPST when using wheat gluten.
An overview of the clinical characteristics of the ω5-gliadin-positive, challenge-confirmed WALDA patients when divided into two groups based on the absence or presence of sIgE to total wheat extract (below or above 0.35 kUA/L) is shown in Table 1. No significant differences in clinical characteristics like delay until diagnosis, reaction severity, or threshold in OCT could be observed. A trend towards fewer atopic comorbidities in the ω5-gliadin sIgE < 0.35 kUA/L group was observed, but it did not reach statistical significance (Table 1). However, patients without sIgE to wheat showed significantly lower values in total IgE and sIgE to ω5-gliadin, gliadins, and wheat gluten (all P < 0.001; Table 1 and Figure 2A) as well as lower wheal diameters in the gluten PPST (P = 0.006; Table 1). Additionally, lower sIgE to grass pollen was observed in the group without sIgE to wheat (P = 0.03; Table 1 and Figure 2B). Interestingly, the PPST was not significantly more often positive in patients with sIgE to wheat as compared to those without, reflecting a discrepancy between these two allergy tests and/or allergen extracts. However, wheal diameters in the wheat gluten PPST were lower in patients without sIgE to wheat (median 4.5 mm vs. 6 mm; P = 0.006).
Clinical characteristics of included WALDA patients dependent on the absence or presence of wheat sIgE
| Clinical characteristics | Wheat sIgE < 0.35 kUA/L (n = 14) | Wheat sIgE > 0.35 kUA/L (n = 22) | P-value |
|---|---|---|---|
| Age, median (range) | 49.5 years (30–79 years) | 51.0 years (23–81 years) | ns |
| Sex, male/female, n (%) | 6/8 (57%/43%) | 11/11 (50%/50%) | ns |
| Any self-reported atopic comorbidities, n (%) | 4 (29%) | 9 (41%) | ns |
| Years to diagnosis, median (range) | 3 (1–20) | 5 (0.5–24) | ns |
| Severity at most severe reaction*, n (%) | Grade I: 5 (36%) | Grade I: 4 (18%) | ns |
| Grade II: 2 (14%) | Grade II: 4 (18%) | ||
| Grade III: 7 (50%) | Grade III: 13 (59%) | ||
| Grade IV: 0 (0%) | Grade IV: 1 (5%) | ||
| Total IgE, median (range) | 154 kUA/L (32–324 kUA/L) | 519 kUA/L (65–2,848 kUA/L) | < 0.001 |
| SIgE to wheat, median (range) | 0.12 kUA/L (< 0.10–0.32 kUA/L) | 0.87 kUA/L (0.47–4.65 kUA/L) | < 0.001 |
| SIgE to ω5-gliadin, median (range) | 2.6 kUA/L (0.5–8.8 kUA/L) | 9.5 kUA/L (1.8–34.6 kUA/L) | < 0.001 |
| SIgE to gliadins, median (range) | 3.2 kUA/L (0.3–10.3 kUA/L) | 0.4 kUA/L (0.2–1.6 kUA/L) | < 0.001 |
| SIgE to wheat gluten, median (range) | 0.6 kUA/L (< 0.1–1.6 kUA/L) | 3.3 kUA/L (0.5–9.9 kUA/L) | < 0.001 |
| SIgE to grass pollen, median (range) | 0.1 kUA/L (< 0.1–12.9 kUA/L) | 0.8 kUA/L (< 0.1 kUA/L–> 100 kUA/L) | 0.03 |
| Positive PPST with native wheat flour, n (%) | 10 (71%) | 19 (86%) | ns |
| Positive PPST with wheat gluten, n (%) | 11 (79%) | 20 (91%) | ns |
| Basal serum tryptase, median (range) | 4.3 μg/L (3.4–9.8 μg/L) | 4.8 μg/L (2.3–10.7 μg/L) | ns |
| OCT reaction threshold#, n (%) | 1 (1, 7.1%) | 1 (3, 13.6%) | ns |
| 2 (0, 0%) | 2 (4, 18.2%) | ||
| 3 (2, 14.3%) | 3 (2, 9.1%) | ||
| 4 (5, 35.7%) | 4 (6, 27.3%) | ||
| 5 (1, 7.1%) | 5 (3, 13.6%) | ||
| 6 (2, 14.3%) | 6 (1, 4.5%) | ||
| 7 (0, 0%) | 7 (1, 4.5%) | ||
| 8 (3, 21.4%) | 8 (1, 4.5%) | ||
| 9 (0, 0%) | 9 (0, 0%) | ||
| 10 (0, 0%) | 10 (0, 0%) | ||
| - |
* According to Ring and Messmer scale; # see OCTs section above. -: one missing value due to external OCT with a different protocol
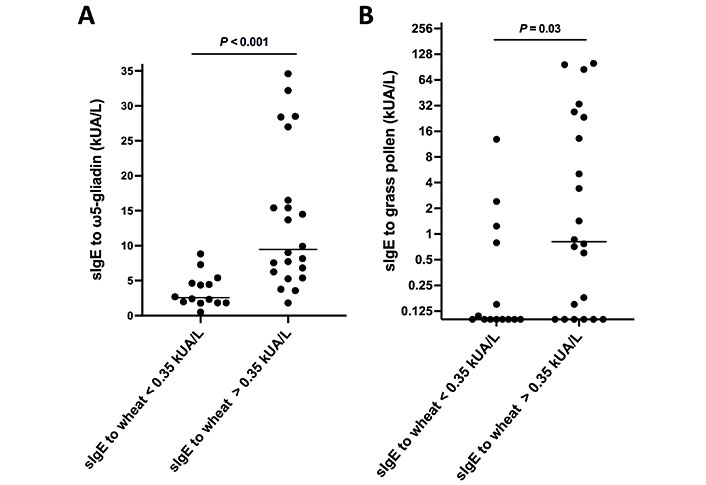
SIgE to different allergens in patients with challenge-confirmed WALDA, stratified by their sIgE to wheat. A. SIgE to ω5-gliadin in patients with challenge-confirmed WALDA, stratified by their sIgE to wheat; B. sIgE to grass pollen in patients with challenge-confirmed WALDA, stratified by their sIgE to wheat (Y-axis was plotted at log2-scale, data shown as median and individual values)
As shown by this study and in line with the literature [4, 8, 22], WALDA patients often experience a long delay until the correct diagnosis, leading to numerous and potentially life-threatening reactions as well as reduced quality of life.
In this study, 39% of challenge-confirmed patients with WALDA showed sIgE to total wheat extract below the threshold of 0.35 kUA/L (corresponding to a sensitivity of 61%) and 19.4% even below the detection limit of 0.1 kUA/L. This finding aligns with previous studies [8, 15]. It highlights the need for direct detection of sIgE to recombinant ω5-gliadin as a screening parameter for WALDA, as this has shown a far better sensitivity [8, 14, 15].
In this study, no longer duration until diagnosis was observed in patients without sIgE to wheat, however, this study was performed at a specialized center where sIgE to ω5-gliadin is assessed as part of the clinical routine in all patients presenting with a clinical history typical for WALDA or suspected idiopathic anaphylaxis. As experienced in the clinical routine and according to the history of some patients, exclusive testing for sIgE to whole wheat extract is still widespread common practice—in this case, 39% of patients would have fallen off the grid due to their missing sensitization to the whole extract. Whole wheat extracts are unsuitable in the diagnosis of classical WALDA as they are poor in gluten, which contains the most important allergens in WALDA, including ω5-gliadin [1]. Thus, the main allergens eliciting WALDA are underrepresented when testing with the whole wheat extract. SIgE to the whole wheat extract rather appears to be associated not only with sensitization against gluten allergens but also reflects total IgE production and concomitant grass pollen allergy making it an insensitive and unspecific biomarker for WALDA.
No clinical differences were found between WALDA patients with and without sensitization to total wheat extract. As expected, sIgE levels to gluten proteins, including ω5-gliadin, were associated with higher sIgE levels to wheat. Although the presence of any atopic condition did not show a statistically significant impact on the presence of sIgE to wheat in WALDA patients, associations with higher total IgE and sIgE to grass pollen were found.
Cross-reactivity between sIgE to grass pollen in patients with allergic rhinoconjunctivitis and total wheat extract has previously been demonstrated [18, 19]. Although single patients with ω5-gliadin-negative WALDA due to grass pollen cross-reactivity have been reported [23], this remains a rarity. In the vast majority of patients, cross-sensitization to wheat due to grass pollen remains clinically irrelevant [18, 19]. Among others, this factor leads to a low specificity of sIgE to wheat as a marker of a possible WALDA. With respect to the already mentioned low sensitivity, sIgE to total wheat extract has a very low value in the diagnostic work-up for patients with classical, ω5-gliadin-positive WALDA.
Overall, sIgE to wheat may be positive especially in WALDA patients with high sIgE levels to the culprit gluten allergens or those with a concomitant grass pollen sensitization, however, irrespective of the clinical presentation or the reaction threshold, nearly 40% of WALDA patients show no sIgE to wheat.
While the majority of WALDA patients react against allergens in the gluten fraction of wheat [1, 15, 24] and testing for sIgE to total wheat extract may be irrelevant in these patients, this may not be the case in rare cases of ω5-gliadin-negative WALDA. Examples may comprise the above mentioned sporadic cases of grass pollen-related WALDA [23], patients sensitized by hydrolyzed wheat proteins [25], or WALDA caused by the wheat lipid transfer protein Tri a 14 (direct identification of sIgE to Tri a 14 should be performed if the latter is suspected) [12, 26]. However, these cases are rare, and the diagnostic workup for patients with suspected ω5-gliadin-negative WALDA should be performed in specialized centers.
Of course, this study has limitations. First, the patient number in this monocentric study is limited. However, WALDA is presumably underdiagnosed, with small patient populations in most centers and studies. Second, this study focused on the classical subtype of ω5-gliadin-positive WALDA and no conclusions can be drawn for patients with ω5-gliadin-negative WALDA, as explained above. Third, further studies are needed to explore the potential impact of sIgE levels to wheat on the time to diagnosis, as the recruitment at a specialized university center with the testing of sIgE to ω5-gliadin as clinical routine may not reflect the diagnostic process in other institutions.
ASA: acetylsalicylic acid
IgE: immunoglobulin E
ns: not significant
OCT: oral challenge test
PPST: prick-to-prick skin tests
sIgE: specific immunoglobulin E
WALDA: wheat allergy dependent on augmentation factors
The authors thank the WALDA patients for participating in the study.
VF: Conceptualization, Data curation, Formal analysis, Validation, Investigation, Methodology, Writing—original draft, Project administration, Writing—review & editing. CK: Conceptualization, Validation, Methodology, Writing—review & editing. RKB: Conceptualization, Methodology, Writing—review & editing. TB: Supervision, Writing—review & editing. KB: Supervision, Funding acquisition, Validation, Writing—original draft, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The study received approval from the ethics committee of the Technical University of Munich (approval number 477/21 S-NP) and complies with the Declaration of Helsinki.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
This work was supported by the authors’ institute (Department of Dermatology and Allergy Biederstein, School of Medicine, Technical University of Munich, Germany) and by a research and development grant from the German Federal Ministry of Education and Research (BMBF), project
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Angel Mazon ... Antonio Nieto
Polina Kostova ... Guergana Petrova
Ana Muñoz-Urribarri
Leonel Pereira, Ana Valado
Purificación González-Delgado ... Anna Nowak-Wegrzyn
