Affiliation:
1Unit of Pediatric Allergy and Pneumology, University and Polytechnic Hospital La Fe, Health Research Institute Hospital La Fe, 46026 Valencia, Spain
Email: amazon@comv.es
ORCID: https://orcid.org/0000-0001-5639-1037
Affiliation:
1Unit of Pediatric Allergy and Pneumology, University and Polytechnic Hospital La Fe, Health Research Institute Hospital La Fe, 46026 Valencia, Spain
ORCID: https://orcid.org/0000-0002-3791-4389
Affiliation:
1Unit of Pediatric Allergy and Pneumology, University and Polytechnic Hospital La Fe, Health Research Institute Hospital La Fe, 46026 Valencia, Spain
ORCID: https://orcid.org/0000-0001-5975-7348
Affiliation:
1Unit of Pediatric Allergy and Pneumology, University and Polytechnic Hospital La Fe, Health Research Institute Hospital La Fe, 46026 Valencia, Spain
Affiliation:
1Unit of Pediatric Allergy and Pneumology, University and Polytechnic Hospital La Fe, Health Research Institute Hospital La Fe, 46026 Valencia, Spain
Affiliation:
1Unit of Pediatric Allergy and Pneumology, University and Polytechnic Hospital La Fe, Health Research Institute Hospital La Fe, 46026 Valencia, Spain
Affiliation:
3Service of Allergy, University Hospital La Plana, 12540 Villarreal, Spain
ORCID: https://orcid.org/0000-0003-3968-510X
Affiliation:
1Unit of Pediatric Allergy and Pneumology, University and Polytechnic Hospital La Fe, Health Research Institute Hospital La Fe, 46026 Valencia, Spain
ORCID: https://orcid.org/0000-0002-6302-6115
Explor Asthma Allergy. 2024;2:2–8 DOI: https://doi.org/10.37349/eaa.2024.00025
Received: June 19, 2023 Accepted: December 19, 2023 Published: January 30, 2024
Academic Editor: Mario Di Gioacchino, Italian Society of Allergy and Clinical Immunology, G. d’Annunzio University, Italy
The article belongs to the special issue The Different Faces of Food Allergy
Desensitization (DSZ) or oral tolerance induction is increasingly used in children who do not outgrow their food allergies. Off-label omalizumab (OMZ) is used as adjuvant therapy for those with severe reactions, but there is little information on outcomes when OMZ is withdrawn. The long-term outcome in a group of children with severe milk or egg allergy who had undergone an OMZ-assisted DSZ procedure is here described. Clinical data from 21 children from the time they started DSZ until database closure were retrospectively collected, to assess the appearance of symptoms and response to clinical decisions under real-life conditions. Patients received OMZ before, during, and after the DSZ procedure itself and OMZ was subsequently discontinued. The scheduled treatment protocol had to be changed in almost all patients due to reactions or individual needs. Three of 21 patients had to prematurely abandon the procedure due to DSZ failure. The other 18 patients were able to tolerate the target dose of food, but nine of them developed symptoms when eating the food 1.5 to 6 months after stopping OMZ. These patients underwent a second course of OMZ-assisted DSZ, which was successful in six, but three had a second relapse 3 to 8 months after stopping OMZ and decided to quit. OMZ-assisted DSZ failed in almost a third of patients with severe allergy even after a second course of OMZ, almost 40% had a successful outcome with one course of OMZ, while almost a third required a second course. Relapses of symptoms occurred up to six months after stopping OMZ.
Allergy to milk and egg does not always disappear spontaneously and desensitization (DSZ) or oral tolerance induction can change its natural course. In severe allergy, DSZ can provide protection against accidental food ingestion and improve the quality of life of patients and their families [1]. However, DSZ is not risk-free; frequent reactions appear, especially in children with severe allergy [2, 3]. These patients are the ones who could benefit the most from this procedure, but, if they do not tolerate it, they remain at risk of severe, even fatal, reactions. Omalizumab (OMZ) is currently approved for severe asthma and chronic urticaria, but its off-label use has been evaluated to improve safety and shorten the DSZ procedure [4–6]. The first report of symptom recurrence after drug discontinuation in three children who had undergone OMZ-assisted DSZ was published in 2014 [7]. In this manuscript a longer follow-up of the clinical course of this approach, under real-life conditions, in a larger series of children with severe allergy is reported.
Patients with cow’s milk or hen’s egg allergy, who had had severe reactions, and who were being followed at our center for OMZ-assisted DSZ were retrospectively evaluated. They received OMZ, in doses and intervals recommended by the manufacturer, on an off-label basis, with prior informed consent from the parents. The original plan was to administer OMZ for at least 2 to 3 months, then perform DSZ while patients were on OMZ, and maintain this for at least 2 to 3 months after reaching the target dose.
The DSZ protocol commonly used in Spain was applied [8, 9]. Briefly, whole cow’s milk (3 g of protein per 100 mL) was administered, starting with 1 mL diluted 1/100 (0.3 mg of protein), and doubling the dose every hour until reaching a dose of 2.5 mL, undiluted (75 mg protein) on the second day. For the egg, raw egg white (10 g of protein per 100 mL) was used, starting with 0.5 mL diluted 1/100 (0.5 mg of protein) and doubling the dose every hour until reaching a dose of 1 mL, undiluted (100 mg protein) on the second day. Patients were instructed to take the dose twice daily and were subsequently visited weekly at our clinic, where the dose was increased 40% to 50% up to a target amount of 200 mL of milk (6 g of protein), or 35 mL of raw egg white (3.5 g of protein). Once the goal was achieved, patients were instructed to take 200 mL of milk (or an equivalent amount of dairy products) twice a day or an omelet (or its equivalent) three times a week. The procedure was modified in doses, increases, duration, or administration intervals according to reactions and individual needs. Electronic medical records were reviewed to retrospectively collect data on clinical response and medical decision-making based on individual assessment.
Twenty-one patients (three from the initial report) [7] aged between 3.5 years and 16.6 years, with allergy to cow’s milk (n = 11) or hen’s egg (n = 10), who had presented severe reactions are here described. A DSZ procedure without OMZ had previously failed in thirteen patients (6/11 in cow’s milk allergy, 7/10 in hen’s egg allergy). OMZ was used in the other eight because symptoms were anticipated due to the severity of their condition. One patient (#1) was already previously receiving OMZ for 2.1 years for severe asthma; twenty received OMZ for off-label use, with informed parental consent. Basal specific immunoglobulin E (sIgE) ranged from 1 kUA/L to 2,040 kUA/L for casein and from 3 kUA/L to > 100 kUA/L for ovomucoid (OVM). The data are shown in Table 1 and Figure 1.
Baseline characteristics and current state of patients
| # | Gender | Age (years) | Failed DSZ w/o OMZ | Casein sIgE | α-LA sIgE | β-LG sIgE | OVM sIgE | OVA sIgE | Courses of OMZ | Current OMZ | Current tolerance |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 8 | No | 15 | 7 | 0 | - | - | 1 | Yes | No |
| 2 | F | 4.3 | Yes | 34 | 9 | 10 | - | - | 1 | Yes | No |
| 3 | M | 5.4 | No | 2,040 | 84 | 294 | - | - | 1 | Yes | Yes |
| 4 | F | 3.7 | Yes | 1 | 7 | 2 | - | - | 1 | No | Yes |
| 5 | M | 16.6 | No | 58 | 11 | 18 | - | - | 1 | No | Yes |
| 6 | F | 3.7 | Yes | 64 | 21 | 9 | - | - | 1 | No | Yes |
| 7 | M | 4.1 | No | 128 | 34 | 14 | - | - | 1 | No | Yes |
| 8 | F | 4.1 | Yes | 308 | 43 | 12 | - | - | 1 | No | Yes |
| 9 | M | 6 | No | 869 | 41 | 12 | - | - | 1 | No | Yes |
| 10 | M | 7.5 | Yes | - | - | - | 4 | 2 | 1 | No | Yes |
| 11 | M | 6.2 | Yes | - | - | - | > 100 | 78 | 1 | No | Yes |
| 12 | F | 8.6 | Yes | - | - | - | 3 | 1 | 1 | No | No |
| 13 | F | 4 | Yes | 75 | 28 | 28 | - | - | 2 | No | Yes |
| 14 | M | 3.5 | Yes | 833 | - | - | - | - | 2 | No | Yes |
| 15 | F | 10.7 | No | - | - | - | 340 | 690 | 2 | No | Yes |
| 16 | M | 10.6 | No | - | - | - | 95 | 335 | 2 | No | Yes |
| 17 | F | 10.1 | Yes | - | - | - | 135 | 155 | 2 | No | Yes |
| 18 | M | 6.3 | No | - | - | - | 48 | 9 | 2 | No | Yes |
| 19 | F | 8.8 | Yes | - | - | - | > 100 | 14 | 2 | No | No |
| 20 | F | 10.8 | Yes | - | - | - | 18 | 32 | 2 | No | No |
| 21 | M | 6.7 | Yes | - | - | - | 30 | 32 | 2 | No | No |
sIgE in kUA/L. M: male; F: female; w/o: without; α-LA: α-lactalbumin; β-LG: β-lactoglobulin; OVA: ovalbumin. -: not applicable. Diluted serum was used, when enough sample was available, to measure sIgE > 100 kUA/L
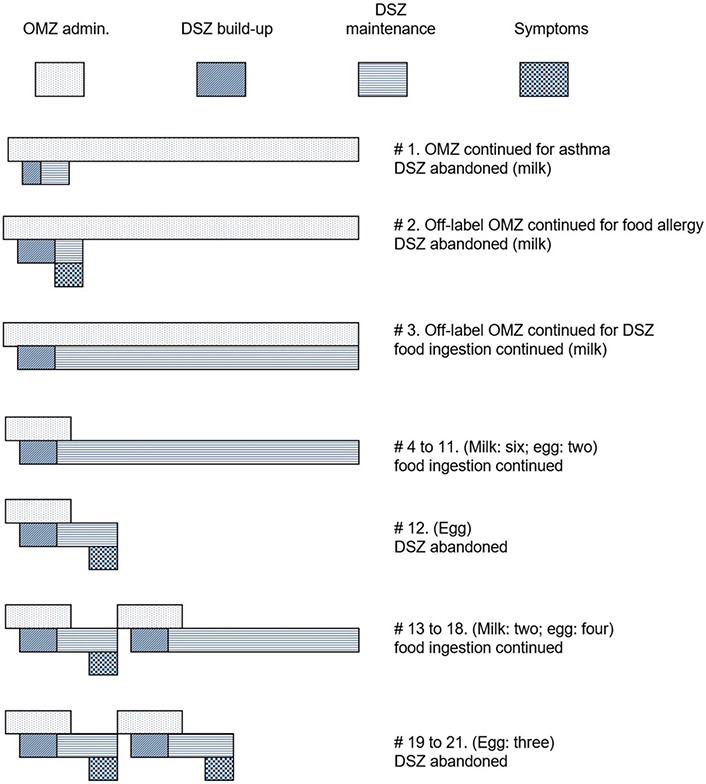
Scheme of the chronology of OMZ administration (first row for each patient), phases of build-up and maintenance of DSZ (second row), and appearance of symptoms (third row). The length of the bars is not proportional since there was a large variation in periods from one patient to another
Patients received OMZ for periods of two months to 2.1 years before starting DSZ. The DSZ procedure lasted between one month and nine months. DSZ was abandoned early in three patients: the one (#1) with severe asthma (before target achievement) due to worsening asthma possibly caused by milk, another (#2) who developed severe digestive symptoms shortly after reaching 200 mL of milk; the symptoms persisted despite progressively reducing the amount until finally withdrawing the milk. In the third (#21) it was due to intense and repeated vomiting with very small amounts of egg white.
Eighteen patients reached the target dose and OMZ was discontinued between 18 days and 1.6 years later in 17 patients. The other (#3) is still receiving OMZ and taking milk after 4.6 years (initially 2,040 kUA/L sIgE to casein, still 389 kUA/L after 3.9 years), as stopping OMZ was considered too risky.
Eight of the 17 patients (#4 to #11) continued to tolerate the food, which they are taking regularly between 1.2 years and 7 years after OMZ was discontinued; three of them presented some transient minor symptoms (abdominal pain and isolated vomiting) that resolved spontaneously.
One of the 17 patients (#12) had minor symptoms three months after stopping OMZ; she was very reluctant to take eggs and decided to abandon the procedure.
The other eight patients (#13 to #20) presented symptoms again with the food they were taking regularly for 1.5 to 6 months after stopping OMZ. The symptoms were anaphylactic reactions, or mainly vomiting, which prevented continued DSZ. Symptoms reappeared in challenge tests performed at our hospital to confirm causality in five patients. In the other three, causality was assumed due to a clear history and accumulated experience in those first five patients.
OMZ was resumed in these eight patients as well as in patient #21 (n = 9). After one day to 5.5 months of treatment, six patients underwent a new rush DSZ procedure, and three challenge tested with the food. All tolerated the food and continued to take it regularly. OMZ was discontinued again in them 0.7 to 1.2 years later.
Six of the nine patients (#13 to #18) are currently taking the food regularly, between 1.3 years and 5.1 years after the second course of OMZ. Two patients (#19 and #20) had severe symptoms again at 3 months and 8 months after the second course of OMZ and discontinued the procedure. Patient #21 also decided to drop out even though he only had minor symptoms.
Reports on OMZ-assisted DSZ have found no significant differences in non-high-risk children, who usually tolerate DSZ procedures well [10]. Children at high risk, using OMZ, have been able to reach the target dose in 75–100% of cases [6, 11]. In general, the follow-up time has been short. In one study, food challenges were performed 12 weeks after discontinuing OMZ and 43% of patients failed to pass [12]. There is a lack of information about the evolution and attitude after these relapses.
Our report is not a controlled trial useful to understand the mechanisms, the evolution of sIgE, or the factors that predict failure/success [3, 13–16], but a description of the clinical response in real life over the long term. Some authors do not recommend DSZ as a routine procedure [2], but in the meantime, children with severe allergies are at high risk of reactions and even death. Off-label OMZ, in these cases, could give them a chance to overcome this allergy. There is currently no agreed protocol for DSZ, with or without the use of OMZ, and several approaches can be found [17].
Substantial changes had to be made in the duration and doses of the procedure compared to the initial planned schedule, in nearly all the patients, adapting to individual real-life circumstances (reactions, availability, distance to hospital, comorbidities, etc.), and additional changes were made according to ongoing experience. When a new course of OMZ was used, patients tolerated a rapid DSZ procedure or even a direct challenge to resume food intake. A slower approach was made in the first patients, avoiding the food for 2–3 months, and that avoidance period between OMZ courses was gradually shortened or even suppressed in later patients. For clinical practice and patient information, OMZ-assisted DSZ failed in six (28%) of high-risk patients, even after two courses of OMZ, especially with eggs. Eight (38%) patients were able to tolerate the food after one course of OMZ, six (28%) required a second course of treatment and the response in another (#3) still remains to be evaluated. Patients and parents should be given information about the results and likely changes before performing this procedure, especially for older children and adolescents. Commitment is important as it takes a lot of dedication and effort and failure is not uncommon, even in patients with not too high sIgE levels. Additionally, they should be informed that the planned schedule will require changes and adaptation in most patients.
Furthermore, our findings suggest that a period of 12 weeks after discontinuing OMZ is too short to ensure continued tolerance and that longer intervals, at least four or rather six months, would be necessary before diagnosing tolerance or sustained unresponsiveness rather than DSZ or temporary hyporesponsiveness [18].
DSZ: desensitization
OMZ: omalizumab
sIgE: specific immunoglobulin E
AM: Conceptualization, Formal analysis, Investigation, Supervision, Visualization, Writing—original draft, Writing—review & editing. DTJ: Data curation, Formal analysis, Supervision, Visualization, Writing—review & editing. BF, MPS, LI, EB, and MN: Data curation, Investigation, Writing—review & editing. SU: Conceptualization, Investigation, Supervision, Writing—review & editing. AN: Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The research in this manuscript complies with the Declaration of Helsinki. The study was approved by the Institutional Ethics Committee of the Health Research Institute Hospital La Fe (no. 2017/0435).
Not applicable.
Not applicable.
Datasets are available from the corresponding author (amazon@comv.es) on reasonable request.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Valentina Faihs ... Knut Brockow
Polina Kostova ... Guergana Petrova
Ana Muñoz-Urribarri
Leonel Pereira, Ana Valado
Purificación González-Delgado ... Anna Nowak-Wegrzyn
