Affiliation:
1Alicante Institute for Health and Biomedical Research (ISABIAL), 03010 Alicante, Spain
2Clinical Medicine Department, University Miguel Hernández, 03202 Alicante, Spain
3Allergy Department, General University Hospital, 03010 Alicante, Spain
Email: purifica60@gmail.com
ORCID: https://orcid.org/0000-0002-1192-8475
Affiliation:
4Allergy Department, Hospital General Universitario Gregorio Marañón, 28007 Madrid, Spain
ORCID: https://orcid.org/0000-0001-8937-7311
Affiliation:
5Allergy Department, Hospital Universitario Lucus Augusti, 27003 Lugo, Spain
ORCID: https://orcid.org/0009-0002-2875-486X
Affiliation:
6Allergy Department, Complejo Hospitalario Universitario de Toledo, 45007 Toledo, Spain
ORCID: https://orcid.org/0009-0002-2317-5359
Affiliation:
1Alicante Institute for Health and Biomedical Research (ISABIAL), 03010 Alicante, Spain
2Clinical Medicine Department, University Miguel Hernández, 03202 Alicante, Spain
3Allergy Department, General University Hospital, 03010 Alicante, Spain
ORCID: https://orcid.org/0000-0003-1065-7199
Affiliation:
7Department of Pediatrics, NYU Grossman School of Medicine, Hassenfeld Children’s Hospital, New York, NY 10016, USA
8Department of Pediatrics, Gastroenterology and Nutrition, Collegium Medicum, University of Warmia and Mazury, 10719 Olsztyn, Poland
ORCID: https://orcid.org/0000-0002-0960-9854
Explor Asthma Allergy. 2024;2:148–160 DOI: https://doi.org/10.37349/eaa.2024.00036
Received: October 19, 2023 Accepted: February 26, 2024 Published: April 23, 2024
Academic Editor: Erminia Ridolo, University of Parma, Italy
The article belongs to the special issue The Different Faces of Food Allergy
Food protein-induced enterocolitis syndrome (FPIES) is an allergic disorder that manifests as reproducible gastrointestinal symptoms within hours of ingestion of the causative food, which can progress to dehydration and hypotension. Historically, FPIES has been recognized as a disease affecting the pediatric population but it can also develop de novo in adults. The pathophysiology is not well understood; however, the local adaptive immune system and gene expression linked to innate immune activation are implicated. Adult-onset FPIES has some differences with pediatric FPIES. Vomiting may be absent, while abdominal pain is the most common manifestation. A clear predominance in women occurs, being seafood the most common trigger, although many other foods have also been implicated. Diagnosis of adult-onset FPIES is based on a thorough clinical history but in many cases, it should be followed by an oral food challenge (OFC), due to the absence of vomiting in some patients and the lack of confirmatory diagnostic test. The first-line treatment for acute FPIES reactions is fluid replacement, by the oral route in mild to moderate reactions or via the intravenous route in severe reactions. Ondansetron may be effective in shortening the duration of emesis. Management of patients after diagnosis includes dietary advice and follow-up with supervised OFC at regular intervals to monitor for resolution. Tolerance to the trigger food in children is commonly achieved, a finding not so common in adult-onset FPIES. The aim of this article is to review the most important current concepts in epidemiology, pathophysiology, diagnosis, and management of FPIES.
Food protein-induced enterocolitis syndrome (FPIES) is a non-immunoglobulin E (IgE) mediated food allergy, which normally debuts in infancy and childhood and manifests as repetitive vomiting, sometimes followed by diarrhea. It can be accompanied by lethargy, hypotonia, hypothermia, and hypotension [1].
In recent years, an increasing number of cases of adult-onset FPIES have been described; however, data from these reports suggest some differences with pediatric FPIES [2–11], so the diagnostic criteria proposed in the international consensus may lead to underdiagnosis in adults.
This review aims to describe clinical characteristics, epidemiology, and differences between adult and pediatric FPIES and to propose diagnostic criteria to improve the diagnosis in adults.
Powell [12] first described FPIES in 1976, but it was not until 2015 that FPIES was recognized and codified in the International Classification of Diseases, 10th edition (ICD-10, code K52.2). Although FPIES is regarded as a rare food allergy disorder, the prevalence reported for children in the USA is 0.51% [13], a figure in line with other studies from Israel and Spain [14, 15]. In adults, a cross-sectional, population-based survey of the USA population carried out by Nowak-Wegrzyn et al. [13] from 2015 to 2016 estimated a prevalence of 0.22% [95% confidential interval (CI), 0.17–0.28] based on data from 40,443 adults, 113 of whom reported physician-diagnosed FPIES.
The true prevalence of adult FPIES is likely underestimated due to the combination of underdiagnosis, under-reporting, and non-attendance to healthcare centers for mild cases. In fact, adult case series report a median of 5 episodes to 10 episodes prior to diagnosis, with a median delay of four years [10, 11]. Regional variations in prevalence may also occur, as described in children [16].
Prevalence data for chronic FPIES are unknown and complicated by the similar clinical presentation to food protein enteropathies in childhood [17]. In adults, the disease is rarely recognized, and only one clinical case was communicated in the literature in 2020 [18].
The average age at the onset of adult FPIES is around 35 years but may present anytime from the late teenage years to old age. A clear predominance of women has been observed in all adult series reported (Table 1). Moreover, most series show high rates of atopic diseases, especially IgE mediated food allergy, rhinitis, asthma, and atopic dermatitis, in adults with FPIES, similar to children [3, 6, 9–11].
Adult-onset FPIES series reported
| Study | Sample size (n) | Study type | % Women | Mean age at 1st reaction (years) | Emergency department visits (%) | Foods | Patients who reacted to > 1 food or food group | Patients with other atopic diseases associated with FPIES (%) | Patients with sIgE to the trigger food (%) |
|---|---|---|---|---|---|---|---|---|---|
| Tan and Smith [3] (2014, Australia) | 31 | Prospective | 77% | 29 | 6% | Crustaceans, mollusks, fish, and egg | 16% | 52% | 0% |
| Du et al. [5] (2018, Canada) | 20 | Retrospective | 90% | 38 | - | Shellfish, dairy, wheat, and egg | 30% | 25% | 0% |
| Gonzalez-Delgado et al. [6] (2019, Spain) | 25 | Prospective | 88% | 28 | - | Crustaceans, fish, cephalopods, and bivalves | 40% | 72% | 0% |
| Li et al. [7] (2020, Canada) | 19 | Retrospective | 68% | 34 | 16% | Crustaceans | - | 28% | 5% |
| Crespo et al. [8] (2021, Spain) | 24 | Prospective and retrospective | 71% | 37 | 33% | Mollusks, crustaceans, fish, oats, and bean | 42% | 50% | 8% |
| García Paz et al. [9] (2023, Spain) | 28 | Retrospective | 75% | 32 | - | Bivalves, crustaceans, and fish | 7% | 50% | 7% |
| González-Delgado et al. [10] (2022, Spain) | 107 | Prospective | 94% | 30 | 14% | Crustaceans, fish, bivalves, cephalopods, and egg | 44% | 65% | 4% |
| Crespo et al. [11] (2022, Spain) | 42 | Prospective and retrospective | 83% | 40 | 21% | Shellfish, fish, vegetables, mushroom, and egg | 14% | 60% | 3% |
-: not reported. sIgE: specific IgE
A higher incidence of gastrointestinal disorders including eosinophilic esophagitis, inflammatory bowel disease, and celiac disease has also been reported in the adult population [10]. As awareness of FPIES grows, future studies will probably report higher incidence rates, especially in the adult population.
The mechanism(s) behind the characteristic symptoms of FPIES is unknown, although some evidence indicates the involvement of the immune system [19–26]. However, these studies were performed in children; there are no data on the adult population.
In adult FPIES, skin tests are usually negative, and food-sIgE is negative or low. Although some children with positive IgE evolve to have a classic IgE mediated disease [27], this has not been reported in adults [3–11]. Moreover, there is a working assumption that type I hypersensitivity has no role in the pathophysiology. In the early research on the disease mechanisms, some authors tried to show the possible implication of food-specific antibodies other than IgE, but casein-specific IgG, IgG1, IgG4, IgM, and IgA levels were not elevated in children with active disease compared with controls in whom the disease was resolved [19, 20].
Given the lack of a consistent antibody response, FPIES is traditionally considered a cell-mediated disease. Duodenal biopsies in affected children show increased mononuclear cells and tumor necrosis factor (TNF)-α expression in the epithelial cells [21]. TNF-α is known to increase intestinal permeability, and it can play a significant role in some FPIES symptoms [21, 22], as can diminished levels of transforming growth factor β (TGF-β) [19].
Morita et al. [22] showed that reactivated peripheral blood mononuclear cells (PBMCs) in patients with non-IgE cow’s milk allergy produced high levels of T helper type 2 (Th2) and Th1 cytokines. Caubet et al. [20] also evaluated PBMC cytokine responses to casein stimulation, comparing milk FPIES versus IgE mediated milk allergy versus outgrown IgE mediated milk allergy. They reported that TNF-α, interleukin 6 (IL-6), and Th2 cytokines were produced in response to casein stimulation in FPIES individuals. However, this response was also observed in current and outgrown IgE mediated milk allergy.
In a preliminary study of intestinal biopsy samples, Adel-Patient et al. [23] reported activated Th1, Th2, and Th17 cells in the mucosa of an FPIES patient, suggesting the involvement of the local adaptive immune system in the pathophysiology of the disease. This may explain the specificity of the clinical response. They also observed the presence of other cell types, including innate lymphoid cells (ILCs), in line with the recently reported systemic antigen-specific activation of innate cells associated with a positive food challenge [24, 25].
Furthermore, Goswami et al. [24] demonstrated profound activation of monocytes, neutrophils, natural killer cells, and eosinophils after oral food challenge (OFC), which might contribute to FPIES symptoms. Likewise, Mehr et al. [25] also communicated the expression of genes related to innate immune activation, including matrix metallopeptidase 9 (responsible for neutrophil migration), IL-1B, intercellular adhesion molecule, signal transducer, and activator of transcription-3, during positive OFC. Berin et al. [26] corroborated these findings, reporting that symptomatic FPIES challenges were associated with significant elevation of cytokines and chemokines, including IL-17 family markers, T cell activation (IL-2), and innate inflammatory markers. Mass cytometry demonstrated activation of non-conventional T cell populations, including γδ T cells and CD3+CD4–CD8–CD161+ cells.
Using a CD154-based detection approach, Goswami et al. [24] could not identify an antigen-specific T cell response in FPIES, though they observed a global loss of lymphocytes from the peripheral circulation following positive OFC.
These data do not necessarily rule out a role for T cells in FPIES. It is possible that antigen-specific cells can be located in the gastrointestinal tract and do not recirculate [26].
There is little research on gut microbiota alterations in non-IgE mediated food allergy disorders. Boyer and Scuderi [28] reported that the microbiota from stool in infants with FPIES was enriched with bacteria from the Gammaproteobacteria and Porphyromonadaceae families. Caparrós et al. [29] also reported a different gut microbial signature in FPIES patients compared with controls. Further gut microbiome research is required to know the precise mechanism of intestinal dysbiosis in FPIES.
A growing body of evidence supports a role for serotonin, a key mediator of the gut-brain axis, in the pathogenesis of FPIES. Ondansetron, a selective 5-hydroxytryptamine type-3 (5-HT3) antagonist, seems to improve the symptoms of acute FPIES [30]. Serotonin also interferes with the balance between Th17 and regulatory T cells [31]. Furthermore, the rise in serotonin activates cholinergic enteric and vagus nerves, causing increased intestinal motility [32].
In adults, FPIES symptoms generally appear after years of previous tolerance, but in some cases, symptoms begin in childhood and persist into adulthood. Currently, no risk factors associated with the development of adult FPIES have been identified, though some authors have speculated that the loss of oral tolerance may be due to environmental influences like diet, use of antimicrobials, infections, pollutants, and detergents driving a shift towards microbial dysbiosis and immune dysregulation [33]. The classic presentation of acute FPIES in children consists of profuse vomiting within 1 h to 4 h after ingestion of the suspected food, accompanied by pallor and lethargy; however, in adults, the onset of symptoms may be delayed up to 6 h [11].
The clinical presentation of FPIES in adults differs considerably from that of children, especially with regard to the presence of vomiting. With the exception of the series by García Paz et al. [9] (where all patients reported this symptom), the most recent published series estimates that only about 64.5% of adults with FPIES experience vomiting (Table 2). The heterogeneous inclusion criteria between the various studies may explain these differences. In adults, the predominant symptom in the published series is abdominal pain, followed by diarrhea. Only around 30% of adult patients experience all three symptoms [5]. Nausea is also common, with 69% of adults reporting this in the Crespo et al. [8] series. Other frequent symptoms in adults include weakness (50%), chills (29%), and lethargy (26%). However, hypothermia, hypotension, and dehydration are less common in adults than in children [10, 11]. Reactions usually resolve within approximately 15 h (range 4–48 h) [11].
The most common symptoms reported in adults
| Case series | Sample size (n) | Abdominal pain | Vomiting | Diarrhea |
|---|---|---|---|---|
| Tan and Smith [3] 2014 | 31 | 77.4% | 71.0% | 58.1% |
| Gleich et al. [4] 2016 | 8 | 87.5% | 62.5% | 75% |
| Du et al. [5] 2018 | 20 | 90% | 50% | 55% |
| Gonzalez-Delgado et al. [6] 2019 | 25 | 100% | 64% | 76% |
| Crespo et al. [8] 2021 | 24 | 87.5% | 75% | 91.6% |
| García Paz et al. [9] 2023 | 28 | 89.3% | 100% | 89.3% |
| Crespo et al. [11] 2022 | 42 | 71.4% | 59.5% | 92.9% |
| González-Delgado [10] 2022 | 107 | 96.3% | 60.7% | 71.9% |
The chronic form of FPIES, less common and less well known than the acute form, has been described in infants and is characterized by chronic emesis, watery diarrhea, and failure to thrive [17]. This form has scarcely been described in adults but is associated with weight loss, chronic diarrhea, and episodic vomiting, with some unrelated foods implicated as triggering symptoms [18]. This form in adults is probably frequently misdiagnosed as irritable bowel disease.
Crustaceans, fish, bivalves, and cephalopods are the common triggers described in different adult populations of Australia, Canada, and Spain [3–11]. However, in recent years many other foods have also been reported, including eggs, fruits (e.g., avocado, banana), oats, and other foods that are not usually implicated in IgE mediated reactions, like mushrooms, meats, garlic, and red pepper. It seems that any food may be implicated, suggesting the emergence of new triggers or possibly a higher index of suspicion [10].
Du et al. [5] reported that up to 20% of patients in a series of 25 had symptoms with wheat, and in the Crespo et al. [11] series, up to 4.8% reported symptoms after eating oats. In cases where cereals are the triggers, lack of awareness probably results in patients being underdiagnosed or misdiagnosed with non-celiac gluten sensitivity.
As with cereals, some patients initially labeled as lactose intolerant showed on OFC that milk can also induce FPIES symptoms [5, 10]; however, in adults, most cases of milk FPIES are probably labeled as lactose intolerance.
In some patients, more than one group of unrelated foods are responsible for symptoms, ranging from 7% in García Paz et al. [9] series to 44% in Crespo et al. [11]. The most common association observed was between different seafoods, such as crustaceans, mollusks, and cephalopods [6, 10, 11]. This clustering pattern has also been observed in the pediatric population, probably due to taxonomical relationships [34].
The lack of vomiting observed in many adults with FPIES is not the only difference with respect to children (Table 3). Unlike the pediatric population, in adults, there is a clear predominance of women in all the series reported [3, 5–11]. A hormonal influence has been postulated, although a recent study failed to demonstrate this fact [10]. The profile of implicated foods constitutes another difference between the pediatric and adult population. This might be a result of different dietary patterns; however, in older children as in adults, seafood is an important trigger [35, 36].
Comparison of FPIES in children and adults
| Variable | Children | Adults |
|---|---|---|
| Prevalence (USA) | 0.34–0.70% | 0.22% |
| Risk factors | Genetic: trisomy 21, father or sibling with FPIES; Environmental: birth via cesarean section, perinatal antibiotics, gut dysbiosis, and timing and pattern of solid food introduction in infancy [1, 14, 16] | Unknown |
| Sex | Equal or slight male predominance | Female 80% |
| Food triggers | Cow’s milk, oat, rice, egg, peanut, fish, avocado, and sweet potato | Seafood (shellfish and fish); rarely cow’s milk, egg, and wheat |
| Symptoms | Repetitive, projectile emesis, lethargy, and pallor | Severe abdominal pain, vomiting, and diarrhea |
| Time to onset of symptoms | 1–4 h | 1–6 h |
| Atypical FPIES (IgE-food sensitization) | About 5–20% | < 5% |
| Progression to immediate IgE mediated reactions in those with atypical FPIES | Reported by Onesimo et al. [27] | Not reported |
| Chronic FPIES | About 10% | Unknown, only 1 case published [16] |
| Natural history | Generally favorable, majority resolve by age 3–5, except fish FPIES, only 30–40% resolve by age 3–5Peanut FPIES, unknown natural history | Less favorable |
| Allergic comorbidities | High | High |
The severity of the disease also seems to differ between populations. Up to 31% of children were treated in the emergency department in Caubet et al. [37] series, while just 6.4% to 21.1% of adults required emergency care in the published series [3, 10, 11]. However, variations in the severity of FPIES are difficult to prove due to the many confounders. For example, adults carry less risk of food transgressions than children. Moreover, care seeking may be more frequent at the pediatric age, regardless of the severity of the reaction.
There are no published cases of adults treated in intensive care unit, unlike the pediatric population. Dehydration and metabolic changes also seem uncommon in adults; however, some severe cases have been reported [2–4, 6, 10, 11].
OFC outcomes also provide information about disease severity. In the largest adult series, 31 of 49 OFCs were positive, and only 33.3% of cases required treatment [10]. In the same series, 12.9% presented hypotension, weakness, and lethargy as a reaction to fish and eggs, in addition to digestive symptoms [10].
In another recent series [11], 9 of 21 OFCs were positive; in addition to digestive symptoms, three patients presented lethargy and three chills. Strikingly, none had emesis, and only four received intravenous saline solution. These data contrast with Wang et al. [38] results in a large pediatric population, in whom 169 OFCs were performed and 30 were positive; up to 50% of the children required intravenous fluid, and 3 required hospitalizations, including one in the intensive care unit. However, as Crespo et al. [11] note, adults with a history of severe symptoms often refuse OFC.
The natural history of the syndrome may also differ by age. Although in adults this is not well known, some authors have described a worse prognosis than in children [10, 11].
Otherwise, unlike the pediatric population, in which up to 20% of patients may show sIgE, especially in milk-induced FPIES, the percentage in adults is lower. Around 4% of patients showed sIgE to the offending food [10, 11]. Skin testing or sIgE can be considered in patients with FPIES at follow-up visits before OFC; however, the prognostic value in adults is unknown.
As in the pediatric population, diagnosis is based on a clinical history of typical signs and symptoms after the ingestion of the trigger food and remission after avoiding it. In the clinical history, aspects like reproducibility of symptoms after exposure, symptoms with other foods, and the latency period must be considered. In most cases, the first episodes are misdiagnosed as viral gastroenteritis or food poisoning.
In a significant percentage of adults, the main diagnostic criteria proposed in the international consensus [1], vomiting, is absent, while abdominal pain or cramping is the most common finding, usually associated with nausea or diarrhea. Due to the lack of any specific biomarkers for diagnosing and monitoring the disease, and given the unspecific nature of symptoms, a thorough clinical history should be followed by an OFC to confirm the diagnosis of FPIES in adults.
If no food transgressions have occurred over several previous years, an OFC should be performed at regular intervals to determine whether the disease has resolved.
In patients with multiple implicated foods and recurrent symptoms, a gastroenterology study is recommended. Once other causes are excluded, an elimination period for suspected triggers, followed by an OFC to confirm the diagnosis is recommended.
Differential diagnosis must consider anisakiasis; food poisoning; fructose, lactose, and sorbitol intolerances; and celiac disease (Table 4). In cases where multiple foods trigger FPIES symptoms, differential diagnosis with irritable bowel diseases may be difficult.
Differential diagnosis of FPIES in adults
| Diseases | Signs and symptoms that can distinguish the condition from FPIES |
|---|---|
| Small intestinal bacterial overgrowth (SIBO) | Bloating, flatulence, and abdominal discomfort with diarrhea or constipation. Diagnosis with a carbohydrate breath test or jejunal aspirate culture |
| Inflammatory bowel disease | Diarrhea, abdominal pain, rectal bleeding, and weight loss, but not related to intake of a specific food |
| Irritable bowel syndrome (IBS) | Abdominal pain and changes in bowel habits (constipation and/or diarrhea) and in stool form |
| Lactose intolerance | Bloating, cramps, diarrhea, borborygmi, and vomiting after ingestion of milk or dairy products with lactose |
| Fructose intolerance | Bloating, flatulence, or diarrhea after ingestion of fruit and fruit-based sweeteners |
| Celiac disease | No temporal relationship between symptoms and specific food intake, progressive malabsorption, and positive celiac serology |
| Eosinophilic gastroenteritis | Symptoms more chronic than episodic, vomiting less severe, and more likely to have positive IgE test results, usually not associated with specific food intake |
| FPIES | Abdominal pain, diarrhea, vomiting related to specific foods (especially seafood, eggs, etc.) |
| Intolerance of short-chain fermentable carbohydrates | Flatulence, abdominal pain, bloating, or diarrhea after foods containing fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) is variably referred to as carbohydrate intolerance |
Although episodes in adults seem less severe than in children, OFC should be performed in a medically supervised setting with access to intravenous rehydration, as some patients may develop repetitive vomiting and dehydration.
The total dose and dosing regimen for FPIES-related OFCs have not been systematically studied, so practices vary. Publications on procedures in adults are scarce because most studies are retrospective, and OFC is performed only in a small number of patients [6, 10, 11].
The international consensus proposes administering the challenge food, usually 0.3 g of the food protein per kilogram of body weight, in three equal doses over 30 min [1]. Infante et al. [39] propose a method with a single daily dose on two non-consecutive days (25% and 100% of a serving size, respectively). Other authors [10] propose a shorter protocol, dividing the total serving size into two doses: 25% of the total amount in the first dose and the rest 2 h later. In both protocols, a 4 h observation period is recommended. The recommendation is that in patients with severe symptoms, a two-day protocol is called for, while in patients with milder symptoms, the shorter protocol may be carried out. Ultimately, it is at the physician’s discretion to modify the regimen depending on the patient’s individual circumstances, as proposed by the international consensus [1].
After OFC, a milder increase in neutrophilia was observed in adults compared with children [6, 10, 11]. The absolute neutrophil count, measured from 31 positive OFCs, showed a median increase of 632.84 cells/mm3 (interquartile range 300–1,300 cells/mm3). Only four patients presented with a neutrophil count of more than 1,500 cells/mm3 [10]. These findings are similar to those reported by Crespo et al. [11], who observed neutrophilia in 3 out of 9 patients. Therefore, although neutrophilia is an uncommon finding, its presence supports the diagnosis.
The proposed algorithm is reflected in Figure 1. In patients with repeated episodes of abdominal pain, with or without nausea, vomiting, or diarrhea that appears 30 min to 6 h after the ingestion of food, and resolution of symptoms after avoiding the offending food, FPIES must be suspected. In adults, due to the nonspecific symptoms, with the absence of vomiting in a relevant percentage of cases, an OFC to confirm the diagnosis is proposed, especially in cases in which diagnosis is uncertain and also after patients have avoided the food for an interval to determine if tolerance has developed.
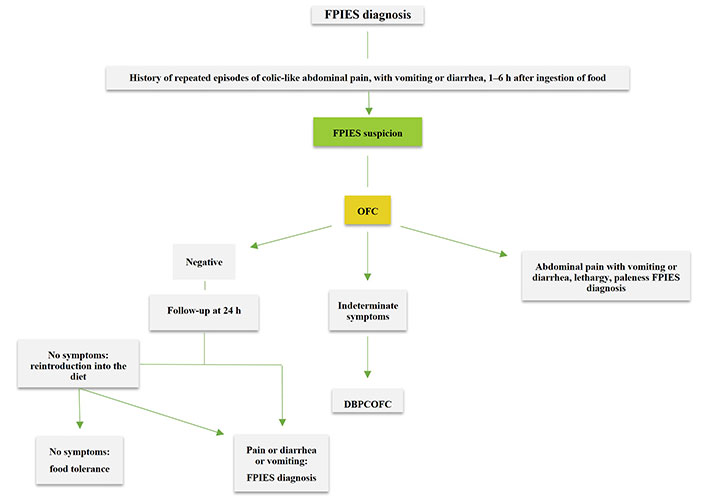
Diagnostic algorithm for adult FPIES. DBPCOFC: double-blind, placebo-controlled OFC
OFC must be carried out with the food prepared in the same manner that causes symptoms and with the appropriate doses [1]. In patients with recurrent symptoms in whom FPIES is suspected, a gastroenterology study is necessary to rule out other diseases such as SIBO, inflammatory bowel disease, and celiac disease, among others [40]. After the exclusion of other causes, a food diary may be useful to identify possible culprit foods, which can then be tested by OFC.
In cases in which symptoms reported after an OFC are equivocal or difficult to evaluate, and in patients who are anxious, a DBPCOFC is advised. Collaboration between allergology and gastroenterology services is essential to diagnose FPIES, especially in patients with more than one food or group of foods implicated.
Management of acute FPIES reactions consists of symptom-directed treatment, and the approach will differ depending on the severity of the symptoms [41]. First-line treatment is rehydration with fluids. For mild reactions, an oral replacement will be sufficient, whereas in moderate or severe reactions, intravenous fluid replacement (1,500–2,000 mL of physiological saline) is recommended. If nausea or vomiting occurs, the treatment of choice is orodispersible ondansetron (a serotonin 5-HT3 receptor antagonist with central and peripheral effects shown to be safer than other antiemetics) [30].
For abdominal pain, analgesics such as paracetamol 500 mg can be used. Adrenaline is not indicated [1]. The use of corticosteroids is controversial and usually reserved for severe reactions. A single dose of intravenous methylprednisolone (1 mg/kg, maximum 60–80 mg) may be given in severe reactions. In life-threatening reactions, intravenous vasopressors may be necessary [41].
If FPIES is suspected, the patient should be referred to the allergist, who will make the diagnosis and recommend an avoidance diet, which will be personalized for each patient, preferably through shared decision-making. Patients are usually advised to avoid the entire food group that has produced the reaction. However, avoidance of other associated food groups (e.g., fish and shellfish), is generally not recommended unless the patient reports symptoms with both [1]. Some patients may benefit from an OFC with a fish or shellfish different from the causative one to avoid unnecessary restrictions.
Currently, there are no biomarkers or data to help predict the development of tolerance, so OFC is the only available diagnostic tool to test tolerance and thus avoid prolonged exclusion diets. The OFC should be performed every 2–3 years, due to the relatively lower tendency of symptom remission in adults. However, some patients with severe reactions refuse the test and prefer to continue with the exclusion diet, so management should be individualized.
Most FPIES cases with childhood-onset resolve by 3–5 years of age. Age at resolution depends mainly on the type of food. Milk and cereals are usually tolerated earlier, while the period for achieving tolerance is longer for egg and fish FPIES. However, patients with multiple FPIES triggers do not appear to become tolerant at an older age than those with a single trigger [16]. Data on the acquisition of tolerance in adults are scarce, but it seems to take longer than in children, and in many cases, it may be persistent [6, 8, 10, 11].
Recent studies [10, 11] in adults have provided more insights into the natural history of FPIES in this age group. One prospective study [10] with a median follow-up period of 6.2 years observed that 16.8% of patients achieved tolerance after a median of 3.5 years. However, other patients in the cohort had not overcome the disease even after 40 years, a finding also described elsewhere [2]. An inverse correlation between disease duration and resolution was observed [10]. Crespo et al. [11] reported that in a population of 15 patients in which OFC was performed, 40% tolerated the offending food after a median of 7 years from the first reaction.
The international consensus [1] provides a useful tool for the diagnosis and management of FPIES; however, a considerable proportion of adults go undiagnosed using the criteria proposed.
Due to the lack of any specific biomarkers for diagnosing and monitoring the disease, and given the unspecific nature of symptoms, a thorough clinical history should be followed by an OFC to confirm the diagnosis of FPIES in adults. The latest reports [10, 11] confirm that in adults, some patients achieve tolerance after a period of avoidance.
More studies are needed to better understand the characteristics of adult FPIES, its pathogenesis, and its natural history, and it is essential to identify a biomarker to diagnose and monitor the disease.
FPIES: food protein-induced enterocolitis syndrome
IgE: immunoglobulin E
IL-6: interleukin 6
OFC: oral food challenge
sIgE: specific immunoglobulin E
Th2: T helper type 2
TNF: tumor necrosis factor
PGD and ANW: Conceptualization, Investigation, Writing—original draft, Writing—review & editing, Supervision. AE and RNO: Conceptualization, Investigation, Writing—original draft. EM: Investigation, Writing—original draft. JF: Conceptualization, Investigation, Supervision, Resources. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 7107
Download: 58
Times Cited: 0
Angel Mazon ... Antonio Nieto
Valentina Faihs ... Knut Brockow
Polina Kostova ... Guergana Petrova
Ana Muñoz-Urribarri
Leonel Pereira, Ana Valado
